COVID-19 and Breast Cancer Management in a Small Healthcare Setting: A Retrospective Study
Download
Abstract
Aims: To evaluate the impact of the COVID-19 pandemic on breast cancer presentation, surgical management, and lymph node involvement in a small, understudied population in Brčko District, Bosnia and Herzegovina.
Methods: A retrospective cohort study conducted at the General Hospital of Brčko District, Bosnia and Herzegovina, from March 2018 to March 2022. We included 113 patients with histopathologically confirmed breast cancer. We divided the patients into two groups: the before-COVID group (65 patients, including one male) treated from March 2018 to March 2020, and the during-COVID group (47 patients) treated from March 2020 to March 2022. We analyzed patient data (age, gender, menopausal status) along with tumor and lymph node histopathological characteristics.
Results: The number of surgeries performed decreased by 16.9% during the COVID period. Patients in the pre-COVID group mostly had grade II tumors, while those in the post-COVID group had predominantly grade III tumors. The average number of lymph nodes sampled was similar between groups (10.70 ± 4.12 before COVID vs. 10.39 ± 4.66 during COVID). Still, the average number of positive lymph nodes was higher in the COVID group (2.5±3.82) compared to the pre-COVID group (1.64±2.90). For patients without neoadjuvant therapy, the average time to surgery increased from 1.04 months (±0.66) before the COVID-19 pandemic to 2.59 months (±2.88) during the COVID-19 pandemic. For those receiving neoadjuvant therapy, time to surgery increased from 5.45 months (±2.97) to 7.29 months (±5.15).
Conclusion: The COVID-19 pandemic led to delayed breast cancer management, higher tumor grade, and increased lymph node involvement in a small, resource-limited healthcare setting. Findings highlight the need for resilient oncology services in smaller districts during public health crises.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in patients in Wuhan, Hubei Province, in December 2019 [1]. The World Health Organization (WHO) declared the disease caused by this virus to be coronavirus disease 2019 (COVID-19). According to WHO data, as of early February 2021, COVID-19 had caused over 2.2 million deaths and 103 million confirmed cases worldwide [2].
Breast cancer is the most commonly diagnosed cancer globally, with an estimated 2.3 million new cases annually. It represents a significant public health burden, requiring enhanced efforts in both primary and secondary prevention worldwide [3]. Delayed diagnosis has resulted in breast cancers detected after quarantine exhibiting worse prognoses, with larger tumor sizes and higher rates of nodal involvement. This suggests that, during potential future lockdowns, breast cancer screening and follow-up examinations should continue without interruption, and patients should be encouraged to seek medical attention promptly if clinical symptoms arise [5]. The full impact of the COVID-19 pandemic on global oncology remains to be seen. Countries with high SARS-CoV-2 prevalence and limited healthcare infrastructure are expected to experience more profound effects [6].
Several studies have reported a substantial impact of the COVID-19 pandemic on breast cancer care, including the suspension of screening programs [4-9]. For example, in Canada, the Netherlands, Germany, Italy, the United Kingdom, and Australia, national screening programs were suspended for periods ranging from one to six months [4]. Hower, data from small regional hospitals and underrepresented areas are scarce. The Brčko District in Bosnia and Herzegovina represents a small healthcare setting with limited oncology resources, where treatment delays, diagnostic constraints, and capacity limitations may have uniquely influenced patient outcomes.
This study aimed to evaluate the impact of the COVID-19 pandemic on breast cancer management in this small, understudied population. Unlike previous studies from large tertiary centers, our work captures real-world challenges in a small hospital, highlighting the clinical consequences of delays and resource limitations during a global pandemic. These findings can inform regional healthcare planning, prioritization of oncology care, and strategies to mitigate the impact of future crises.
Materials and Methods
This retrospective cohort study was conducted at Brčko District General Hospital, Bosnia and Herzegovina, between March 2018 and March 2022. March was chosen as the starting point to ensure two full years before and two years after the first confirmed case of COVID-19 in Bosnia and Herzegovina, which was registered in March 2020. The study included 113 female patients who underwent surgical treatment for histopathologically confirmed primary breast cancer. Diagnosis was established through imaging, including mammography, ultrasound, or breast MRI, and confirmed either by core biopsy or intraoperative ex tempore analysis following review by the multidisciplinary oncology council. Patients with stage IV disease not eligible for surgery, those requiring subcutaneous mastectomy with reconstruction, and those with incomplete medical records or missing histopathological data were excluded. Demographic, clinical, and tumor-related data were extracted from electronic and paper-based hospital records and independently verified by two investigators to ensure accuracy.
Patient characteristics included age, menopausal status, and comorbidities. Tumor characteristics encompassed tumor size, histological type, receptor status (ER, PR, HER2), Ki-67 proliferation index, histological grade, clinical TNM stage, and number of positive axillary lymph nodes. Primary outcomes were histological grade, axillary lymph node involvement, and time from diagnosis to surgery, while secondary outcomes included type of surgical procedure performed, use of neoadjuvant therapy, and Ki-67 index. Subgroup analyses were performed based on age, menopausal status, and molecular subtype where appropriate.
All patients underwent standardized preoperative evaluation, including clinical examination, imaging, laboratory tests (complete blood count, biochemistry, tumor markers CEA, CA 15-3, and CA 125), and additional staging investigations such as chest X-ray, skeletal survey, CT scans, and abdominal ultrasound when indicated. Internal medicine and anesthesiology assessments ensured surgical eligibility under general anesthesia. Treatment decisions were made by the oncology council. Neoadjuvant therapy was administered to patients with palpable axillary lymph nodes, tumor size greater than 2 cm, triple-negative or HER2-positive tumors, high Ki-67 index, unresectable tumors, or inflammatory carcinoma. Surgery was performed either after tumor downstaging or immediately in early-stage disease.
Surgical procedures included radical and simple mastectomy, partial breast resection with sentinel lymph node biopsy, axillary dissection, and tumorectomy. Sentinel lymph nodes were identified using 0.5 ml of subdermal methylene blue in the periareolar region and sent for ex tempore analysis; positive nodes prompted axillary dissection. Tumor excision aimed for negative margins, confirmed intraoperatively. Extent of surgery followed TNM staging guidelines, and definitive histopathology guided adjuvant therapy recommendations. All patients were screened for SARS-CoV-2 using rapid immunochromatographic tests prior to admission, and only those testing negative were admitted for surgery. Oncology patients were prioritized for surgery during pandemic-related capacity limitations.
Continuous variables were summarized as mean ± standard deviation or median with range, and categorical variables as counts and percentages. Between-group comparisons were performed using Student’s t-test or Mann–Whitney U test for continuous variables and chi-square or Fisher’s exact test for categorical variables. Multivariate logistic regression was conducted to identify independent predictors of higher histological grade and lymph node positivity, adjusting for age, tumor size, and receptor status. Statistical significance was defined as p≤ 0.05. Analyses were performed using SPSS version 24 and JASP version 0.16.
Results
The total number of patients in the pre-COVID group was 66 (58.4%), while in the during-COVID group it was 47 (41.5%). The number of surgeries performed during the COVID period decreased by 16.9%. The average age of patients before the COVID-19 pandemic was 62.02 years (SD = 11.97), and during the pandemic, it was 61.96 years (SD = 12.53) (Table 1).
| Observed Groups | Before COVID-19 | During COVID-19 |
| Number of patients | 66 (58.4%) | 47 (41.5%) |
| Average age (years) | 62.02 (SD = 11.97) | 61.96 (SD = 12.53) |
Regarding neoadjuvant therapy, the absolute number of patients receiving it was the same (n = 16), but the proportion differed. Before the COVID-19 pandemic, 24.24% of patients received neoadjuvant therapy, compared to 34.04% during the pandemic, representing a 9.8% increase (Table 2).
| Neoadjuvant Therapy | Before COVID-19 | During COVID-19 | Chi-square Value | df | Significance (p) |
| YES | 16 (24.24%) | 16 (34.04%) | 1.299 | 1 | 0.254 |
| NO | 50 (75.76%) | 31 (65.96%) | |||
| Total | 66 (100%) | 47 (100%) |
These results suggest that more advanced tumor stages occurred during the pandemic, although the difference was not statistically significant (p = 0.254).
Types of surgical procedures were similar before and during the pandemic (p > 0.05). Radical mastectomy was the most common surgery in both periods (53.03% vs. 61.70%) (Table 3).
| Type of surgery | Before COVID-19 (%) | During COVID-19 (%) |
| Radical mastectomy | 35 (53.03) | 29 (61.70) |
| Simplex mastectomy | 4 (6.06) | 2 (4.26) |
| Partial breast resection with axillary dissection | 17 (25.76) | 4 (8.51) |
| Partial breast resection with SLNB | 8 (12.12) | 9 (19.15) |
| Partial breast resection | 2 (3.03) | 3 (6.38) |
| Total | 66 (100) | 47 (100) |
Histological tumor grade showed significant differences (Figure 1).
Figure 1. Distribution of Histological Tumor Grades before and During COVID-19.
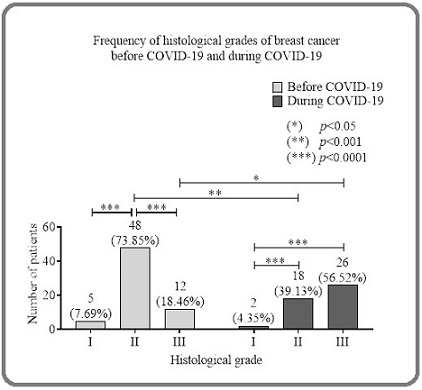
Before the COVID-19 pandemic, grade II tumors predominated (73.85%), followed by grade III (18.46%) and grade I (7.69%). During COVID, grade III tumors were most frequent (56.52%), followed by grade II (39.13%) and grade I (4.35%). These results indicate a shift towards more aggressive tumors during the pandemic.
Comparison between groups showed significantly more grade III tumors during COVID (p < 0.05) and more grade II tumors before COVID (p < 0.001).
Regarding axillary lymph nodes, the median number removed was similar between groups (10 vs. 12, p=0.357), but the number of positive (metastatic) lymph nodes was significantly higher during COVID (median one vs. 0, p=0.04) (Table 4).
| Status of Axillary Lymph Node | Before COVID-19 | During COVID-19 | p value |
| Axillary lymph nodes removed | Median: 10 (range 6–22) | Median: 12 (range 6–19) | 0.3565 |
| Positive axillary lymph nodes | Median: 0 (range 0–10) | Median: 1 (range 0–14) | 0.04 (*) |
(*) The difference was statistically significant
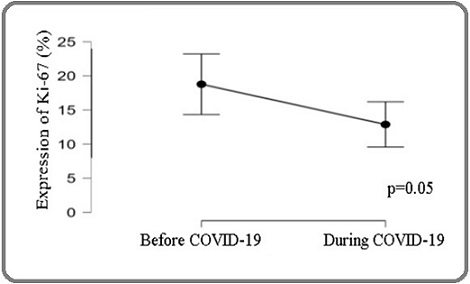
Ki-67 expression was higher before COVID, but this difference was not statistically significant (p = 0.05) (Figure 2).
Figure 2. Ki-67 Expression before and During COVID-19..
Time from diagnosis to surgery increased during the pandemic. Among patients without neoadjuvant therapy, the mean time to surgery was 1.04 months before the COVID-19 pandemic and 2.59 months during the COVID-19 pandemic (p = 0.05). For patients receiving neoadjuvant therapy, the time increased from 5.45 to 7.29 months; however, this difference was not statistically significant (p = 0.337) (Tables 5 and 6).
Discussion
This study provides unique insights from a small regional hospital with limited oncology resources, a setting rarely represented in COVID-19 literature. Our study found a 16.8% reduction in breast cancer surgeries during the COVID-19 pandemic, consistent with other reports [7, 8]. These results likely reflect decreased hospital admissions and limitations in surgical capacity. Similar studies reported increased stage IV diagnoses and reduced surgery volumes during early pandemic months [7, 8].
The proportion of patients receiving neoadjuvant therapy increased by nearly 10% during the pandemic. However, this increase was not statistically significant, supporting the findings of Chapgar et al. and Corke et al., who observed an increase in the use of neoadjuvant therapy as a strategy to manage delays and surgical limitations [9, 10].
Axillary lymph node involvement was significantly higher during COVID-19, confirming more advanced disease at presentation, a critical prognostic factor associated with recurrence risk and survival [11, 12].
This trend aligns with the observed increase in high histological grade tumors during the pandemic, suggesting a shift toward more aggressive disease [13, 14].
Although Ki-67 is a recognized marker of tumor proliferation and worse prognosis [15], we did not find significant differences in its expression before and during the pandemic.
We observed prolonged time to surgery during the pandemic, especially in patients without neoadjuvant therapy. This delay may contribute to tumor progression and worse outcomes, as also reported in other studies [16, 17].
Our study has several limitations: we used a retrospective design, enrolled a relatively small sample, and did not collect data on radiation therapy or all surgical procedures (e.g., subcutaneous mastectomy, which our institution does not perform).
In conclusion, the COVID-19 pandemic negatively impacted breast cancer management in the Brčko District, with patients presenting with more advanced and aggressive tumors, increased lymph node involvement, higher neoadjuvant therapy use, and longer delays to surgery. These findings underscore the importance of maintaining timely cancer care even during public health crises. Findings underscore the importance of maintaining timely oncology care in small, resource-limited hospitals during public health emergencies.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- A Novel Coronavirus from Patients with Pneumonia in China, 2019 Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, et al . The New England Journal of Medicine.2020;382(8). CrossRef
- Clinical Characteristics of Coronavirus Disease 2019 in China Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, et al . The New England Journal of Medicine.2020;382(18). CrossRef
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- The benefits and harms of breast cancer screening: an independent review Marmot M. G., Altman D. G., Cameron D. A., Dewar J. A., Thompson S. G., Wilcox M.. British Journal of Cancer.2013;108(11). CrossRef
- Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre Linck P, Garnier C, Depetiteville M, MacGrogan G, Mathoulin-Pélissier S, Quénel-Tueux N, Charitansky H, et al . European Radiology.2022;32(3). CrossRef
- Has the COVID-19 Pandemic Affected Breast Cancer Stage and Surgical Volume? Kara H, Arikan AE , Dulgeroglu O, Tutar B, Tokat F, Uras C. Frontiers in Surgery.2022;9. CrossRef
- The Impact of the COVID-19 Pandemic on Breast Cancer Patients İlgün AS , Özmen V. European Journal of Breast Health.2022;18(1). CrossRef
- Effect of the COVID-19 Pandemic on Surgical Breast Cancer Care in the Netherlands: A Multicenter Retrospective Cohort Study Filipe MD , Deukeren D, Kip M, Doeksen A, Pronk A, Verheijen PM , Heikens JT , Witkamp AJ , Richir MC . Clinical Breast Cancer.2020;20(6). CrossRef
- Lannin, Sarah Schellhorn Mougalian, Elizabeth Rapp Berger, Cary Philip Gross, Nina Ruth Horowitz, Tara B. Sanft, Michael DiGiovanna, Mehra Golshan, and Lajos Pusztai Anees B, Chagpar , Donald R. Journal of Clinical Oncology.2021;39(15_suppl):e18708-e18708.
- Lauren Corke, Omar Hajjaj, Kaylie Willemsma, Stephen Chia, Christine Simmons. Impact of COVID-19 on patients undergoing neoadjuvant therapy: A pre/post pandemic analysis and assessment of quality of care delivered [abstract] In: Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7-10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82(4 Suppl):Abstract nr P2-11-08...
- Prognostic value of extracapsular extension of axillary lymph node metastases in T1 to T3 breast cancer Neri A, Marrelli D, Roviello F, De Stefano A, Guarnieri A, Pallucca E, Pinto E. Annals of Surgical Oncology.2005;12(3). CrossRef
- Association between Lymph Node Ratio and Disease Specific Survival in Breast Cancer Patients with One or Two Positive Lymph Nodes Stratified by Different Local Treatment Modalities Hong R, Dai Z, Zhu W, Xu B. PloS One.2015;10(10). CrossRef
- Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index Henson D. E., Ries L., Freedman L. S., Carriaga M.. Cancer.1991;68(10). CrossRef
- Prognostic significance of Nottingham histologic grade in invasive breast carcinoma Rakha EA , El-Sayed ME , Lee AHS , Elston CW , Grainge MJ , Hodi Z, Blamey RW , Ellis IO . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(19). CrossRef
- Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients Azambuja E., Cardoso F., Castro G., Colozza M., Mano M. S., Durbecq V., Sotiriou C., et al . British Journal of Cancer.2007;96(10). CrossRef
- Time to Treatment Initiation for Breast Cancer During the 2020 COVID-19 Pandemic Hawrot K, Shulman LN , Bleiweiss IJ , Wilkie EJ , Frosch ZAK , Jankowitz RC , Laughlin AI . JCO oncology practice.2021;17(9). CrossRef
- Delay in Breast Cancer Treatments During the First COVID-19 Lockdown. A Multicentric Analysis of 432 Patients Vanni G, Tazzioli G, Pellicciaro M, Materazzo M, Paolo O, Cattadori F, Combi F, et al . Anticancer Research.2020;40(12). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times