Identification of the Relation between Uterine Manipulator and the Pathological Factors and Oncologic Outcome in Patients with Uterine Cancer
Download
Abstract
Background: Endometrial cancer (EC) is the most common gynecological cancer in developed countries, and its incidence is increasing. The uterine manipulator (UM) increases the ergonomics of surgical movement during laparoscopic total hysterectomy.
Objectives: The aim of this study is to compare the surgical and oncological results of total laparoscopic hysterectomy with or without UM.
Methods: One hundred seventeen EC patients who underwent total laparoscopic hysterectomy between 2018 and 2024 were retrospectively evaluated.
Results: One hundred five (89.7%) of the patients were over 50 years of age, 102 (87.2%) were in the postmenopausal period, and 79 (67.5%) had a BMI >30. Tumor size was larger than 2 cm in 70 (59.8%) of the patients, and FIGO stage was I in 105 (89.7%) patients. Fifty-eight (49.6%) patients had myometrial invasion >50%, 4 (3.4%) patients had cervical involvement, 77 (65.8%) patients had lymphovascular space invasion (LVSI), 3 (2.6%) patients had adnexal involvement, and 9 (7.7%) patients had lymph node metastases. Recurrence was observed in 15 patients, and 15 patients died. Of the 95 (81.2%) patients whose procedure utilized UM, 17 had LVSI, 12 patients experienced recurrence, and 12 patients died. No significant relationship was observed between UM use and LVSI (p=0.100), recurrence (p=0.838), or survival (p=0.838).
Conclusion: The use of UM in laparoscopic surgery of EC did not appear to affect LVSI, recurrence, or survival in EC.
Introduction
Endometrial cancer (EC), one of the most common gynecological malignancies with increasing incidence and mortality rates, is the fourth most common cancer among women worldwide [1]. In 2023, 66,200 new EC cases and 13,030 EC deaths were estimated in the United States [2]. According to European Cancer Information System (ECIS) 2020 data, EC is the fourth most common cancer in Europe after breast, colorectal, and lung cancer and accounts for 6.8% of all cancers [3]. The incidence of EC has been increasing in recent years due to increased obesity, hormonal factors, and advances in cancer detection and diagnosis, but the incidence of EC varies between countries due to differences in risk factors. Risk factors for EC have been reported as age, family history of EC, use of exogenous estrogen, use of tamoxifen, prolonged menstruation, diabetes, obesity, low physical activity, and poor diet [4-8]. More than 50% of women diagnosed with EC are diagnosed at an early stage. The standard surgical protocol for early-stage EC is lymph node dissection (LND) with total hysterectomy and bilateral salpingo-oophorectomy (TH-BSO). Even though the five-year overall survival (OS) in EC is over 90%, recurrence or metastasis occurs in approximately 10-15% of early-stage ECs and 40% of late-stage ECs [9, 10]. Therefore, identifying patients at high risk of recurrence is important for developing postoperative treatment protocols to prevent recurrence. Numerous factors such as tumor histological grade, tumor stage, tumor size, depth of myometrial invasion (MI), lymphovascular space invasion (LVSI), local spread, and presence of metastasis have been identified as prognostic biomarkers for disease-free survival (DFS), recurrence, and overall survival (OS) in patients with EC [11-13]. The use of a uterine manipulator (UM) in minimally invasive surgery offers several intraoperative advantages, even if oncological outcomes remain unaffected. UMs enhance surgical ergonomics by improving visualization of anatomical structures (e.g., vaginal fornices, vesicouterine fold) and facilitating uterine mobilization, particularly in patients with high BMI or complex anatomy. These benefits may contribute to shorter operative duration, reduced blood loss, and lower conversion rates to laparotomy. While the absolute reduction in surgical time may seem modest, even small decreases in operative duration can have cumulative benefits, particularly in a high-volume surgical setting. Shorter procedures reduce exposure to anesthesia, which is independently associated with lower risks of postoperative complications such as pneumonia, venous thromboembolism, and ileus, especially in older patients or those with comorbidities. Some meta-analyses demonstrated that every additional 30 minutes of anesthesia time correlated with a 14% increase in postoperative pulmonary complications. Short duration may contribute to reduced physiological stress and faster mobilization, which are critical for recovery in endometrial cancer patients, who are often elderly or obese.
The minimally invasive approach, including laparoscopic surgery, has become more attractive in the treatment of EC due to faster recovery, less pain, and shorter hospital stay [14-16]. Although it has been reported that the minimally invasive surgery in EC does not affect the incidence of recurrence or OS, there are concerns that UM use causes retrograde spread of endometrial cancer cells and iatrogenic LVSI, resulting in an increased incidence of positive peritoneal cytology [17-19]. It has been emphasized that laparoscopy in the treatment of EC is associated with a higher incidence of positive peritoneal cytology [20-24]. Additionally, studies have shown that LVSI increases due to UM use [25, 26]. On the contrary, it has been documented that the use of UM does not significantly affect LVSI and cytology and does not have a negative impact on oncological outcomes [27, 28]. However, there is inconsistency between study results regarding the development of LVSI, recurrence, and OS associated with the use of UM in EC. Therefore, this study aimed to retrospectively evaluate the effect of UM used for the treatment of patients with EC on surgical and oncological outcomes.
Materials and Methods
This retrospective study was conducted in the Oncology Department of Azerbaijan Medical University. The files of 117 endometrial cancer cases who underwent surgical treatment between 2018 and 2024 were retrospectively evaluated (Figure 1).
Figure 1. Chart of the Study Population.
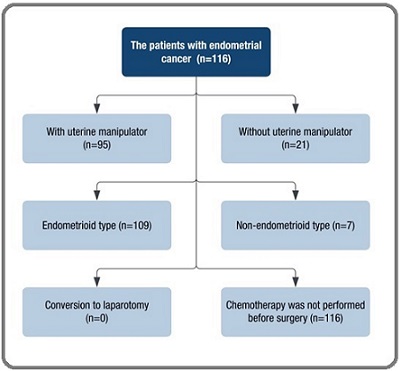
The inclusion criteria for the study were cases with endometrioid and non-endometrioid histological type tumors, patients with histological grade I-III, and patients who underwent pelvic and para-aortic lymph node dissection. Approval from the local ethics committee of the Oncology Department of the Azerbaijan Medical University (Approval № 238/2024.06.01).
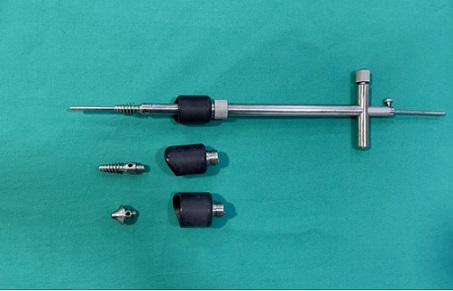
All surgical procedures were performed by one gynecologic oncologist. All patients underwent total abdominal hysterectomy and bilateral salpingo- oophorectomy. We used uterine manipulator Figure 2.
Figure 2. Hohl-type Uterine Manipulator.
Patients with EC were staged using the revised 2009 FIGO staging system. Clinical and pathological characteristics of the patients, patients’ age, use of uterine manipulator, body mass index, menopausal status, CA-125 level, surgery duration, FIGO stage, histological subtype, tumor size, depth of MI, LVSI, cervical and adnexal involvement, and presence of LND were evaluated.
Statistical analysis
Statistical evaluation was done using SPSS 20 statistical software. The Kolmogorov-Smirnov test was used to evaluate the suitability of the measured data to the normal distribution. Variables are expressed as frequency and percentage. Fisher’s Exact Test was used to compare categorical data. Logistic regression analysis was performed to determine the effect of participants’ parameters on recurrence and survival. For statistical analysis results, a p-value of less than 0.05 was considered significant.
Results
Demographic and clinical data of the patients included in the study are presented in Table 1.
| Variables | Test Statistics (%) |
| Age | |
| ≤50 | 12 (10.3) |
| >50 | 105 (89.7) |
| Menopausal Status | |
| Premenopause | 15 (12.8) |
| Postmenopause | 102 (87.2) |
| Body Mass Index | |
| ≤30 | 38 (32.5) |
| >30 | 79 (67.5) |
| Preoperative Diagnostic Examination | |
| Transvaginal Ultrasound | 2 (1.7) |
| Computed Tomography | 19 (16.2) |
| Magnetic Resonance Imaging | 96 (82.1) CA-125 (n:88) |
| ≤35 | 67 (76.1) |
| >35 | 21 (23.9) |
| Preoperative Histology Diagnosis (N:115) | |
| Endometrial Intraepithelial Neoplasia | 11 (9.6) |
| Endometrial Cancer | 104 (90.4) |
| Type Of Surgery | |
| Laparoscopy | 95 (81.2) |
| Laparotomy | 22 (18.8) |
| Type of Surgery | |
| TH-BSO | 2 (1.7) |
| TH-BSO + Frozen | 1 (0.9) |
| TH-BSO + BPLND | 88 (75.2) |
| TH-BSO + BPLND +Frozen | 8 (6.8) |
| TH-BSO + BPPALND + Omentectomy | 4 (3.4) |
| TH-BSO + BPLND + Omentectomy | 13 (11.1) |
| TH-BSO + BPLND + Right inguinofemoral LND | 1 (0.9) |
| Surgery Duration | |
| ≤120 min | 33 (28.2) |
| >120 min | 84 (71.8) |
| Uterine Manipulator (n:116) | |
| Yes | 95 (81.2) |
| No | 21 (17.9) |
| Regional Lymph Node Dissection | |
| Yes | 115 (98.3) |
| No | 2 (1.7) |
| Grade | |
| 1 | 29 (24.8) |
| 2 | 44 (37.6) |
| 3 | 41 (35.0) |
| Unknown | 3 (2.6) |
| Histology Type of Tumor | |
| Endometrioid | 109 (93.2) |
| Clear Cell | 2 (1.7) |
| Serous | 5 (4.3) |
| Carcinosarcoma | 1 (0.9) |
| Tumor size | |
| ≤2 cm | 47 (40.2) |
| >2 cm | 70 (59.8) |
| FIGO | |
| IA | 50 (42.7) |
| IB | 55 (47.0) |
| IIA | 1 (0.9) |
| IIIA | 2(1.7) |
| IIIC1 | 7 (6.0) |
| IIIC2 | 1(0.9) |
| IVB | 1 (0.9) |
| Myometrial Invasion | |
| No | 13 (11.1) |
| ≤50% | 46 (39.3) |
| >50% | 58 (49.6) |
| Lympho-Vascular Space Invasion | |
| No | 40 (34.2) |
| Yes | 77 (65.8) |
| Adnexal Involvement | |
| No | 114 (97.4) |
| Yes | 3 (2.6) |
| Cervical Involvement | |
| No | 113 (96.6) |
| Yes | 4 (3.4) |
| Lymph Node Metastasis | |
| No | 108 (92.3) |
| Yes | 9 (7.7) |
| Hospitalization Duration | |
| ≤4 days | 88 (75.2) |
| >4 days | 29 (24.8) |
| Perioperative Complication | |
| No | 114 (97.4) |
| Yes | 3 (2.6) |
| Treatment Applied | |
| Surgery | 34 (29.1) |
| Surgery + Radiotherapy | 60 (51.3) |
| Surgery + Chemotherapy | 8 (6.8) |
| Surgery + Radiotherapy + Chemotherapy | 15 (12.8) Adjuvant Therapy |
| No | 35 (29.9) |
| Yes | 82 (70.1) |
| Type of Adjuvant Therapy (n:82) | |
| Pelvic Radiotherapy | 2 (2.4) |
| Pelvic Radiotherapy + Brachytherapy | 29 (34.9) |
| Pelvic Radiotherapy + Brachytherapy+ Chemotherapy | 11 (13.3) |
| Brachytherapy | 32 (39.8) |
| Pelvic Radiotherapy + Chemotherapy | 1 (1.2) |
| Chemotherapy | 7 (8.4) |
| Recurrence | |
| No | 102 (87.2) |
| Yes | 15 (12.8) |
| Survival | |
| Alive | 102 (87.2) |
| Dead | 15 (12.8) |
TH-BSO, Total Hysterectomy and Bilateral Salpingo-Oophorectomy; BPLND, Bilateral Pelvic Lymph Node Dissection; BPPALND, Bilateral Pelvic and Para- Aortic Lymph Node Dissection; LND, Lymph Node Dissection
One hundred five (89.7%) patients were >50 years old, 102 (87.2%) patients were postmenopausal, and 79 (67.5%) patients had a BMI >30. CA-125 was >35 in twenty-one (23.9%) patients, preoperative histological diagnosis was EC in 104 (90.4%) patients, and endometrioid type was the histological diagnosis in 109 (93.2%) patients. The most frequently performed surgery was TH-BSO+Bilateral Pelvic Lymph Node Dissection (BPLND) in eighty-eight (75.2%) patients, and a uterine manipulator was used in 95 (81.2%) patients. The size of the tumor was larger than 2 cm in 70 (59.8%) patients, and FIGO stage was I in 105 (89.7%) patients. Fifty-eight (49.6%) patients had MI >50%, 46 (39.3%) patients had MI <50%, and 3 (11.1%) patients had no MI. Seventy-seven (65.8%) patients had LVSI, 3 (2.6%) patients had adnexal involvement, 4 (3.4%) patients had cervical involvement, and 9 (7.7%) patients had lymph node metastases. No complications were observed in the perioperative period in 114 (97.4%) patients, and 88 (75.2%) patients stayed in the hospital for less than four days. While 34 (29.1%) patients underwent surgery alone, 60 (51.3%) patients underwent surgery+radiotherapy. Adjuvant therapy was guided by NCCN/ESGO guidelines for the study group and does not depend on the application of UM. Chemotherapy regimens included carboplatin (AUC 5-6) and paclitaxel (175 mg/m²) every 3 weeks for 4–6 cycles. Radiotherapy involved external beam radiation (EBRT: 45–50.4 Gy in 25–28 fractions) and/ or brachytherapy (21–30 Gy in 3–5 fractions). High-risk cases received combined modalities. Adjuvant treatment was applied to 82 (70.1%) patients, and the most common adjuvant treatment was brachytherapy in 32 (39.8%) patients and pelvic radiotherapy + brachytherapy in 29 (34.9%) patients. Recurrence was observed in a total of 15 (12.8%) patients, and 15 (12.8%) patients died during the follow-up period.
The relationship between UM and other parameters is given in Table 2.
| Variables | Uterine Manipulator (n:116) (%) | ||
| Yes | No | Test statistics | |
| Age | |||
| ≤50 | 9 (9.5) | 3 (14.3) | X 2 :0.429 |
| >50 | 86 (90.5) | 18 (85.7) | p: 0.512 |
| Menopausal Status | |||
| Premenopause | 11 (11.6) | 4 (19.0) | X 2 :0.852 |
| Postmenopause | 84 (88.4) | 17 (81.0) | p:0.356 |
| Body Mass Index | |||
| ≤30 | 26 (27.4) | 12 (57.1) | X 2 :6.922 |
| >30 | 69 (72.6) | 9 (42.9) | p:0.009 |
| Preoperative Diagnostic Examination | |||
| Computed Tomography | 8 (8.6) | 10 (47.6) | X 2 : 19.614 |
| Magnetic Resonance Imaging | 85 (91.4) | 11 (52.4) | p< 0.001 |
| CA-125 (n:88) | |||
| ≤35 | 56 (81.2) | 11 (61.1) | X 2 : 3.241 |
| >35 | 13 (18.8) | 7(38.9) | p: 0.072 |
| Preoperative Histology Diagnosis (N:115) | |||
| Endometrial Intraepithelial Neoplasia | 11 (11.7) | 0 (0.0) | X 2 : 2.590 |
| Endometrial Cancer | 83 (88.3) | 20 (100.0) | p: 0.108 |
| Type of Surgery | |||
| TH-BSO | 2 (2.1) | 0 (0.0) | X 2 : 90.261 |
| TH-BSO + Frozen | 1 (1.1) | 0 (0.0) | P<0.001 |
| TH-BSO + BPLND | 83(87.4) | 4 (19.0) | |
| TH-BSO + BPLND +Frozen | 8 (8.4) | 0 (0.0) | |
| TH-BSO + BPPALND + Omentectomy | 0 (0.0) | 4 (19.0) | |
| TH-BSO + BPLND + Omentectomy | 0 (0.0) | 13 (61.9) | |
| TH-BSO + BPLND + Right inguinofemoral LND | 1 (1.1) | 0 (0.0) | |
| Surgery duration | |||
| ≤120 min | 30 (31.6) | 2 (9.5) | X 2 : 4.188 |
| >120 min | 65 (68.4) | 19 (90.5) | p: 0.041 |
| Regional Lymph Node Dissection | |||
| Yes | 93 (97.9) | 21 (100.0) | X 2 : 0.450 |
| No | 2 (2.1) | 0 (0.0) | p: 0.502 |
| Grade | |||
| 1 | 25 (26.3) | 4 (19.0) | X 2 : 2.708 |
| 2 | 38 (40.0) | 6 (28.6) | p: 0.439 |
| 3 | 20 (31.6) | 10 (47.6) | |
| Unknown | 2 (2.1) | 1 (4.8) | |
| Histology Type of Tumor | |||
| Endometrioid | 90 (94.7) | 18 (85.7) | X 2 : 6.059 |
| Clear Cell | 1 (1.1) | 1 (4.8) | p: 0.109 |
| Serous | 4 (4.2) | 1 (4.8) | |
| Carcinosarcoma | 0 (0.0) | 1 (4.8) | |
| Tumor size | |||
| ≤2 cm | 41 (43.2) | 5 (23.8) | X 2 : 2.691 |
| >2 cm | 57 (56.8) | 16 (76.2) | p: 0.101 |
| FIGO | |||
| IA | 45 (47.4) | 5 (23.8) | X 2 : 15.006 |
| IB | 43 (45.3) | 11 (52.4) | p: 0.020 |
| IIA | 0 (0.0) | 1 (4.8) | |
| IIIA | 2 (2.1) | 0 (0.0) | |
| IIIC1 | 4 (5.7) | 3 (14.3) | |
| FIGO | |||
| IIIC2 | 0 (0.0) | 1 (4.8) | |
| IVB | 1 (1.1) | 0 (0.0) | |
| Myometrial Invasion | |||
| No | 12 (12.6) | 1 (4.8) | X 2 : 3.348 |
| ≤50% | 40 (42.1) | 6 (28.6) | p: 0.187 |
| >50% | 43 (45.3) | 14 (66.7) | |
| Lympho-Vascular Space Invasion | |||
| No | 36 (37.9) | 4 (19.0) | X 2 : 2.704 |
| Yes | 59 (62.1) | 17 (81.0) | p: 0.100 |
| Adnexal Involvement | |||
| No | 92 (96.8) | 21 (100.0) | X 2 : 0.681 |
| Yes | 3 (3.2) | 0 (0.0) | p: 0.409 |
| Cervical Involvement | |||
| No | 94 (98.9) | 18 (85.7) | X 2 : 9.046 |
| Yes | 1 (1.1) | 3 (14.3) | p: 0.003 |
| Lymph Node Metastasis | |||
| No | 89 (93.7) | 18 (85.7) | X 2 : 1.526 |
| Yes | 6 (6.3) | 3 (14.3) | p: 0.217 |
| Hospitalization Duration | |||
| ≤4 days | 74 (77.9) | 13 (61.9) | X 2 : 2.345 |
| >4 days | 21 (22.1) | 8 (38.1) | p: 0.126 |
| Perioperative Complication | |||
| No | 93 (97.9) | 20 (95.2) | X 2 : 0.482 |
| Yes | 2 (2.1) | 1 (4.8) | p: 0.488 |
| Treatment Applied | |||
| Surgery | 29 (30.5) | 5 (23.8) | X 2 : 3.191 |
| Surgery + Radiotherapy | 50 (52.6) | 9 (42.9) | p: 0.363 |
| Surgery + Chemotherapy | 6 (6.3) | 2 (9.5) | |
| Surgery + Radiotherapy + Chemotherapy | 10 (10.5) | 5 (23.8) | |
| Adjuvant Therapy | |||
| No | 29 (30.5) | 6 (28.6) | X 2 : 0.031 |
| Yes | 66 (69.5) | 15 (71.4) | p: 0.860 |
| Type of Adjuvant Therapy (n:82) | |||
| Pelvic Radiotherapy | 1 (1.5) | 1 (6.2) | X 2 : 10.435 |
| Pelvic Radiotherapy + Brachytherapy | 21 (31.8) | 7 (43.8) | p: 0.064 |
| Pelvic Radiotherapy + Brachytherapy+ Chemotherapy | 8 (12.1) | 3 (18.8) | |
| Brachytherapy | 31 (47.0) | 2 (12.5) | |
| Pelvic Radiotherapy + Chemotherapy | 0 (0.0) | 1 (6.2) | |
| Chemotherapy | 5 (7.6) | 2 (12.5) | |
| Recurrence | |||
| No | 83 (87.4) | 18 (85.7) | X 2 : 0.042 |
| Yes | 12 (12.6) | 3 (14.3) | p: 0.838 |
| Survival | |||
| Alive | 83 (87.4) | 18 (85.7) | X 2 : 0.042 |
| Dead | 12 (12.6) | 3 (14.3) | p: 0.838 |
X2, Fisher's Exact Test; TH-BSO, Total Hysterectomy and Bilateral Salpingo-Oophorectomy; BPLND, Bilateral Pelvic Lymph Node Dissection; BPPALND, Bilateral Pelvic and Para-Aortic Lymph Node Dissection; LND, Lymph Node Dissection
The use of UM was significantly higher in patients with BMI>30 (p=0.009), in patients using MRI for preoperative diagnosis (p<0.001), in patients who underwent surgical TH-BSO+BPLND (p<0.001), in patients with surgery time <120 minutes (p=0.041), and in patients without cervical involvement (p=0.003). While LVSI was observed in 17 (81.0%) patients whose procedure did not use UM, 59 (62.1%) patients whose procedure utilized UM had LVSI (p = 0.100). There was recurrence in 12 (12.6%) patients whose procedure used UM, while there was recurrence in 3 (14.3%) patients who did not undergo UM (p=0.838). Similarly, the mortality rate was 12.6% (n=12) in patients with UM and 14.3% (n=3) in patients without UM.
Factors associated with recurrence are given in Table 3, and factors associated with survival are given in Table 4.
| B | SE | Wald | df | p | OR | 95% CI | ||
| Uterine Manipulator | -0.142 | 0.696 | 0.042 | 1 | 0.838 | 0.867 | 0.222 | 3.393 |
| CA-125 | 0.482 | 0.77 | 0.391 | 1 | 0.532 | 1.619 | 0.358 | 7.331 |
| Regional Lymph Node Dissection | 18.034 | 25861.919 | 0 | 1 | 0.999 | 67929325.66 | 0 | . |
| Tumor Size | -0.459 | 0.76 | 0.364 | 1 | 0.546 | 0.632 | 0.142 | 2.804 |
| Myometrial Invasion | 18.678 | 13140.106 | 0 | 1 | 0.999 | 129364546.7 | 0 | . |
| Lympho-Vascular Space Invasion | -1.565 | 1.103 | 2.015 | 1 | 0.156 | 0.209 | 0.024 | 1.815 |
| Adnexal Involvement | 2.041 | 1.632 | 1.563 | 1 | 0.211 | 7.7 | 0.314 | 188.808 |
| Cervical Involvement | -18.924 | 25088.991 | 0 | 1 | 0.999 | 0 | 0 | . |
| Lymph Node Metastasis | 20.71 | 15836.07 | 0 | 1 | 0.999 | 986786540.7 | 0 | . |
| B | SE | Wald | df | p | OR | 95% CI | ||
| Uterine Manipulator | -0.142 | 0.696 | 0.042 | 1 | 0.838 | 0.867 | 0.222 | 3.393 |
| CA-125 | 0.466 | 0.702 | 0.441 | 1 | 0.507 | 1.594 | 0.402 | 6.313 |
| Regional Lymph Node Dissection | 17.561 | 25651.084 | 0 | 1 | 0.999 | 42330157.18 | 0 | . |
| Tumor Size | 0.443 | 0.762 | 0.338 | 1 | 0.561 | 1.558 | 0.35 | 6.94 |
| Myometrial Invasion | 18.816 | 13123.811 | 0 | 1 | 0.999 | 148478077.1 | 0 | . |
| Lympho-Vascular Space Invasion | -0.964 | 0.847 | 1.295 | 1 | 0.255 | 0.381 | 0.072 | 2.007 |
| Adnexal Involvement | -19.261 | 21313.016 | 0 | 1 | 0.999 | 0 | 0 | . |
| Cervical Involvement | -19.426 | 25824.727 | 0 | 1 | 0.999 | 0 | 0 | . |
| Lymph Node Metastasis | 19.241 | 16556.06 | 0 | 1 | 0.999 | 227214190.8 | 0 | . |
UM, CA-125 level, LND, tumor size, MI, LVSI, adnexal involvement, cervical involvement, and LNM were not associated with recurrence and survival.
Discussion
In this study, we examined the effects of UM on surgical and oncological outcomes in patients with EC. UM was used more frequently in patients with BMI>30, patients with FIGO stage I, and patients who underwent TH-BSO+BPLND, and the duration of surgery was less in patients using UM. However, no association of UM use with LVSI, recurrence, and survival was observed.
UMs used during laparoscopic and robotic hysterectomies can reveal the superficial and retroperitoneal anatomy more clearly [29]. UMs facilitate the work of surgeons by providing easier identification of the fornices, better development of the vesicouterine fold, greater exposure of the cul-de-sac in cases of endometriosis or adhesion, and easier mobilization of the uterus [30]. However, there are concerns that this device placed in the endometrial space may disrupt EC cells and thus affect patient outcomes. In particular, there is insufficient evidence regarding the effect of UM use on long-term outcomes in EC patients. As a result, debate regarding the use of UM continues in the gynecologic oncology community. Hypotheses for local recurrence risk and the potential ways of intraoperative tumor spillage during minimally invasive surgery were illustrated in Figure 3.
Figure 3. Potential Theoretical Side Effects of the Uterine Manipulator: The potential ways of intraoperative tumor spillage during minimally invasive surgery. To begin with, direct manipulation of the uterus during surgery may cause the fragmentation of the tumor. Then, fragmented tumor cells in the vaginal fornices may backflow into the pelvic peritoneal surfaces at the time of intra-corporeal colpotomy (A). Furthermore, during a laparoscopic procedure, the use of an intrauterine manipulator may lead to iatrogenic uterine perforation, which causes the direct spread of the tumor (B). Moreover, tumor cells may be spread into the peritoneal cavity via trans-tubal retrograde flow (C).
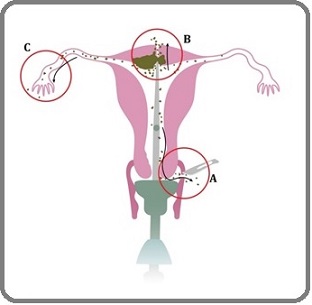
To begin with, direct manipulation of the uterus during surgery may cause the fragmentation of the tumor. Then fragmented tumor cells in the vaginal fornices may back-flow into the pelvic peritoneal surfaces at the time of intra-corporeal colpotomy. Furthermore, during laparoscopic procedure, the use of an intrauterine manipulator may lead to uterine perforation, which causes the direct spread of tumor. Moreover, tumor cells may be spread into the peritoneal cavity via transtubal retrograde flow. However, distant metastases are more likely driven by tumor biology (e.g., molecular subtype, LVSI) rather than UM use.
The use of UM in EC patients is thought to be associated with transtubal tumor spread and an increased rate of LVSI. Studies have documented that UM causes retrograde seeding of tumor cells into the peritoneal cavity due to the pressure effect and a higher positive peritoneal cytology [31, 32]. Krizova et al. [26] reported that in the histopathological examination of hysterectomy specimens of 160 oncological patients, the use of UM showed a higher rate of lymph vascular pseudo-invasion and positive peritoneal cytology. However, Kitahara et al. [25] suggested that pseudo-invasion results from artifacts in the gross pathological process rather than a true invasion. Therefore, there are studies showing that the use of UM increases the rate of LVSI and positive peritoneal cytology [31, 33, 34]. However, unlike these studies, Machida et al. [35] noted that the application of UM during laparoscopic hysterectomy in patients with EC was not associated with a higher frequency of LVSI. Frimer et al. [36] did not observe a relationship between the use of UM and an increase in micrometastasis and isolated tumor cells during surgical treatment in 175 EC patients. Similarly, Lee et al. [18] determined in a randomized study that the use of UM did not show an increase in peritoneal tumor cells or an increase in positive LVSI. Tinelli et al. [27] found that the use of UM did not affect peritoneal cytology and LVSI in 110 patients with stage I EC. Another recent study found that the use of UM in minimally invasive surgery for EC was not associated with LVSI [37]. In our study, LVSI was determined in 77 (65.8%) of 117 EC patients who underwent laparoscopic surgery. LVSI was observed in 81.0% of patients without UM and in 62.1% of patients with UM, but there was no significant difference between the groups. The LVSI results in this current study are consistent with previous study results showing that the use of UM in laparoscopic surgery in EC patients is not associated with LVSI, although overall observed LVSI rates in this current study were higher than in previous studies. The elevated LVSI rate (65.8%) contrasts with SEER data (LVSI ~20%-40 %) and may reflect differences in histopathological criteria, centralized pathology review, population heterogeneity, or a high-risk cohort (49.6% with deep myometrial invasion). This study also evaluated the impact of UM on survival and likelihood of recurrence. In our study, recurrence was observed in 15 (12.8%) patients and 15 (12.8%) patients died. Ten of the 15 patients with recurrence died. The recurrence rate and mortality rate in patients with UM was 12.6% and was not significantly different from the recurrence rate and mortality rate of 14.3% in patients without UM. Padilla-Iserte et al.’s [38] multicenter cohort study determined that in patients undergoing minimally invasive surgery for early-stage EC, UM exhibited a higher recurrence rate, lower recurrence-free survival, and lower overall survival rate. In a recent study, it was determined that the general recurrence rate was similar in patients with and without UM, but local vaginal recurrence rates were higher in patients using UM [37]. When researchers excluded patients who received adjuvant therapy, they observed that patients with UM showed a higher rate of vaginal vault recurrence and overall recurrence and worse disease-free survival. Researchers reported that UM demonstrated worse outcomes for the subgroup of low-risk patients who did not receive adjuvant therapy [37]. A subgroup analysis of patients who did not receive adjuvant therapy (n=35) revealed no significant difference in recurrence (14.3% vs. 15.4%, p=0.912) or survival (14.3% vs. 15.4%, p=0.912) between UM and non-UM groups. However, the small sample size limits definitive conclusions. While prior studies suggest UM may worsen outcomes in untreated subgroups, our analysis found no significant association. This discrepancy may reflect differences in risk stratification or adjuvant therapy eligibility criteria. However, contrary to these data, in a multicenter study conducted by the Italian Gynecological Endoscopy Society in which 951 EC patients were retrospectively evaluated, it was determined that there was no relationship between the use of UM in EC treatment and disease-free, disease-specific, and overall survival. [28] Gueli Alletti et al. [39], in their multicenter prospective randomized clinical study evaluating 154 patients with G1-G2 early-stage EC, noted that UM did not affect the relapse pattern or DFS. According to the results of a large meta-analysis evaluating 18 studies, the use of UM during laparoscopic surgery for EC does not increase the recurrence rate [40]. Similarly, in a recent meta-analysis evaluating 14 studies including 5019 patients, no statistically significant relationship was determined between the use of UM during hysterectomy for EC and recurrence-free and overall survival [41]. The results of our study that UM does not affect recurrence and survival are consistent with previous results. Moreover, in our study, the hospitalization period and perioperative complication rate of patients with UM were similar to patients without UM. Additionally, in patients with UM, the rate of patients with surgery time >120 minutes was less than in patients without UM.
There are possible explanations for the different results. 1. Heterogeneity in patient populations. Studies vary in baseline risk factors (e.g., grade, tumor stage, histologic subtype). For instance, populations with higher rates of advanced-stage disease or aggressive histologies (e.g., grade 3) may show a high prevalence of tumor seeding with UM use. Conversely, studies focusing on early-stage, low-grade tumors may report no significant association with recurrence or survival.
2. Differences in UM design and technique. UM devices vary in rigidity, cervical occlusion mechanisms, and intrauterine pressure creation. For example, balloon-tipped UMs (e.g., Cohen manipulator) may exert lower pressure compared to fixed-volume systems (e.g., RUMI), potentially influencing the risk of retrograde tumor spillage. Procedural differences, such as cervical sealing or minimal colpotomy, could further confound outcomes.
3. Surgical expertise and learning curves. A surgeon’s experience with UM-assisted minimally invasive surgery (MIS) may impact outcomes. Inexperienced operators may inadvertently increase intrauterine pressure or cause uterine perforation. This variability is rarely accounted for in retrospective studies.
4. Adjuvant therapy protocols. Discrepancies in adjuvant treatment (e.g., chemotherapy, brachytherapy) across institutions could mask or modify the true oncologic impact of UM use. Studies failing to control for adjuvant therapy may report conflicting survival outcomes despite similar surgical practices.
2. Methodologic limitations. Retrospective studies dominate this field, introducing selection bias and confounding. For example, studies comparing UM users to non-users may overlook inherent differences in tumor biology or surgical complexity. Additionally, short follow- up durations in some series may underestimate recurrence rates.
One limitation of our study is that it is a retrospective, single-center study with a small number of patients. The grade, stage, and histological subtype heterogeneity of EC is another limitation. This heterogeneity regarding EC may have affected the study results. According to the study, after controlling for confounders, the application of a uterine manipulator did not independently predict survival or recurrence. Deficiencies such as the type of UM used, indications for use, duration of UM placement, and the application of different treatment methods are also limitations.
In conclusions, in this retrospective study, the surgical and oncological outcomes of the use of uterine manipulators during endometrial cancer surgery were evaluated. Firstly, the HOHL type uterine manipulator with an atraumatic tip was used in all cases included in this study. This selection was based on its ergonomic design, which facilitates optimal uterine maneuver and colpotomy under direct visualization while minimizing tumor fragmentation. While other UM types (eg, Clermont- Ferrand) are available in our institution, they were not used in this cohort to maintain procedural standardization. Secondly, mitigating the risk of intraoperative tumor spillage was a critical focus of our surgical procedure. At the initiation of operation, bilateral fallopian tubes were coagulated and sealed to prevent retrograde transtubal tumor seeding. Under direct laparoscopic visualization, with careful avoidance of tumor fragmentation, the HOHL type manipulator’s colpotomy cup was employed to stabilize the cervix and ensure a circumferential, en-bloc resection. No instances of gross tumor fragmentation were documented intraoperatively. Thirdly, UM fixation is realized by experienced doctors. The HOHL manipulator was inserted transvaginally after catching the cervix with a tenaculum. Fixation was achieved using the manipulator’s adjustable cervical cup, which was tightened to occlude the cervical os and minimize retrograde tumor spillage. No additional sutures were required for stabilization. To sum up, we can give information on our routine key steps to prevent dissemination, which include:
Preoperative vaginal disinfection with povidone-iodine.
Circumferential colpotomy was performed laparoscopically using monopolar electrocautery, maintaining a 1–2 cm margin from the cervical cup.
Immediate closure of the vaginal vault following uterine extraction to isolate the surgical field.
Minimal uterine manipulation in cases with suspected fundal or adnexal tumor involvement.
For avoidance of morcellation, all specimens were extracted intact via the vagina.
Lavage of the peritoneal cavity after surgical material removal in cases with suspected microscopic spillage was carried out.
The concern that unmeasured criteria (e.g., BMI, tumor size, stage) may have influenced both the use of a uterine manipulator and oncological outcomes may occur. In the original analysis, baseline demographic and clinical characteristics of the UM and non-UM groups are presented in the table. While key variables, such as tumor stage, histology, and surgical approach, were balanced between groups, other factors, including BMI, tumor size, and surgeon preference, were not formally compared. Baseline characteristics differed between UM and non-UM groups: UM patients had higher BMI and shorter surgery duration. Our findings suggest that, within the limitations of a retrospective analysis, the use of uterine manipulators in minimally invasive endometrial cancer surgery does not appear to significantly impact lymphovascular space invasion, recurrence, or survival outcomes. While our study found no association between UM use and survival, its role in optimizing surgical precision and efficiency underscores its utility in select cases. However, due to potential confounding factors, selection bias, and variability in surgical techniques inherent to retrospective studies, these results should be interpreted with caution. Moreover, debates regarding the use of uterine manipulators seem to continue. Therefore, multicenter, randomized, prospective studies on the use of uterine manipulators during endometrial cancer surgery are needed.
Acknowledgements
We want to acknowledge the AGP editorial board for their scientific opportunity and outstanding support that was provided in this manuscript.
Author contributions
Conceptualization: [AI]; Methodology: [AI]; Formal analysis and investigation: [AI]; Writing - original draft preparation: [AI]; Writing - review and editing: [AI]; Supervision: [AI].
Competing interests
The authors have no conflict of interest in this study.
Data availability statements
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
Approval from the local ethics committee of the Oncology Department of the Azerbaijan Medical University (Approval № 238/2024.06.01). Since the study was retrospective and observational, the Clinical Research Ethics Committee of Azerbaijan Medical University waived the need for written informed consent.
Funding
This study was not funded by any organization.
References
- Cancer Statistics, 2021 Siegel RL , Miller KD , Fuchs HE , Jemal A. CA: a cancer journal for clinicians.2021;71(1). CrossRef
- American Cancer Society: Key statistics for endometrial cancer. Available from: https://www.cancer.org/cancer/endometrialcancer/about/key-statistics.html Access year: 2023.
- ECIS-European Cancer Information System: Estimates of cancer incidence and mortality in 2020. Available from: https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-AEE$2-All$4-2$3-All$6-0,85$5-2020,2020$7-7$CEstByCancer$X0_8-3$CEstRelativeCanc$X1_8-3$X1_9-AE27$CEstBySexByCancer$X2_8-3$X2_-1-1 Access year: 2022.
- Lifetime number of years of menstruation as a risk index for postmenopausal endometrial cancer in the Norwegian Women and Cancer Study Gavrilyuk O, Braaten T, Weiderpass E, Licaj I, Lund E. Acta Obstetricia Et Gynecologica Scandinavica.2018;97(10). CrossRef
- Diet and exercise in uterine cancer survivors (DEUS pilot) - piloting a healthy eating and physical activity program: study protocol for a randomized controlled trial Koutoukidis DA , Beeken RJ , Manchanda R, Burnell M, Knobf MT , Lanceley A. Trials.2016;17(1). CrossRef
- Impact of Type 2 Diabetes Mellitus on Endometrial Cancer Survival: A Prospective Database Analysis Njoku K, Agnew HJ , Crosbie EJ . Frontiers in Oncology.2022;12. CrossRef
- Menopausal Hormone Therapy and Risk of Endometrial Cancer: A Systematic Review Tempfer CB , Hilal Z, Kern P, Juhasz-Boess I, Rezniczek GA . Cancers.2020;12(8). CrossRef
- Statistical Meta-Analysis of Risk Factors for Endometrial Cancer and Development of a Risk Prediction Model Using an Artificial Neural Network Algorithm Hutt S, Mihaies D, Karteris E, Michael A, Payne AM , Chatterjee J. Cancers.2021;13(15). CrossRef
- Development and validation of a circulating tumor DNA-based optimization-prediction model for short-term postoperative recurrence of endometrial cancer Liu Y, Lu X, Guo H, Bao C, Zhang J, Jin Y. World Journal of Clinical Cases.2024;12(18). CrossRef
- ARG1 is a potential prognostic marker in metastatic and recurrent endometrial cancer Tran DN , Rozen V, Hunter MI , Kim TH , Jeong JW . Research Square.2023. CrossRef
- Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer Tortorella L, Restaino S, Zannoni GF , Vizzielli G, Chiantera V, Cappuccio S, Gioè A, et al . Journal of Gynecologic Oncology.2021;32(2). CrossRef
- Association of Myometrial Invasion With Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies With 68,870 Patients Wang J, Xu P, Yang X, Yu Q, Xu X, Zou G, Zhang X. Frontiers in Oncology.2021;11. CrossRef
- Characteristics of patients with late recurrence endometrial cancer Çakır İ, Gülseren V, Özdemir IA , Abacı H, Talu ECK , Çakır ZE , Ata Can, et al . Journal of Cancer Research and Therapeutics.2024;20(1). CrossRef
- Surgical outcomes of robotic-assisted surgical staging for endometrial cancer are equivalent to traditional laparoscopic staging at a minimally invasive surgical center Cardenas-Goicoechea J, Adams S, Bhat SB , Randall TC . Gynecologic Oncology.2010;117(2). CrossRef
- Postoperative pain medication requirements in patients undergoing computer-assisted (“Robotic”) and standard laparoscopic procedures for newly diagnosed endometrial cancer Leitao MM , Malhotra V, Briscoe G, Suidan R, Dholakiya P, Santos K, Jewell EL , et al . Annals of Surgical Oncology.2013;20(11). CrossRef
- Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2 Walker JL , Piedmonte MR , Spirtos NM , Eisenkop SM , Schlaerth JB , Mannel RS , Spiegel G, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(32). CrossRef
- Total laparoscopic hysterectomy in early-stage endometrial cancer using an intrauterine manipulator: is it a bias for frozen section analysis? Case-control study Fanfani F., Gagliardi M. L., Zannoni G. F., Gallotta V., Vizzielli G., Lecca A., Scambia G., Fagotti A.. Journal of Minimally Invasive Gynecology.2011;18(2). CrossRef
- Effects of uterine manipulation on surgical outcomes in laparoscopic management of endometrial cancer: a prospective randomized clinical trial Lee M, Kim YT , Kim SW , Kim S, Kim JH , Nam EJ . International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2013;23(2). CrossRef
- Total laparoscopic hysterectomy without uterine manipulator at big uterus weight (>280 g) Mebes I, Diedrich K, Banz-Jansen C. Archives of Gynecology and Obstetrics.2012;286(1). CrossRef
- Laparoscopy versus laparotomy for the management of early stage endometrial cancer Galaal K, Bryant A, Fisher AD , Al-Khaduri M, Kew F, Lopes AD . The Cochrane Database of Systematic Reviews.2012;(9). CrossRef
- Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer--a prospective randomized trial Malur S., Possover M., Michels W., Schneider A.. Gynecologic Oncology.2001;80(2). CrossRef
- Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: a prospective randomized study Malzoni M, Tinelli R, Cosentino F, Perone Ci, Rasile M, Iuzzolino D, Malzoni C, Reich H. Gynecologic Oncology.2009;112(1). CrossRef
- Laparoscopic versus laparotomic approach to endometrial cancer Perrone A. M., Di Marcoberardino B., Rossi M., Pozzati F., Pellegrini A., Procaccini M., Santini D., De Iaco P.. European Journal of Gynaecological Oncology.2012;33(4).
- Advantages of laparoscopy versus laparotomy in extremely obese women (BMI>35) with early-stage endometrial cancer: a multicenter study Tinelli R, Litta P, Meir Y, Surico D, Leo L, Fusco A, Angioni S, Cicinelli E. Anticancer Research.2014;34(5).
- Vascular pseudoinvasion in laparoscopic hysterectomy specimens for endometrial carcinoma: a grossing artifact? Kitahara S, Walsh C, Frumovitz M, Malpica A, Silva EG . The American Journal of Surgical Pathology.2009;33(2). CrossRef
- Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review Krizova A, Clarke BA , Bernardini MQ , James S, Kalloger SE , Boerner SL , Mulligan AM . The American Journal of Surgical Pathology.2011;35(1). CrossRef
- Laparoscopic treatment of early-stage endometrial cancer with and without uterine manipulator: Our experience and review of literature Tinelli R, Cicinelli E, Tinelli A, Bettocchi S, Angioni S, Litta P. Surgical Oncology.2016;25(2). CrossRef
- The effect of a uterine manipulator on the recurrence and mortality of endometrial cancer: a multi-centric study by the Italian Society of Gynecological Endoscopy Uccella S, Bonzini M, Malzoni M, Fanfani F, Palomba S, Aletti G, Corrado Gi, et al . American Journal of Obstetrics and Gynecology.2017;216(6). CrossRef
- Clermont Ferrand uterine manipulator Nassif J, Wattiez A. Surgical Technology International.2010;20.
- Clinical Relevance of Uterine Manipulation on Oncologic Outcome in Robot-Assisted versus Open Surgery in the Management of Endometrial Cancer Eoh KJ , Kim Y, Nam EJ , Kim SW , Kim YT . Journal of Clinical Medicine.2023;12(5). CrossRef
- Does the use of a uterine manipulator with an intrauterine balloon in total laparoscopic hysterectomy facilitate tumor cell spillage into the peritoneal cavity in patients with endometrial cancer? Lim S., Kim H. S., Lee K. B., Yoo C. W., Park S. Y., Seo S. S.. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2008;18(5). CrossRef
- High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy Sonoda Y., Zerbe M., Smith A., Lin O., Barakat R. R., Hoskins W. J.. Gynecologic Oncology.2001;80(3). CrossRef
- Associated characteristics and impact on recurrence and survival of free-floating tumor fragments in the lumen of fallopian tubes in Type I and Type II endometrial cancer Albright BB , Black JD , Passarelli R, Gysler S, Whicker M, Altwerger G, et al . Gynecologic Oncology Reports.2018;23. CrossRef
- Tumoral displacement into fallopian tubes in patients undergoing robotically assisted hysterectomy for newly diagnosed endometrial cancer Delair D, Soslow RA , Gardner GJ , Barakat RR , Leitao MM . International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists.2013;32(2). CrossRef
- Intrauterine Manipulator Use During Minimally Invasive Hysterectomy and Risk of Lymphovascular Space Invasion in Endometrial Cancer Machida H, Hom MS , Adams CL , Eckhardt SE , Garcia-Sayre J, Mikami M, Matsuo K. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2018;28(2). CrossRef
- Micrometastasis of endometrial cancer to sentinel lymph nodes: is it an artifact of uterine manipulation? Frimer M, Khoury-Collado F, Murray MP , Barakat RR , Abu-Rustum NR . Gynecologic Oncology.2010;119(3). CrossRef
- The Impact of Intrauterine Manipulators on Outcome and Recurrence Patterns of Endometrial Cancer Patients Undergoing Minimally Invasive Surgery Laskov I, Michaan N, Zeng X, Salvador S, Lau S, Gilbert L, Gotlieb WH , Kessous R. Journal of Women's Health (2002).2024;33(3). CrossRef
- Impact of uterine manipulator on oncological outcome in endometrial cancer surgery Padilla-Iserte P, Lago V, Tauste C, Díaz-Feijoo B, Gil-Moreno A, Oliver R, Coronado P, et al . American Journal of Obstetrics and Gynecology.2021;224(1). CrossRef
- A Multicentric Randomized Trial to Evaluate the ROle of Uterine MANipulator on Laparoscopic/Robotic HYsterectomy for the Treatment of Early-Stage Endometrial Cancer: The ROMANHY Trial Gueli Alletti S, Perrone E, Fedele C, Cianci S, Pasciuto T, Chiantera V, Uccella S, et al . Frontiers in Oncology.2021;11. CrossRef
- Influence of uterine manipulator on oncological outcome in minimally invasive surgery of endometrial cancer: A systematic review and meta-analysis Scutiero G., Vizzielli G., Taliento C., Bernardi G., Martinello R., Cianci S., Riemma G., Scambia G., Greco P.. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology.2022;48(10). CrossRef
- Intrauterine manipulator during hysterectomy for endometrial cancer: a systematic review and meta-analysis of oncologic outcomes Zorzato PC , Uccella S, Biancotto G, Bosco M, Festi A, Franchi M, Garzon S. American Journal of Obstetrics and Gynecology.2024;230(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times