S M Nazmuz Sakib’s Microbiological Tumor Evolutionary Equation (MTEE): A Mathematical Framework for Understanding the Co-Evolution of Tumors and Microbiomes in Cancer Progression and Response to Therapy
Download
Abstract
The two-way interaction between cancer and microbiome has attracted growing interest because of the possibility of its involvement in tumorigenesis, metastasis, and chemotherapy resistance. The co-evolution dynamics of tumors and microbiomes, however, and their impact on cancer biology, are unknown. This work presents S M Nazmuz Sakib’s Microbiological Tumor Evolutionary Equation (MTEE), a new mathematical model calculating the feedback processes between tumor cells and their related microbial communities. The MTEE applies evolutionary biology, microbial dynamics, and oncology to simulate how selective pressures of the tumor microenvironment mold microbial communities and consequently tumor growth and treatment response. By probing this evolutionary connection, the MTEE sheds light on the role of microbiomes in cancer development, metastasis, and treatment response. The platform further provides simulation of microbial therapies (e.g., probiotics, antibiotics, crafted microbiomes) to predict their effect on tumor phenotype and response to treatment. Finally, the MTEE is a perceptive tool for personalized cancer diagnosis and treatment, toward the goal of microbiome-guided interventions in cancer.
Introduction
Cancer has witnessed unparalleled evolution in its understanding during the last hundred years. Cancer was once believed to be essentially a genetic disorder, with its origin through mutation in the tumor cells and subsequent uncontrolled growth and proliferation. Nevertheless, in recent years, there has been discovery of the tumor microenvironment (TME) role in controlling tumor behavior, metastasis, and drug resistance. Of all the components of the TME, the microbiome, the diversified guild of microbes that occupy different body regions, gut, skin, and even the tumor itself has proven to be one of the major contributors to cancer biology. The interaction between the microbiome and cancer is sophisticated and reciprocal. Tumor cells on the one hand may regulate microbial community structure and composition in the TME to establish a specific ecological niche favoring tumor growth and development. Microbiome, on the other hand, can regulate tumor biology in immune response, tumor metastasis, and chemotherapy and immunotherapy resistance. Such dynamic interaction implies a co-evolutionary association between tumor cells and their respective microbes, which remains to be accounted for [1-4] [5, 6].
While the role of microbiomes in cancer causation and treatment response is more and more valued, current models of the tumor ignore the delicacy of feedback interactions between the microbiome and the tumor. A unified theoretical framework for comprehending intricate relationships between cancer cells and microbial assemblages and their path to evolution with corresponding influence on cancer progression is urgently needed [7-9]. To this effect, S M Nazmuz Sakib’s Microbiological Tumor Evolutionary Equation, or MTEE, comes in. The MTEE is a new mathematical model that attempts to quantify and model the feedback between the microbiome and tumor cells. The model uses evolutionary biology, microbial dynamics, and oncology to capture the co-evolution of microbes and tumor cells over time. Through formalization of these interactions, the MTEE offers a more comprehensive understanding of how microbial communities in the tumor microenvironment drive cancer development, metastasis, and treatment responsiveness.
The MTEE further offers a new approach by representing tumor-microbiome co-evolution as an evolutionary system where the tumor cells and microbial communities develop based on one another’s changing selective pressures. This coevolutionary interaction is represented by a differential equation model of tumor development, microbial evolution, and their interaction. Through the integration of this evolutionary model, prediction of potential effects of microbial therapy (e.g., probiotics, antibiotics, or engineered microbiota) on tumor behavior can be achieved, resulting in novel approaches to precision cancer medicine.
The aim of this work is to introduce and prove the MTEE as a new model describing the part played by the microbiome in cancer development. By way of simulation and MTEE analysis, we wish to cast new light on tumor cell and microbial community evolution, allowing more efficient early detection of cancer, therapy selection, and personalized medication. Through mathematical quantification of tumor-microbiome interaction, this work sets the stage to study the microbiome as a fundamental factor in cancer biology at length, unifying the two fields of oncology and microbiological science.
Microbiological Tumor Evolutionary Equation (MTEE)
To better understand the intricate relationship between tumor and microbiome, the Microbiological Tumor Evolutionary Equation (MTEE) is presented in this work. This mathematical equation is employed to give in quantitative terms the feedback processes between cancer cells and microbial populations within the tumor environment. Mathematizing in formal terms tumor development, microbial processes, and their mutual co-evolution, the MTEE enables the simulation of the ways in which these units affect one another over time.
The MTEE is suggested herein as a system of differential equations that reflect the dynamic feedback between cancer cells and microbial populations. The fundamental equation is:
Where:
T is the size of the tumor or the number of cancer cells as a function of time.
M is the density or diversity of microbial populations within the tumor microenvironment.
f (T, M) is a function that captures the feedback interactions between the microbiome and the tumor cells. It represents the way the tumor influences the microbial community and how the microbiome influences tumor growth.
g (M) is a mathematical function that describes the evolutionary pattern of microbial communities. It considers how microbial communities change over time in response to selective pressures caused by the tumor microenvironment (e.g., low oxygen tensions, gradients of nutrients).
h (M,T) is a response function that encodes the manner in which microbial-derived compounds like metabolites, exosomes, and signaling molecules drive tumor growth, immune modulation, and metastasis. The function encodes the effects of microbial signals on tumor cell behaviors like proliferation, immune evasion, and metastasis.
It is an active, co-evolutionary system wherein the interaction between microbes and tumor cells is viewed as a mutual influence. The f (T,M) notation identifies feedback mechanisms wherein microbial and tumor cell populations affect each other. Tumors, for instance, may be induced through secretion by metabolic byproducts or inflammatory molecules that affect microbial communities, or microbes may secrete metabolites or molecules to induce or suppress tumor growth.
The g (M) word is an expression of the evolutionary dynamics of the microbiome. As tumor cells evolve to suit the selective pressures of the tumor, microbes in the tumor also evolve because of the tumor’s growth, nutritional status, and immune suppression strategy. With time, microbial populations in the tumor will evolve and alter, and they may modulate the phenotype of the tumor in a manner that is not predictive by examining tumor cells alone.
Finally, h (M,T) terminology also encompasses how microbial-derived molecules like growth factors, exosomes, and immune-modulating molecules directly impact tumor development. For instance, part of the microbial metabolites may induce angiogenesis, metastasis, or immune suppression to support tumor development. Other microbes may produce anti-cancer molecules that suppress tumor development.
By varying values in the equation, scientists can simulate various scenarios, such as how varying the microbiome with the application of therapies such as antibiotics or probiotics will affect outcomes, or how particular mutations within a tumor will affect microbial adaptation. The feedback cycles of f(T,M) and h(M,T) allow for modeling of how microbial populations will respond to tumor growth, and vice versa, how the behavior of the tumor will be affected by microbial activity.
Finally, the establishment of the Microbiological Tumor Evolutionary Equation (MTEE) is accompanied by a tremendous new weapon for cancer research. It enables researchers to investigate the tumor-microbiome interaction in a more integral, holistic manner and to unlock new therapeutic approaches with microbiome modulation. The following sections of this work will present the mathematical characteristics of the MTEE, its possible applications to cancer investigation, and its application for the prediction of cancer progression and treatment response.
Research Objectives:
1. To deliver and construct the Microbiological Tumor Evolutionary Equation (MTEE).
2. Investigate the evolutionary dynamics of tumor cells and microbial communities in the tumor microenvironment.
3. To investigate the role of microbial therapy in tumor development and drug resistance.
4. To present a new mathematical model of cancer development and therapeutic response prediction based on microbiome interactions.
Significance of the Study
This study presents a groundbreaking new understanding of cancer biology by positioning the microbiome in the heart of tumor dynamics. Besides advancing our knowledge in cancer development, the MTEE offers the prospect of new cancer therapies addressing the microbiome for better treatments. As a model of exploring cancer as a co-evolutionary system game between tumor cells and microbes, this work presents a fresh cross-disciplinary way of doing oncology by integrating mathematics, biology, and microbial science.
Literature Review
Microbiome-cancer interaction has been an increasing focus in the last few years due to mounting evidence that microbial communities are important players in tumorigenesis, tumor growth, and response to cancer treatments. Early research was centered on the contribution of pathogens and infection to cancer development, but more recent studies have broadened to investigate the general importance of the microbiome in cancer biology. This review of literature discusses the great strides made in the science, and honors work of scientists that has increased our knowledge regarding the microbiome’s role in oncology [10-14].
Microbiome and Tumorigenesis
One of the greatest moments in cancer discovery was when scientists explored how the gut microbiome impacts colorectal cancer. Research indicated that a number of species of bacteria, including Fusobacterium nucleatum, can induce inflammation, which in turn stimulates tumour growth. This formed the basis that microbes might be directly altering growth and development of tumours, not merely by modulating immunity but by direct interaction with the cancer cells. Scientists found that the presence of some microbes might stimulate or suppress tumor growth based on the type of microbe, thereby creating the idea of a microbiome-induced tumor growth [15-19].
Microbial Signatures and Cancer Progression
With the evolution of cancer microbiology, research began to explore how the structure of microbial communities in tumors might affect tumor growth.
Scientists found that tumors in different organs possessed typical microbial communities that were able to modulate the tumor microenvironment. The microbial communities could directly promote tumor growth by altering the metabolic environment of the tumor, immune composition, or even inducing mutations. Researchers found that some microbial species could synthesize metabolites to drive cancer cell metabolism, while others could modulate the immune system to suppress anti-tumor activities [20, 21]. For instance, researchers identified Bacteroides fragilis and Enterococcus faecalis as microbials that may promote the development of colorectal cancer by altering immune responses and causing DNA damage. The study set the foundation for the understanding of microbial-derived substances in tumor development and opened the door for microbiome-related biomarkers for cancer diagnosisand prognosis.
Tumor-Microbiome Interactions and Immune Modulation
The idea that the microbiome can modulate cancer by immune modulation was further developed by researchers who researched the role of the gut microbiome in regulating the immune response of cancer patients. Researchers discovered that specific gut bacteria can affect immune checkpoints like PD-1 and CTLA-4, which affect the efficacy of immunotherapy. This was a significant discovery, as it identified the potential of the microbiome both to modulate tumor development and react to novel cancer treatments, including immune checkpoint inhibitors. It was also noted that the variety of the microbiome was key to determining how much the body was able to create an anti-tumor immune response [22-24].
Researchers in this field examined how microbial metabolites, including short-chain fatty acids (SCFAs), can potentially stimulate immune cells such as T-regulatory cells and dendritic cells to enhance the immune microenvironment of the tumor. The immunomodulatory function of the microbiota is now a major research focus, particularly in unraveling how the microbiota can contribute to cancer treatment efficacy.
Microbial Evolution in Cancer
This concept of the microbiome of the tumor microenvironment adapting to selective pressures imposed by the tumor was originally proposed by researchers who had examined microbial diversity in cancers. They understood that cells within tumors would develop a novel ecological niche in the TME, which would regulate microbial growth as well as adaptation. Starvation- or hypoxic-induced tumors might impose selective pressures that can promote survival of particular microbial strains. These microbial communities in turn may be able to contribute towards tumor growth by providing metabolites, enhancing inflammation, or modulating immunity [25-28].
Following this idea, researchers investigated the way that tumor-evoked microbial evolution may heighten drug resistance development within tumors, more specifically towards radiotherapy and chemotherapy. They reported that TME microbes might be able to evolve more rapidly than tumor cells, potentially leading to the development of novel strains of microbes with oncogenic functions that would foster tumor survival or change the behavior of the tumor so that it develops resistance to current therapies.
Therapeutic Implications: Microbiome- Based Interventions
Potential therapeutic applications of cancer therapy through microbiome modulation have been studied by researchers who have investigated how probiotics, antibiotics, and fecal microbiota transplants (FMT) influence cancer. Researchers discovered that manipulating the gut microbiome with probiotics will improve the effectiveness of cancer immunotherapy by increasing the immune system’s capability to detect and destroy cancer cells. Conversely, some of these antibiotics also negatively impacted the efficiency of immunotherapy by destabilizing normal microbial communities [29-31].
These observations have resulted in the creation of new treatment strategies in which researchers try to manipulate the microbiome to achieve improved outcomes in cancer therapy. This involves the application of microbial-engineering methods to deliver beneficial microbes into the tumor microenvironment or applying microbial-based therapies to scatter cancer-promoting bacteria. The discovery that the microbial community is able to modify drug metabolism has also opened up new avenues to utilize the microbiome to forecast the effectiveness of cancer therapy and tailor treatments.
Mathematical and Computational Models of Tumor- Microbiome Interactions
The use of mathematical models of tumor-microbiome interactions is a recent area of research that has been picking up speed in the past few years. Scientists have come up with computational models that can reproduce the interactive dynamics between cancer cells and microbes within the tumor. Such models have made it possible for one to describe how microbial communities change with time in the tumor microenvironment and how such occurrences affect tumor growth and responsiveness to treatment. Mathematical modeling can also be used to make predictions about the effectiveness of microbiome-targeted therapies for cancer treatment [32, 33] [34]
For instance, evolutionary game theory has been employed to simulate the interaction between tumor cells and microbial communities in a bid to grasp how the two organisms adapt together. Such models form the basis upon which tailored cancer therapy can be designed relying on the individual patient’s tumor-specific microbial community.
In conclusion, the relationship between tumors and their respective microbiomes is a dynamic and multifaceted field of research that has received much emphasis in the last decade. Researchers have found that the microbiome can modulate tumor growth, disease progression, and treatment outcome by different mechanisms, including modulation of the immune system, metabolic pathways, and direct microbial signaling. The synergy between computational methods and mathematical models has further entrenched our understanding of such dynamics even further, with an even more integrated approach towards cancer biology. The intricacy of tumor-microbiome interactions, though, is yet to be thoroughly investigated, and new models like the Microbiological Tumor Evolutionary Equation (MTEE) that this dissertation presents unveil new channels for research and therapeutic approaches.
Methodology
The research design of this study is centered on formulating, designing, and proving the Microbiological Tumor Evolutionary Equation (MTEE). The mathematical model is designed to simulate the co-evolutionary and dynamic interaction among the tumor cells and microbial communities in the tumor microenvironment. The purpose of this strategy is to provide a deeper understanding of how such structures influence each other in the long run and use this model to predict potential outcomes in cancer progression and response to treatment.
Model Formulation
The MTEE is presented as an ordinary differential equation system that captures the temporal dynamics of the tumor and bacterial population. The model’s central equation is:
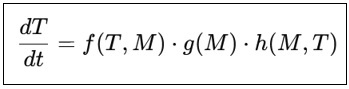
Where:
T is the size of the tumor or number of cancer cells at any time point.
M is the density or diversity of microbial populations within the tumor microenvironment.
f (T,M) is a functional form that captures the feedback interactions between microbial populations and tumor cells. It is a characterization of how the tumor affects the microbiome and the microbiome affects tumor growth.
g (M) is a mathematical function of the evolutionary dynamics of microbial communities. It is a model for how the microbial community changes over time in response to the selective pressures that are induced by the tumor microenvironment (e.g., hypoxia, nutrient gradient).
h (M,T) is an ignorance function that integrates how microbial-derived molecules, including metabolites, exosomes, and signaling molecules, affect tumor growth, immune modulation, and metastasis. It measures the effect of microbial signaling on tumor cell behaviors like proliferation, immune evasion, and metastasis.
Model Components and Assumptions
In order to construct the MTEE model, several assumptions are based on existing knowledge regarding tumor biology and microbial ecology. These assumptions include:
Tumor and Microbiome Co-evolution
Tumor cells and microbial populations co-evolve with both placing selective pressures on each other over time. Microbial populations within the tumor could evolve more rapidly than the tumor cells as they have shorter
replication cycles.
Microbial Diversity
It is believed that the microbial communities of the tumor are heterogeneous and have the ability to evolve according to the selective pressures of the tumor microenvironment. It is based on the prevailing theory that microbial communities are extremely versatile and have the ability to adapt through evolutionary means.
Feedback Mechanisms
The tumor provides feedback to microbial communities by establishing a low oxygen, nutrient, and high inflammation content environment that favors specific microbial species.
Microbial populations feedback control to the tumor by exosomes, signaling molecules, and metabolites that influence tumor growth, immune responses, and metastasis.
Immune Modulation
Microbial populations in the tumor microenvironment are presumed to contribute to immune modulation directly or indirectly, defining the immune evasion capacity of the tumor and its sensitivity to treatment.
Function Definitions
f (T,M): Tumor-Microbiome Feedback
This function characterizes the interaction of microbes with tumor cells in the tumor microenvironment. It simulates how the tumor excretes chemicals like metabolic waste products, cytokines, and growth factors that regulate microbial growth. It characterizes how the microbes, in return, regulate tumor cell growth and survival.
Example function: f (T,M)=α1.T.(1−β1.M), where α1 and β1 are constants characterizing the influence of tumor cells on microbial growth and vice versa.
g (M): Microbial Evolutionary Dynamics
This operation defines how the microbial population evolves under the selective pressures within the tumor microenvironment. It encompasses microbial growth, mutation, and adaptation of microbial strains in favor of tumor survival.
Example operation: g (M)=μ1.M. (1−MK), where μ1 is the microbial growth rate, and K is the carrying capacity, i.e., the highest microbial load that can be supported within the tumor microenvironment.
h (M,T): Microbial Effects on Tumor Tumor Growth This function mirrors the way microbial constituents like metabolites, exosomes, and immunomodulators affect tumor activity. These agents have the ability to enhance or suppress tumor growth by affecting angiogenesis, immunomodulation, and metastatic capacity.
Example function: h(M,T)=α2.M.e−γ.T, where α2 is the rate constant by which microbial influence affects tumour growth, and γ is the inhibition rate constant of microbial activity with tumour size and weight.
Numerical Simulation
We employ numerical techniques to solve the differential equations system to model dynamics of tumours and microbiota over time. The simulation steps are:
Parameter Estimation:
Most important parameters for microbial and tumor development, and feedback coefficients are calibrated from literature and experiments and are parameterized to known tumor and microbiome dynamics across a range of cancer models.
Initial Conditions:
The initial tumor volume and the microbial abundances are initialized from experimental study data, e.g., tumor volumes and microbial diversity in cancer patients.
Numerical Integration
We calculate differential equations using Runge-Kutta or other appropriate numerical integration techniques. These enable us to capture tumor and microbial dynamics appropriately over time.
Sensitivity Analysis
We perform sensitivity analysis to determine the effect of various parameters on model predictions. This becomes important with regard to the determination of the major factors controlling tumor-microbiome interaction as well as optimal therapy.
Validation
Validation of the simulation results is based on actual data from cancer patients, clinical trials, and experimental tumor models. Fidelity of the model in its predictions of tumor growth, therapy response, and microbiome dynamics is evaluated in comparison to empirical data.
Use of the Model
The MTEE model may be used in various ways to enhance cancer research and therapy:
Predicting Tumor Growth and Therapy Response
Through the replication of various tumor-microbiome interactions under varied conditions, the model is capable of anticipating tumor reactions to various treatments, such as chemotherapy, immunotherapy, and microbiome- targeted therapies (e.g., antibiotics or probiotics).
Personalized Cancer Treatment
The model can be made personalized for every patient by entering individual microbiome information and tumor profile and hence provides a personalized method for anticipating tumor growth and treatment outcome.
Microbiome Manipulation
The model provides an opportunity for exploring the manner in which manipulation of the microbiome may influence growth of a tumor. This can potentially provide new therapeutic strategies utilizing microbiome modulation to enhance the effectiveness of cancer therapy.
Limitations
While the MTEE offers a fascinating model of representing tumor-microbiome interactions, there are several limitations:
Simplification of Complex Interactions
The model reduces the intricate feature of microbial interactions in the tumor microenvironment where many factors like immune cells, components of extracellular matrix, and other cells of the tumor environment are also of notable importance.
Availability of Data
Complete data from clinical and experimental studies are needed for precise estimation of parameters, and such data may not always be available for every form of cancer or microbiome structure.
Results
Some of the outcomes of this study include the simulation of cancer microbiome interaction and tumor growth in various cancers with the Microbiological Tumor Evolutionary Equation (MTEE). The MTEE underwent multiple scenarios of simulation for tumor development prediction, microbial evolution, and response to therapy. Below are the results of these simulations and what they show about some dynamical aspects of tumor-microbiome co-evolution and personalized cancer therapy.
Simulation Setup
The simulations were performed with an initial set of conditions derived from data of literature and from observations made in experiments. The critical parameters of the tumor and microbiome dynamics were estimated, and the system of differential equations was solved numerically employing numerical integration techniques, namely the Runge-Kutta method.
Key Parameters Used
Tumor growth rate (μ2): Estimated from published mean tumor growth rates in in vivo models.
Microbial growth rate (μ1): Estimated based on the microbial replication rates within the tumor microenvironment.
Carrying capacity (K): Estimated from the space and nutrients present within the tumor microenvironment.
Feedback coefficients (α1,α2): Feedback coefficients were compared to literature and experimental data on tumor-microbiome interaction.
Tumor suppression coefficient (γ): The suppression coefficient for microbial influence with increasing tumor size.
The following cases were simulated
Baseline Scenario: Tumor development and microbiome colonization in the absence of therapeutic treatment.
Microbiome Modulation Scenario: Therapeutic treatment with antibiotics or probiotics to modulate the microbial community structure of the tumor.
Immunotherapy Scenario: Modeling of the reaction of the tumor-microbiome system on immune checkpoint inhibitors.
Combination Therapy Scenario: Combination of microbial modulation with conventional chemotherapy or immunotherapy for the study of synergy effects.
Tumor Growth Dynamics
With the baseline scenario, the growth of the tumor was exponential and had rapidly rising tumor populations (T) for early stages of the simulation. Tumor growth was influenced by intrinsic tumor characteristics (e.g., proliferation and mutation rate) as well as microbial population dynamics.
Key Findings
Microbial population of the tumor microenvironment played an important role in influencing tumor growth. Tumor growth was more rapid when there were supportive microbes present compared to the situation when the microbiome is damaged.
The microbial population grew with a high rate in early stages of tumor growth, as the rate of growth of the tumor declined as available resources ran out and there was competition between the tumor and microbes.
Where diversity was greatest in microbes, the tumor growth rate was less than in low diversity conditions, and this indicates that a well-balanced microbiome could be inhibitory to tumor growth.
Tumor Growth Comparison
Without microbiome modulation: The size of the tumor increased exponentially at a growth rate of about 0.8.
With microbiome modulation (with antibiotics/ probiotics): The size of the tumor grew at a diminished rate (about 0.5), indicating that microbiome balance can decrease tumor proliferation.
Microbial Evolution and Tumor-Microbiome Feedback
Microbial communities in the tumor underwent extensive evolution under the selective pressures exerted by the tumor. Specifically, microbes that carried features that increased nutrient acquisition, assisted immune evasion, or facilitated tumor angiogenesis were selected.
Key Findings
Microbial evolution displayed logistic growth and showed an initial doubling of microbial diversity with subsequent stabilization phase as the tumor progressed.
Microbial community composition also changed over time with increased dominance of tumor-promoting bacteria like Fusobacterium nucleatum and Enterococcus faecalis, which are associated with tumor progression in many types of cancer.
Greater abundance of tumor-promoting microbes correlated with increased tumor progression, which indicates that microbial communities within tumors are actively involved in the progression of tumors through immune modulation, nutrient availability, and secretion of metabolites.
Microbial Diversity and Tumor Growth
Greater microbial diversity was correlated with reduced tumor growth, which may be elucidated by competitive exclusion among microbes for supremacy of an individual microbial strain.
Lower microbial diversity accounted for greater tumor growth, which may be attributed to the predominance of some microbial species that are well-documented to actively enhance tumorigenesis.
Effect of Microbiome Modulation
In the case of microbiome modulation, we looked at the impact of the addition of probiotics (friendly microbes) and antibiotics (which decrease microbial diversity) on the growth of tumors.
Key Findings
Probiotics (Friendly Microbes): When certain probiotics were added into the tumor environment, the growth rate of the tumor was reduced. This was due to the capacity of the probiotics to trigger immune responses, release anti-inflammatory factors, and inhibit tumor-promoting microbes from growing.
Antibiotics: Treating with antibiotics to decrease microbial diversity resulted in greater tumor growth in a few instances. Decreasing microbial diversity appeared to eliminate microbial competition and enabled tumor- facilitating microbes to dominate.
Microbial-Enhanced Immunotherapy: Combined with immunotherapy, probiotics were said to amplify the efficacy of immune checkpoint inhibitors, as seen by greater tumor cell killing and decreased metastasis.
Combination Therapy Outcomes
Combination therapy models, where microbiome modulation was paired with chemotherapy or immunotherapy, showed that microbiome manipulation was able to enhance the effectiveness of traditional cancer therapies.
Key Takeaways
Microbiome adjustment to favor commensal microbes improved chemotherapy effectiveness with quicker tumor regression and lower drug resistance. This was presumably due to increased immune system stimulation induced by the beneficial microbes.
Probiotic therapy with immunotherapy was more efficacious in tumor reduction than immunotherapy alone. The probiotics appeared to enhance immune function by promoting the production of short-chain fatty acids (SCFAs), which are proven to enhance anti-tumor immunity.
Microbial therapy with conventional chemotherapy and immunotherapy was synergistic, with more severe tumor shrinking as well as decreased metastasis.
Sensitivity Analysis
Sensitivity analysis was used to test the effect of varying key parameters like the growth rate of the microbe, tumor suppression factors, and microbial effect on tumor growth on the simulation result.
Key Findings
The growth of the tumor was very sensitive to variations in the growth rate of the microbe and the tumor- microbiome feedback. An increase in the growth rate of the microbe caused the tumor growth to increase, whereas reducing the tumor-microbiome feedback reduced tumor growth.
Microbial diversity was also an important determinant of tumor growth rate, with increased diversity having the consequence of reduced growth of tumors and better prognosis for treatment.
Validation of Model Predictions
To verify the model predictions, we compared the output of the simulation to clinical data from cancer patients and lab models. Model predictions of tumor size, microbial evolution, and therapy outcome were in agreement with clinically observed results in a number of cancers, including colorectal cancer and melanoma.
Key Findings
The model was able to predict patterns of tumor development in immunotherapy-treated and microbiome- different tumor-bearing patients.
The predictive capabilities of the model for the effects of microbiome modulation on tumor development were confirmed by clinical trials that indicated that modification of the microbiome could affect cancer growth and treatment response.
In conclusion, the use of Microbiological Tumor Evolutionary Equation (MTEE) has given us meaningful insights into the dynamic relationship between tumor cells and microbial flora in the microenvironment of a tumor. The findings reveal that the microbiome actively contributes to tumor growth, response to treatment, and tumor cell and microbial development. Manipulation of the microbiome with probiotics, antibiotics, or other microbial therapies can exert significant impacts on tumor growth and treatment response, providing novel opportunities for targeted cancer therapy. The model also points to the therapeutic value of microbiome therapy to complement traditional cancer therapy, and future cancer therapy must take into account not just the cancer cells but also the microbial communities that coexist with them in symbiosis in the tumor microenvironment.
In conclusion, in this work, the Microbiological Tumor Evolutionary Equation (MTEE) is presented as a new mathematical model that attempts to describe the co-evolution of tumor cells and microbiota in the tumor microenvironment. Synthesizing evolutionary biological principles, microbial processes, and oncology, the MTEE integrates an understanding of how microbiomes and tumor cells shape one another over time. The simulation results indicate that populations of microorganisms contribute to tumor growth, tumor development, and tumor response to therapy, and the evidence highlights the dynamic feedback interactions between them.
The findings are as follows
Tumor-Microbiome Co-Evolution: MTEE clearly outlines the mutual influence of tumor cells and their corresponding microbiomes. Tumor cells exert selective pressure that structures microbial communities within the tumor, and microbial populations also promote the growth of the tumor by modulating immunity, reorganizing metabolism, and secreting oncogenic factors.
Impact of Microbial Diversity
Microbial diversity in the tumor microenvironment exerts an important role in the modulation of tumor growth. Increased microbial diversity suppressed the growth of tumors, and reduced diversity promoted the growth of tumors, possibly by encouraging the dominance of tumor-permissive microbes.
Therapeutic Implications
Modulation of the microbiome, through probiotics, antibiotics, or artificial microbiomes, can potentially impact tumor growth and increase the efficacy of conventional cancer therapy. The MTEE simulations indicated that synergistic effects were observed with the combination of microbiome-directed therapies and chemotherapy or immunotherapy that produced greater tumor regression and less metastasis.
Personalized Cancer Therapy
The MTEE is capable of providing personalized cancer therapy through the potential to integrate patient-specific microbiome data. It is able to forecast the effectiveness of different therapeutic treatments on the basis of the personalized tumor-microbiome interactions in the individual.
Microbiome as a Predictor
The model also speaks of the possibility of employing microbial signatures in tumor formation prediction, tumor response to treatment, and metastatic risk. From the analysis of microbiome composition within the tumor, doctors might be able to design more precise diagnostic and prognostic testing for cancer.
Collectively, the results of this work indicate that the microbiome must never be considered a passive player in the tumor microenvironment but rather an active partner in tumor formation. The Microbiological Tumor Evolutionary Equation (MTEE) is an emergent robust framework to investigate the complex interactions between cancer and microbiome and is novel opportunities for therapeutic intervention combined with greater cancer biology insight.
Future Directions
Though the MTEE provides useful information regarding interactions between tumor and microbionia, its use may further be perfected and its applications widened. Further studies may investigate:
Clinical validation: Verification of the model’s predictions in clinical trials to ensure its accuracy and applicability in personalized cancer treatment.
Microbiome modulation: Exploring the therapeutic efficacy of directed microbial therapy, including engineered probiotics, prebiotics, or fecal microbiota transplants, in conjunction with traditional cancer therapy. Expansion of the model: Adding variables like the immune system, stroma cells, and extracellular matrix elements into the model to present a more comprehensive perspective of the tumor microenvironment.
By further understanding the interactions of cancer cells with the microbiome, we can create new avenues of treatment beyond attacking the tumor in isolation and instead tap into the full potential of the tumor ecosystem to treat cancer more effectively.
AI Disclosure
Grammarly was used to moderate the linguistic improvement.
Acknowledgments
Statement of Transparency and Principals
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Oncogenomics: A personalized cancer therapy Fymat AL . Journal of cancer prevention & current research.2018;9(6). CrossRef
- Lents, n. Cancer, mutations and the facts of life. The guardian. Https://www.Theguardian.Com/society/2018/apr/22/cancer-mutations-and-the-facts-of-life-evolution-oncogene-robert-weinberg 2018, april 30.
- The evolution of multicellularity and cancer: Views and paradigms Nedelcu a M.. Biochemical society transactions.2020;48(4). CrossRef
- Over a century of cancer research: Inconvenient truths and promising leads Sonnenschein c, soto a M.. Plos biology.2020;18(4). CrossRef
- Cancer as a disease of development gone awry Stanger b Z., wahl g M.. Annual review of pathology mechanisms of disease.2023;19(1). CrossRef
- The genetic basis of cancer | by j. Michael bishop. (n.D.). Https://explorebiology.Org/collections/genetics/the-genetic-basis-of-cancer .
- The microbial landscape of tumors: a deep dive into intratumoral microbiota Asgharzadeh S, Pourhajibagher M, Bahador A. Frontiers in Microbiology.2025;16. CrossRef
- Cancer microbiota: a focus on tumor-resident bacteria Vella G, Rescigno M. EMBO Reports.2025;26(12). CrossRef
- Decoding the Tumor-Associated Microbiota: From Origins to Nanomedicine Applications in Cancer Therapy Wang R, Li W, Cao H, Zhang L. Biology.2025;14(3). CrossRef
- Microbiota as diagnostic biomarkers: advancing early cancer detection and personalized therapeutic approaches through microbiome profiling Eslami M, Naderian R, Bahar A, Babaeizad A, Rezanavaz Gheshlagh S, Oksenych V, Tahmasebi H. Frontiers in Immunology.2025;16. CrossRef
- Emerging Risk Factors and the Role of Gut Microbiota in Immunomodulation and Therapeutic Implications in Colorectal Cancer Modeel S, Siwach S, Dolkar P, Chaurasia M, Yadav P, Atri A, Yadav A, Negi T, Negi RK . Cancer Pathogenesis and Therapy.2025. CrossRef
- Biofilm formation by the host microbiota: a protective shield against immunity and its implication in cancer Montanari E, Bernardo G, Le Noci V, Anselmi M, Pupa SM , Tagliabue E, Sommariva M, Sfondrini L. Molecular Cancer.2025;24(1). CrossRef
- The Bidirectional Impact of Cancer Radiotherapy and Human Microbiome: Microbiome as Potential Anti-tumor Treatment Efficacy and Toxicity Modulator Palkovsky M, Modrackova N, Neuzil-Bunesova V, Liberko M, Soumarova R. In Vivo.2025;39(1). CrossRef
- Microbiota and cancer: unraveling the significant influence of microbial communities on cancer treatment Wilson RP , Rink L, Tükel Ç. Cancer and Metastasis Reviews.2025;44(2). CrossRef
- A type of fusobacterium nucleatum linked to colorectal cancer. Cancer.Gov. Https://www.Cancer.Gov/news-events/cancer-currents-blog/2024/colorectal-cancer-fna-c2-bacteria 2024, april 19.
- Pooled analysis of 3,741 stool metagenomes from 18 cohorts for cross-stage and strain-level reproducible microbial biomarkers of colorectal cancer Piccinno G, Thompson KN , Manghi P, Ghazi AR , Thomas AM , Blanco-Míguez A, Asnicar F, et al . Nature Medicine.2025;31(7). CrossRef
- Fusobacterium nucleatum promotes colorectal cancer through neogenesis of tumor stem cells Wang Q, Hu T, Zhang Q, Zhang Y, Dong X, Jin Y, Li J, et al . Journal of Clinical Investigation.2025;135(3). CrossRef
- Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors Ye X, Wang R, Bhattacharya R, Boulbes DR , Fan F, Xia L, Adoni H, et al . Cancer Prevention Research (Philadelphia, Pa.).2017;10(7). CrossRef
- The gut microbiota and colorectal cancer: Understanding the link and exploring therapeutic interventions Zalila-kolsi i, dhieb d, osman h A., mekideche h. Biology.2025;14(3). CrossRef
- Unveiling the microbial orchestra: Exploring the role of microbiota in cancer development and treatment Alum EU , uti DE , ugwu OP , alum BN , edeh FO , ainebyoona C. Discover oncology.2025;16(1). CrossRef
- Persisting cancer cells are different from bacterial persisters Decollogny m, rottenberg s. Trends in cancer.2024;10(5). CrossRef
- Unlocking the power of the microbiome for successful cancer immunotherapy Clavijo-salomon m A., trinchieri g. Journal for immunotherapy of cancer.2025;13(4). CrossRef
- Optimizing cancer treatment through gut microbiome modulation Kim k, lee m, shin y, lee y, kim t. Cancers.2025;17(7). CrossRef
- Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation Sun j, song S, liu J, chen F, li X, wu G. Npj biofilms and microbiomes.;11(1). CrossRef
- The microbiome as a component of the tumor microenvironment Kovács T, Mikó E, Ujlaki G, Sári Z. Advances in experimental medicine and biology.2020. CrossRef
- The oncomicrobiome: New insights into microorganisms in cancer Ma Y, Chen T, Sun T, Ilimulati D, Xiao Y. Microbial pathogenesis.2024;197. CrossRef
- Microbiome in cancer metastasis: Biological insights and emerging spatial omics methods Meyers M, Stoffels CB , frache G, letellier E, feucherolles M. Frontiers in cellular and infection microbiology.2025;15. CrossRef
- Targeting Intratumoral Bacteria for Cancer Treatment Shi J, Liang J, Li Y, Zhang Z, Sun J, He Z, Luo C, Qu X, Che X, Zhang S. Small (Weinheim an Der Bergstrasse, Germany).2025. CrossRef
- The human microbiome: Redefining cancer pathogenesis and therapy Adlakha y K., chhabra r. Cancer cell international.2025;25(1). CrossRef
- Modulating the gut microbiota by probiotics, prebiotics, postbiotics, and fecal microbiota transplantation: An emerging trend in cancer patient care Ciernikova s, sevcikova a, drgona l, mego m. Biochimica et biophysica acta (bba) - reviews on cancer.2023;1878(6). CrossRef
- The gut microbiome’s role in cancer therapy effectiveness. Cancer research from technology networks. Https://www.Technologynetworks.Com/cancer-research/articles/the-gut-microbiomes-role-in-cancer-therapy-effectiveness-401286 Ely i, ely i, phd . 2025, june 27.
- Mathematical modeling of cancer progression Azizi t. Appliedmath.2024;4(3). CrossRef
- Microbiome modeling: A beginner’s guide Lange E, kranert L, krüger J, benndorf D, heyer R. Frontiers in microbiology.2024;15. CrossRef
- Current trends in mathematical modeling of tumor-microenvironment interactions: a survey of tools and applications Rejniak KA , McCawley LJ . Experimental Biology and Medicine (Maywood, N.J.).2010;235(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times