Clinical, Morphological, and Immunophenotypic Insights into Pediatric Acute Megakaryoblastic Leukemia: A Multifaceted Approach
Download
Abstract
Background: Despite described in literature for decades, many aspects of acute megakaryoblastic leukemia (AMKL) particularly immunophenotypic spectrum remain unclear. Our study of a total of 25 pediatric AMKL cases, present detailed characteristics of AMKL patients, including immunophenotype, morphology and flow cytometric (FCM) properties.
Methods: This is a retrospective study of a total 25 AMKL cases, diagnosed on flow cytometry, performed either on nlood or bone marrow aspirate. Patients were categorized into either DS or non-DS AMKL cases. Their demographics, clinical history, morphological and cytogenetic findings were retrieved from online hospital information portal. Immunophenotypic expression of CD markers and flow cytometric properties were analyzed and recorded.
Results: The study found that 24% of patients were DS-AMKL, while 76% were non-DS-AMKL. The median WBC count was higher in DS-AMKL patients, and blasts were more likely to express CD7, CD36, CD11b, CD13, and CD33. FCM properties showed a median w/h ratio of 1.45 in DS-AMKL patients and 1.20 in non-DS-AMKL patients.
Conclusion: The present findings are a good cohort of pediatric AMKL cases, with in-depth analysis of flow cytometric properties and immunophenotype. No statistical significance was found in the studied categories between DS-AMKL and Non DS-AMKL, which may be due to the fact that DS-AMKL comprised a small subset of the total cases and/or overall, both subtypes share common pathogenetic pathways.
Introduction
The WHO Classification 2022 categorizes AML into various groups, including those with defining genetic abnormalities and differentiation-based classifications, with acute megakaryoblastic leukemia (AMKL) being a rare and aggressive subtype primarily seen in children [1]. AMKL is characterized by at least 20% leukemic cell accumulation in the bone marrow, with 50% showing megakaryocytic lineage. Children with Down syndrome (DS) have a higher incidence of AMKL [2]. Another related category is transient abnormal myelopoiesis (TAM), which has distinct genetic features; unlike AMKL, TAM often resolves spontaneously within weeks [1, 3, 4]. Despite being recognized for decades, AMKL’s immunophenotypic and morphological characteristics remain underexplored [2]. Notably, the differences between Down syndrome-associated AMKL (DS-AMKL) and non-DS AMKL in demographics, immunophenotypic markers, and morphology have not been thoroughly compared. This study presents the hematological features, immunophenotypic profiles, microscopic findings, flow cytometric properties, and cytogenetic abnormalities of AMKL patients diagnosed at a tertiary care center over five years, including a comparison between DS-AMKL and non-DS-AMKL.
Materials and Methods
Study design
This study is retrospective in nature, encompassing all cases of acute megakaryoblastic leukemia (AMKL) diagnosed at the Department of Haematology, Indus Hospital and Health Network in Karachi, Pakistan, between 2019 and 2023. The study cohort comprised 25 patients whose demographics, clinical histories, and morphological findings from specimens were extracted from the institution’s online portal hospital management information system (HMIS). Flow cytometric analyses were conducted and characterized for each patient, alongside the retrieval and documentation of cytogenetic results where available. Patients were categorized into DS-AMKL or Non DS-AMKL subtypes. Ethical approval was obtained from the Institutional Review Board (IRB) of the institute. Inclusion criteria consisted of cases diagnosed as AMKL based on flow cytometry, while exclusion criteria encompassed cases not diagnosed via flow cytometry, non-AMKL acute leukemias, all chronic leukemias, and cases lacking complete demographic or clinical information.
CBC
The specimens were analyzed using an EDTA anticoagulant tube on the Beckman Coulter DxH 900 automated hematology analyzer.
Morphology
Specimen slides were prepared for morphological interpretation and stained with Leishman stain.
Immunophenotyping
Flow cytometric analysis was conducted on peripheral blood specimens from 20 patients, while bone marrow aspirates were analyzed for the remaining 5 patients. Multi-parameter (8 color) flow cytometry was performed using a BD FACS Canto 2 analyzer equipped with DIVA software. A comprehensive panel of markers was utilized, including MPO (myeloperoxidase; intracytoplasmic), TdT (terminal deoxynucleotidyl transferase; intracytoplasmic), CD34, CD45, CD19, CD79a (intracytoplasmic), CD3 (intracytoplasmic), CD7, CD33, CD13, CD11b, CD11c, CD64, CD61, CD41a, CD42b, HLADR, CD117, CD36,
and glycophorin A. Blasts were gated using side scatter (SSC) vs. CD45 dot plot, and the expression of various markers was evaluated within this population. A marker was considered positive if at least 10% of the population exceeded an isotypic control threshold. Staining intensity was categorized as negative, dim positive, positive, or bright positive based on the fluorescence intensity compared to a corresponding normal hematolymphoid cell population. A diagnosis of acute megakaryoblastic leukemia (AMKL) was confirmed when blasts expressed at least two of the platelet glycoproteins (CD42b, CD41, or CD61).
Flowcytometry (FCM) properties
The study involved the examination of side scatter (SSC) against CD45 plots, with CD45 represented on the x-axis and SSC on the y-axis, maintaining consistent plot dimensions across all analyses. Detailed analysis was performed on the visual morphology, CD45 expression, and SSC expression of the blast cluster. CD45 expression levels were classified as negative (100-101), dim positive (102-103) or positive (103 and above) based on forward scatter (FSC) log scale. Morphometric assessment of the blast cluster included measuring the maximum width and height at 100% magnification on an A4-sized printout, calculating the ratio between width and height (w/h), and categorizing SSC expression based on the maximum y-axis scale into low (0-50), moderate (50-100), and high (above 100). In cases where CD45/SSC expression varied, the brighter value was documented.
Cytogenetics
Karyotyping was conducted on specimens collected in sodium-heparin anticoagulant tubes. Cell cycle arrest was achieved using colcemid, followed by fixation with potassium chloride. The G-banding technique (Giemsa staining) was employed for chromosomal analysis. The resulting images were analyzed and interpreted using the semi-automated Cytovision MB8 system from Leica.
Statistical analysis
Data analysis was conducted using SPSS software (Version 23.0, IBM, Armonk, NY, USA). Qualitative variables include gender, clinical presentation, physical examination findings, morphology and shape of the blast cluster, SSC and CD45 expression. Quantitative variables include age, blood cell counts, blast percentage and width-to-height ratio. Descriptive analyses of qualitative data were presented as frequencies and percentages (n%), while quantitative data were expressed as mean ± standard deviation or median (range) if not normally distributed. The Shapiro-Wilk test was used to assess the normality of the dataset. Statistical comparisons of continuous variables between two groups were performed using either the independent samples t-test or the Mann–Whitney U-test, depending on the distribution. Levene’s Test was applied to assess the equality of variances. Categorical data from two groups were analyzed using the χ2-test, with the Monte Carlo test applied for larger contingency tables. Pearson’s Chi-square test was employed, and Fisher’s Exact test was used when the minimum expected count was less than 5. A significance level of α = 0.05 was set, with p-values < 0.05 considered indicative of statistical significance.
Results
Demographics
The study encompassed a total of 25 patients, with 6 (24%) diagnosed with DS-AMKL and 19 (76%) with non-DS-AMKL. The median age at diagnosis for DS-AMKL patients was 38 months (range: 0.03-53 months), compared to 44 months (range: 8-156 months) for non-DS-AMKL patients (p = 0.203). The gender distribution revealed that 5 (35.7%) of the DS-AMKL group were male and 1 (9.1%) was female, whereas the non-DS-AMKL group comprised 9 (64.3%) males and 10 (90.9%) females (p = 0.180) (Table 1).
| VARIABLES | DS-AMKL | NON DS-AMKL | P VALUE |
| Patients, n (%) | 6 (24) | 19 (76) | |
| Age at diagnosis, median months (range) | 38 (0.03-53) | 44 (8-156) | 0.203 |
| Gender | 0.18 | ||
| Male, n (%) | 5 (35.7) | 9 (64.3) | |
| Female, n (%) | 1 (9.1) | 10 (90.9) | |
| Hemogram | |||
| Hemoglobin (Mean ± SD) | 10.33 ± 4.81 | 7.22 ± 1.99 | 0.177 |
| WBC, median (range) | 30.45 (7.50 - 82.40) | 45.0 (8.70 - 2305) | 0.408 |
| Platelets, median (range) | 87.5 (5.0 - 228.0) | 15.0 (2.0 - 519.0) | 0.339 |
| Blasts (Mean% ± SD) | 46.5 ± 21.7 | 43.2 ± 29.9 | 0.803 |
| Clinical Presentation | n (%) | n (%) | |
| Fever | 4 (66.7) | 18 (94.7) | 0.133 |
| Bleeding manifestations | 3 (50.0) | 9 (47.4) | 1 |
| Hepatomegaly | 1 (16.7) | 13 (68.4) | 0.056 |
| Splenomegaly | 2 (33.3) | 12 (63.2) | 0.35 |
| Lymphadenopathy | 1 (16.7) | 10 (52.6) | 0.18 |
| FCM properties | |||
| W/H ratio, Median (Range) | 1.45 (0.9-2.2) | 1.20 (0.7-2.8) | 0.406 |
| SSC expression, n (%) | 0.409 | ||
| Low | 0 (0) | 4 (21.1) | |
| Moderate | 5 (83.3) | 14 (73.7) | |
| High | 1 (16.7) | 1 (5.3) | |
| CD45 expression, n (%) | 0.069 | ||
| Bright | 5 (83.3) | 6 (35.3) | |
| Moderate | 1 (16.7) | 11 (64.7) | |
| Blast Cluster Shape, n (%) | 0.058 | ||
| Circle | 1 (16.7) | 0 (0.0) | |
| Horizontal | 4 (66.7) | 5 (26.3) | |
| Horizontally Oval | 0 (0.0) | 4 (21.1) | |
| Oval | 1 (16.7) | 10 (52.6) |
Clinical presentation
Regarding clinical presentation, fever was observed in 4 (66.7%) DS-AMKL patients and 18 (94.7%) non-DS- AMKL patients (p = 0.133). Bleeding manifestations were noted in 3 (50.0%) DS-AMKL patients and 9 (47.4%) non-DS-AMKL patients (p = 1.000). Hepatomegaly was identified in 1 (16.7%) DS-AMKL patient compared to 13 (68.4%) non-DS-AMKL patients (p = 0.056). Additional clinical findings revealed that splenomegaly was present in 2 (33.3%) DS-AMKL patients and 12 (63.2%) non-DS- AMKL patients (p = 0.350), while lymphadenopathy was observed in 1 (16.7%) DS-AMKL patient and 10 (52.6%) non-DS-AMKL patients (p = 0.180) (Table 1).
Hematological profile
Laboratory characteristics indicated that hemoglobin levels were slightly elevated in the DS-AMKL group (mean ± SD: 10.33 ± 4.81 g/dL) in comparison to the non-DS-AMKL group (7.22 ± 1.99 g/dL) (p = 0.177).
The median white blood cell (WBC) count was 30.45 x 109/L (range: 7.50-82.40) in DS-AMKL and 45.0 x 109/L (range: 8.70-2305) in non-DS-AMKL (p = 0.408). The median platelet count was 87.5 x 109/L (range: 5.0-228.0) in DS-AMKL, compared to 15.0 x 109/L (range: 2.0-519.0) in non-DS-AMKL (p = 0.339). The mean percentage of blasts was 46.5 ± 21.7% in DS-AMKL and 43.2 ± 29.9% in non-DS-AMKL (p = 0.803) (Table 1).
Immunophenotypic expression
Concerning CD45 expression, bright expression was recorded in 5 (83.3%) DS-AMKL and 6 (35.3%) non-DS- AMKL patients, while moderate expression was noted in 1 (16.7%) DS-AMKL and 11 (64.7%) non-DS-AMKL patients (p = 0.069).
In contrast to the usual negativity of tumor cells for CD34 in AMKL, overall 12 out of 25 (48%) cases were positive for CD34. 100% of the cases were positive for CD61, CD 41a and CD42b (whichever applied). 16 (64%) cases were positive for CD117. HLA-DR, which is commonly present on myeloblasts, was only positive in 5 (20%) cases of Non DS and negative in all cases of DS-AMKL. MPO, TdT, CD79a, CD3 and glycophorin A were 100% negative. CD7 showed 100% positivity in DS-AMKL where as 29% positivity in Non DS-AMKL. Similarly, CD36 expression was in 100% of the cases in DS-AMKL while 67% in Non DS-AMKL. Other markers showed variable expression. Compared with Non DS-AMKL, blasts in DS-AMKL cases were more likely to express CD7, CD36 and CD11b, as well as CD13 and CD33. See Tables 2 and 3. Also see Figure 1 for flow cytometric dot plots of a case.
Table 2. Expression of Immunophenotypic Markers (Non DS-AMKL) – Heat Chart.
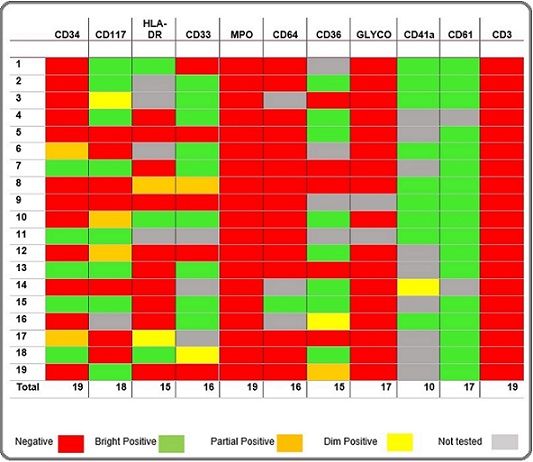
Table 3. Expression of Immunophenotypic Markers (DS-AMKL) – Heat Chart.
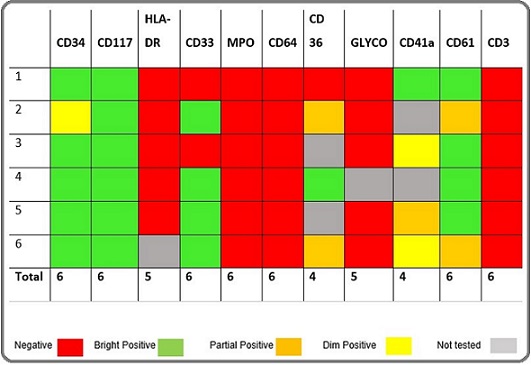
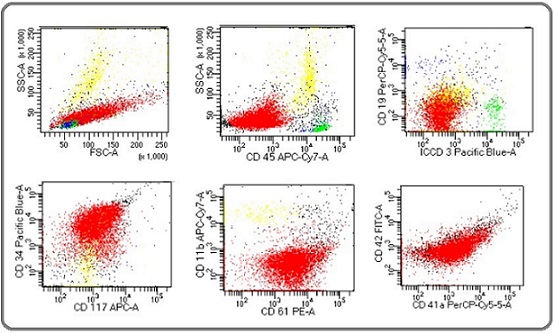
Figure 1. Flow Cytometric Dot Plots of One of Our Case. Color coding – Red: blasts. Green: benign T cells. Blue: benign B cells. Yellow: granulocytes. Interpretation: Blasts are negative for CD45, CD19, CD3 and CD11b, while positive for CD34, CD117, CD61, CD41 and CD42.
Flowcytometric (FCM) properties
Flow cytometry (FCM) properties showed that the width to height (W/H) ratio had a median of 1.45 (range: 0.9-2.2) in DS-AMKL patients and 1.20 (range: 0.7-2.8) in non-DS-AMKL patients (p = 0.406). Side scatter (SSC) expression was low in 0 (0%) DS-AMKL patients and 4 (21.1%) non-DS-AMKL patients, moderate in 5 (83.3%) DS-AMKL patients and 14 (73.7%) non-DS-AMKL patients, and high in 1 (16.7%) DS-AMKL patient and 1 (5.3%) non-DS-AMKL patient (p = 0.409). The shape of the blast cluster was categorized as follows: circle in 1 (16.7%) DS-AMKL patient and 0 (0%) non-DS-AMKL patients, horizontal in 4 (66.7%) DS-AMKL patients and 5 (26.3%) non-DS-AMKL patients, horizontally oval in 0 (0%) DS-AMKL patients and 4 (21.1%) non-DS-AMKL patients, and oval in 1 (16.7%) DS-AMKL patient and 10 (52.6%) non-DS-AMKL patients (p = 0.058).
Cytogenetic findings
Amongst all the cases with available cytogenetic studies, DS-AMKL cases had confirmed trisomy 21, while one case also showed complex karyotype. In Non DS- AMKL patients, 2 (8%) had gain of RUNX1, 4 (16%) had complex karyotype and 3 (12%) had normal karyotype. The majority of cases do not share common cytogenetic abnormalities. These finding support the fact that the AMKL is a biological heterogeneous sub-type of AML.
Discussion
Acute megakaryoblastic leukemia (AMKL) is a diverse form of acute myeloid leukemia (AML) originating in primitive megakaryocytes, defined by over 20% megakaryocytic blasts [1]. AMKL patients are categorized into children with Down syndrome (DS), children without DS, and adults. It represents 3–10% of pediatric AML cases, generally with a poor prognosis, except for DS children, who have a significantly better outlook [1, 3]. AMKL is the most common AML type in DS patients, with a 400-fold higher risk than in non-DS children. In adults, AMKL is rare, accounting for about 1% of AML cases, and its clinical presentation is similar to other AML subtypes, often including peripheral blood cytopenias and, occasionally, thrombocytosis [1].
Among a total of 25 cases in the present study, 6 (24%) of the cases were DS AMKL while 19 (76%) were Non-DS AMKL cases. Patients belonging to the present study were all from the pediatric age group with median age of 3.6 years (ranged from 1 day to 13 years). Ages of the patients were similar and overlapping in either category, except for one DS AMKL case who presented on the first day of life. Most of the patients presented with complaints of fever and bleeding manifestations (see Table 1), comparable to the clinical presentation found by Sharma S. and colleagues, as well as reported in other studies (see Table no. 1 for comparison) [1, 2]. Moreover, no significant difference was found between the initial clinical presentation of DS vs NON-DS AMKL patients in our study, except one Down’s syndrome case who had accompanying congenital heart anomalies. Furthermore, visceromegaly was found in 14 (56%) and lymphadenopathy in 11 (44%) of the cases, most of which belonged to the Non DS-AMKL category. These physical examination findings were similar to those found by Asahito Hama et al [4] in their cohort of 45 pediatric AMKL cases [5].
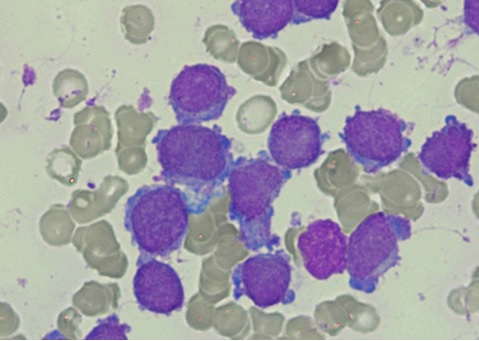
The patients’ hemograms revealed anemia, thrombocytopenia and leucocytosis, with median hemoglobin of 10.33 g/dl in DS-AMKL and 7.22 g/dl in Non DS-AMKL, median platelet count of 87 x 10E9/L in DS-AMKL and 15 x 10E9/L in Non DS-AMKL, median WBC count of 30.5 x 10E9/L in DS-AMKL and 45.0 x 10E9/L in Non DS-AMKL cases. In an Indian study by Sharma S. and colleagues of five AMKL cases, the pediatric patients had anemia and thrombocytopenia, similar to the findings of the present study, while WBC count ranged from 7.1 to 40 x 10E9/L [2]. Another large cohort of 45 patients studied by Asahito Hama et al, found similar hemogram profiles, with no significant difference between DS vs Non-DS AMKL cases [5].However, 10 of our patients presented with WBC count more than 50 x 10E9/L and ranged from 52 to 230.5 x 10E9/L. None of the reported studies show such high counts, to the best of our knowledge [1]. Blast cells were in the range of 15-72% in DS AMKL cases while 8-87% in Non-DS AMKL ones. Characteristic cytoplasmic blebbing was observed in 14 (56%) cases overall while clustering was noted in 2 (8%) cases. See Figure 2 for smear morphology of a case.
Figure 2. One of Our Case, Peripheral Smear Exhibiting Characteristic Megakaryoblasts Morphology with Granular Chromatin and Cytoplasmic Blebbing.
The rest had unremarkable morphology. These findings showed no statistically significant differences between DS-AMKL and Non-DS-AMKL patients. A reason could be due to the less number of patients overall and also particularly in DS-AMKL category. The study of Asahito Hama et al also reported varying blast cells percentage as well as spectrum of morphology ranging from blasts with deep basophilic cytoplasm, blasts having cytoplasmic blebbing or undifferentiated morphology [5]. A study of 29 AMKL patients by Paredes-Aguilera et al [6] reported overall blasts percentage ranging from 30% to 100%. This study included only two DS AMKL cases, so a reliable comparison couldn’t be undertaken. Literature also revealed variable blasts morphological features in AMKL ranging from clustering, cytoplasmic blebbing to simple blasts with round nuclei and scant agranular cytoplasm [1, 2] [5-8].
Flow cytometry has considerably enhanced clinicians’ ability to correctly diagnose AMKL [7]. In the past, numerous flow cytometry laboratories endeavored to identify the least number of antibodies capable of correctly diagnosing acute leukemia [9-11]. To reduce the cost of reagents, efficient triaging methods are being investigated. Objective analysis of SSC versus CD45 expression can be used in an attempt to distinguish between lymphoid and myeloid leukemia [9-11]. In this study, we attempted to identify an objective metric on SSC versus CD45 gated blasts to study parameters of flowcytometric properties which may help to distinguish between lymphoid and myeloid blasts; specifically megakaryoblasts. A limitation encountered was that upon literature search, to the best of our knowledge, no study could be found which have studied these flow cytometric properties parameters in AMKL cases. All our DS AMKL cases showed bright expression of CD45, while it was moderate to bright in the other cohort. Amongst the DS-AMKL cases, shape of the blast cluster was mostly horizontal, while in the Non DS-AMKL cases, it varied from oval to horizontal. SSC expression was moderate in most of the cases in either categories. The median width to height (w/h) ratio in DS AMKL was 1.45, while it was 1.20 in Non-DS AMKL cases. However, no statistical significance could be found in differentiating these two AMKL subtypes. Saksena et al [9] studied such properties in acute leukemia cases (ALL vs AML) and found the median w/h ratio of 2.3 in T-ALL, 3.9 in B-ALL and 0.97 in AML cases. They further studied and recommended that using a w/h cut-off ratio of 1.6 effectively differentiated between ALL and AML. Our study also demonstrated median w/h ratio of less than 1.6, favoring similar results and strengthening the suggestion. Our recommendation is more such comparative studies are needed having a meaningful sample size, in order to statistically analyze flow cytometric properties having impact on these patients diagnosis, management and outcomes.
Detection of megakaryoblasts by immunophenotyping depends on the detection of lineage associated markers which include CD41, CD61 and CD42 [1]. CD61 was 100% positive in our study cases. CD41 was bright positive in all Non-DS AMKL and two DS AMKL cases while it was partial / dim positive in four DS AMKL patients. Expression of CD42 was mostly bright positive in all our cases. Aberrant expression of CD7 was present in 100% of DS-AMKL where as 29% positivity in Non DS-AMKL patients. Similarly, CD36 expression was positive in 100% of the cases in DS-AMKL while 67% in Non DS-AMKL. Compared with Non DS-AMKL, blasts in DS-AMKL cases were more likely to express CD7 and CD36. HLA- DR, which is commonly present on myeloblasts, was only positive in 5 (20%) cases of Non DS and negative in all cases of DS-AMKL. This was in contrast to the findings shown by Rogelio Paredes-Aguilera and colleagues [6]. Their study of 29 prospective AMKL cases revealed HLA- DR positive expression in 73.9% of the cases. Moreover, they reported CD13 expression in 44.8% and CD33 in 20.7% of the cases. In our study CD13 expression was mostly negative in either categories while CD33 showed variable expression; positive in 4 (67%) DS AMKL cases while 15 (79%) Non-DS AMKL cases. This indicates that AMKL generally lacks HLA DR and CD13 expression, similar findings were reported by Nienke Brouwer et al. in their immunophenotypic evaluation of 72 AMKL cases using the EuroFlow AML panel [7]. MPO, TdT, CD79a, CD3 and glycophorin A were 100% negative in our study.
AMKL has a complex cytogenetic profile, reflecting its heterogeneous nature [1, 5]. Our study had limitations due to incomplete karyotyping. When available, results showed trisomy 21 in all DS-AMKL cases, with one case also having a complex karyotype. Among Non DS-AMKL patients, 16% had complex karyotypes and 12% had normal karyotypes. Eliane Duchayne and colleagues found complex karyotypes in 38.5% of pediatric and 58.5% of adult AMKL cases, with adults showing greater complexity [8]. Notably, they identified the t (1;22) (p13;q13) abnormality in 37% of cases, primarily in patients under 1 year old.
In conclusion, this is perhaps the largest such study on AMKL in our region, providing useful insights into pediatric acute megakaryoblastic leukemia with detailed analysis of flow cytometric properties and immunophenotypic markers expression. No statistical significance was found in the studied categories between DS-AMKL and Non DS-AMKL, which may be due to the fact that DS-AMKL comprised a small subset of the total cases and/or overall both subtypes share common pathogenetic pathways.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- World Health Organisation Classification Haematolymphoid Tumors, 5th edition, International Agency for Research on Cancer 2022.
- Clinico-haematological profile of acute megakaryoblastic leukaemia: report of five cases Sharma S, Nangia A, Jain Malhotra S, Narayan S, Harbhajanka A, Singh S. Advances in Hematology.2009;2009. CrossRef
- Acute megakaryocytic leukemia: What have we learned Hahn AW , Li B, Prouet P, Giri S, Pathak R, Martin MG . Blood Reviews.2016;30(1). CrossRef
- Transient myeloproliferative disorder in children with Down syndrome: clarity to this enigmatic disorder Gamis AS , Smith FO . British Journal of Haematology.2012;159(3). CrossRef
- Acute megakaryoblastic leukaemia (AMKL) in children: a comparison of AMKL with and without Down syndrome Hama A, Yagasaki H, Takahashi Y, Nishio N, Muramatsu H, Yoshida N, Tanaka M, et al . British Journal of Haematology.2008;140(5). CrossRef
- Biology, clinical, and hematologic features of acute megakaryoblastic leukemia in children Paredes-Aguilera R, Romero-Guzman L, Lopez-Santiago N, Trejo RA . American Journal of Hematology.2003;73(2). CrossRef
- Immunophenotypic Analysis of Acute Megakaryoblastic Leukemia: A EuroFlow Study Brouwer N, Matarraz S, Nierkens S, Hofmans M, Nováková M, Costa ES , Fernandez P, et al . Cancers.2022;14(6). CrossRef
- Acute megakaryoblastic leukaemia: a national clinical and biological study of 53 adult and childhood cases by the Groupe Français d'Hématologie Cellulaire (GFHC) Duchayne E, Fenneteau O, Pages M, Sainty D, Arnoulet C, Dastugue N, Garand R, Flandrin G. Leukemia & Lymphoma.2003;44(1). CrossRef
- Side scatter versus CD45 flow cytometric plot can distinguish acute leukaemia subtypes Saksena A, Gautam P, Desai P, Gupta N, Dubey A. P., Singh T. The Indian Journal of Medical Research.2016;143(Supplement). CrossRef
- Discriminant function analysis as decision support system for the diagnosis of acute leukemia with a minimal four color screening panel and multiparameter flow cytometry immunophenotyping Ratei R., Karawajew L., Lacombe F., Jagoda K., Del Poeta G., Kraan J., De Santiago M., et al . Leukemia.2007;21(6). CrossRef
- Optimizing antibody panels for efficient and cost-effective flow cytometric diagnosis of acute leukemia Haycocks NG , Lawrence L, Cain JW , Zhao XF . Cytometry. Part B, Clinical Cytometry.2011;80(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times