Plasma 8-Hydroxydeoxyguanosine, 4-Hydroxynonenal and Selenium in Hepatitis B Virus-Infected Adults in Ibadan, Nigeria
Download
Abstract
Introduction: Hepatitis B Virus (HBV) infection is a significant global health challenge, particularly in Sub-Saharan Africa, including Nigeria. Reactive oxidative species (ROS) generated during HBV replication contribute to redox imbalance, leading to oxidative stress. However, data on the levels of oxidative stress markers in Nigerians infected with HBV is scarce. This study aimed to investigate plasma levels of oxidative stress markers [8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxynonenal (4-HNE)] and the trace element, selenium, in HBV-infected adults in Ibadan, Nigeria.
Materials and Methods: In a case-control study, 40 HBV-infected patients served as cases, and 40 HBV-uninfected patients served as controls. Anthropometric data were collected, and plasma 8-OHdG and 4-HNE were determined using the ELISA method. Selenium was determined using atomic absorption spectrophotometry. Data were analysed using descriptive, t-test, and Pearson correlation statistics at p = 0.05.
Results: The mean ages of HBV-infected and uninfected individuals were 35.3±1.5 and 36.0±1.5 years, respectively. The level of 4-HNE was not significantly different between HBV-infected (2.2±1.7 ng/ml) and uninfected (1.8±1.3 ng/ml). There was also no significant difference in 8-OHdG levels between HBV-infected (4.1±2.4 ng/ml) and uninfected (3.6±1.8 ng/ml). However, in HBV-infected patients, 8-OHdG levels showed a significant positive correlation with 4-HNE (r =0.320, p =0.044). Selenium levels were also significantly higher in HBV-infected (78.4±25.4 ug/dl) than uninfected (35.6±11.4 ug/dl).
Conclusion: The levels of markers of lipid peroxidation and DNA oxidation were similar in HBV-infected and uninfected participants, but selenium, the key component of the potent antioxidant glutathione peroxidase was more than double in the infected group. It is plausible that the protective effect of Se may have spared the other markers of oxidative damage. Further studies are needed to elucidate these preliminary findings.
Introduction
Chronic Hepatitis B (CHB) virus infection represents a major global public health issue, affecting an estimated 296 million individuals and contributing to over 800,000 deaths each year. The primary causes of these fatalities are liver cirrhosis and hepatocellular carcinoma (HCC) [1]. Particularly in Sub-Saharan Africa (SSA), CHB exerts an overwhelming impact, with approximately 80 million individuals infected [2]. Within Nigeria, the prevalence of hepatitis B virus (HBV) infection stands at about 12.2% [3], making CHB a leading risk factor for HCC in this region. Hepatocellular carcinoma is globally recognized as the sixth most common cancer, characterized by a dismal 5-year survival rate, primarily due to the late-stage diagnosis of the disease [4].
The intricate molecular nature of HBV infection demands an in-depth and comprehensive approach to understanding its pathogenesis and identifying novel therapeutic targets. Achieving this is vital to meet the United Nations’ objective of diminishing the morbidity and mortality associated with CHB and eradicating it as a global health threat by 2030. Central to the pathogenesis of HBV-related liver disease is the generation of reactive oxygen species (ROS) during viral replication, leading to a redox imbalance. This critical connection is highlighted in several studies. For instance, Kim, Seo [5] demonstrated that ROS, facilitated by Hsp90, enrich the formation of the HBV capsid, thus contributing to redox imbalance and the progression of liver disease. Furthermore, Bender and Hildt [6] observed that both HBV and Hepatitis C Virus (HCV) influence the mechanisms generating and inactivating ROS, impacting the Nrf2/Keap1-signaling pathway and potentially exacerbating redox imbalance. In a similar vein, Wang, Yun [7] discovered that ROS production during HBV replication can intensify liver disease pathogenesis by increasing levels of the viral oncoprotein, HBx. Collectively, these findings emphasize the pivotal role of ROS in the development of HBV-related liver disease. Consequently, oxidative stress markers such as 8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxynonenal (4-HNE), which are indicators of oxidative DNA damage and lipid peroxidation, respectively, are believed to play significant roles in the progression of HBV infection [7].
Selenium, a vital trace element, is renowned for its antioxidant properties that contribute to mitigating oxidative stress and safeguarding cellular structures. This protective role is facilitated by the element’s modulation of cellular responses through activities of selenocysteine-containing selenoproteins [8]. Additionally, selenium enhances the activities of key enzymes such as superoxide dismutase (SOD) and peroxidase (POD), playing a significant role in defending against oxidative damage [9]. Moreover, recent studies indicate a link between diminished selenium levels and heightened vulnerability to HBV infection and its subsequent complications, especially in areas with prevalent HBV infection [10]. Notably, lower selenium concentrations have been associated with an increased risk of hepatocellular carcinoma in patients with chronic hepatitis virus infection [11]. Furthermore, decreased plasma selenium levels are significantly correlated with increased mortality risk in HIV-1-infected individuals, suggesting a parallel in viral infections such as HBV [12]. These findings underscore the imperative for additional research, particularly in African populations, which present unique genetic and environmental contexts.
Despite the crucial link between HBV infection and oxidative stress markers, research specifically addressing this relationship, particularly among the adult population in Nigeria, is notably limited. Existing studies tend to focus predominantly on the virological aspects of HBV or on broad assessments of liver function, often neglecting the detailed molecular insights that could be uncovered through the examination of oxidative stress markers and their interactions with trace elements such as selenium. Therefore, this study seeks to fill this gap in knowledge by analysing the plasma levels of 8-OHdG, 4-HNE, and selenium in individuals infected with HBV in Ibadan, Nigeria.
Gaining a deeper understanding of the intricate relationship between these biomarkers and HBV infection could pave the way for the development of novel diagnostic tools, prognostic methods, and treatment approaches. This might include the creation of targeted therapies, either as standalone treatments or in combination with existing antiviral drugs. Investigating the interplay between oxidative stress markers and HBV infection holds the promise of enhancing the efficacy of therapeutic interventions and delivering substantial public health benefits, particularly in areas like Nigeria, where HBV is prevalent.
Materials and Methods
Study Design and Setting
This case-control study was conducted at the Department of Chemical Pathology, College of Medicine, University of Ibadan. Participants were recruited from the Gastroenterology Clinic of Medical Outpatients and the Blood Bank of the University College Hospital (UCH) in Ibadan, located in the Ibadan North Local Government Area. The majority of the local population is Yoruba, an ethnic group in Nigeria. The hospital serves as a major referral centre for residents and people from the south-western region of Nigeria.
Study Population and Sampling
The study included 40 HBV-infected patients as cases and 40 HBV-uninfected individuals as controls. Cases were adults clinically diagnosed with HBV infection. The control group comprised healthy adults from the blood donor population, negative for hepatitis B, C, and HIV. Eligibility criteria for cases included being an adult (18 years or older) with a diagnosis of chronic HBV infection (treatment-naive) and without major co-morbidities like HIV or chronic kidney disease, as these can affect oxidative stress markers and study outcomes. Controls were selected based on the absence of HBV, HCV, and HIV infections, no ongoing medication, and general good health. Participants were consecutively recruited during weekly clinic sessions, and controls were sourced from blood donors at the hospital’s blood bank.
Sample Size Determination
Based on Shimoda, Nagashima [13], who reported a mean level of 8-hydroxydeoxyguanosine in HBV infection as 3.22±0.94, we utilized a sample size formula for mean comparison in case-control studies. With an 80% power and 95% confidence level, assuming a difference of 0.22, we calculated a required sample size of at least 40 participants per group.
Data Collection and Laboratory Analyses
Demographic and anthropometric data were collected using a structured questionnaire. Participants’ weight and height were measured following standard methods [14]. Five millilitres of venous blood were collected into anticoagulant bottles for stability of peroxidation markers. Plasma obtained after centrifugation was aliquoted and stored at -20°C for subsequent analysis. Plasma levels of 8-hydroxydeoxyguanosine and 4-hydroxynonenal were measured using competitive ELISA kits (Elabscience®, 2021), while plasma selenium levels were determined using Atomic Absorbance Spectrophotometry [15]. Liver enzymes (AST, ALT and GGT), serum bilirubin, albumin and total protein were analysed using standard methods.
Data Analysis
Data were analysed using IBM SPSS Statistics for Windows Version 20.0 (IBM SPSS Inc., Chicago). Descriptive statistics were used to compare demographic and anthropometric characteristics between groups using a two-sample t-test. Pearson correlation analysis assessed relationships between variables, and the Mann-Whitney U test compared non-parametric data between groups. The significance level was set at p = 0.05.
Ethical Consideration
Participation in the study was voluntary and based on informed consent. Patient information collected through questionnaires was de-identified to ensure confidentiality. Ethical approval for the study was granted by the University of Ibadan/University College Hospital (UI/UCH) Joint Ethics Committee with approval number UI/EC/20/0300.
Results
Demographic and Anthropometric Characteristics of Participants
The study comprised 40 patients with HBV infection and 40 individuals without HBV. The participants consisted of 52 males, with 22 (42.3%) being HBV-infected and 30 (57.7%) HBV-uninfected, and 28 females, among whom 18 (64.3%) were HBV-infected and 10 (35.7%) HBV- uninfected (Table 1).
| Characteristics | HBV-infected | HBV-uninfected | p |
| (N = 40) | (N = 40) | ||
| Age (years) | 35.3±1.5 | 36.0±1.5 | 0.745 |
| Gender, n (%) | |||
| Male | 22 (55.0) | 30 (75.0) | |
| Female | 18 (45.0) | 10 (25.0) | 0.061 |
| Marital Status, n (%) | |||
| Single | 10 (25.0) | 12 (30.0) | |
| Married | 30 (75.0) | 28 (70.0) | 0.617 |
| Educational attainment, n (%) | |||
| Primary and lower | 10 (25.0) | 14 (35.0) | |
| Secondary and above | 30 (75.0) | 26 (65.0) | 0.329 |
| Occupation, n (%) | |||
| None/Retired | 6 (15.0) | 6 (15.0) | |
| Unskilled worker | 8 (20.0) | 7 (17.5) | |
| Skilled worker | 12 (30.0) | 15 (37.5) | |
| Professional | 14 (35.0) | 12 (30.0) | 0.907 |
| Ethnic Group, n (%) | |||
| Yoruba | 38 (95.0) | 35 (87.5) | 0.235 |
| Non-Yoruba | 2 (5.0) | 5 (12.5) | |
| Alcohol intake, n (%) | |||
| No | 30 (75.0) | 28 (70.0) | 0.567 |
| Yes | 10 (25.0) | 12 (30.0) | |
| Mean anthropometric indices | |||
| Weight (kg) | 70.8 ± 13.6 | 72.7 ± 12.9 | 0.746 |
| Height (m) | 1.7±0.09 | 1.7±0.1 | 0.928 |
| Body Mass Index (Kg/m 2 ) | 26.5 ±3.7 | 26.1±3.7 | 0.355 |
| Waist Circumference (cm) | 85.9±12.2 | 83.5±11.1 | 0.186 |
| Hip Circumference (cm) | 96.8±10.5 | 96.3±7.9 | 0.406 |
| Waist to Hip Ratio | 0.89±0.10 | 0.87±0.07 | 0.258 |
Results are expressed as a percentage of column total of each subsection. Significant differences between HBV-infected and uninfected groups were compared (p < 0.05). Abbreviations, HBV – Hepatitis B Virus
The mean ages of the HBV-infected group (35.3±1.5 years) and the HBV-uninfected group (36.0±1.5 years) did not show a statistically significant difference (p = 0.745). Additionally, the distribution of participants across various demographic characteristics such as educational attainment, occupation, ethnic group, and alcohol intake, displayed no significant variance between the HBV-infected and uninfected groups (Table 1). Furthermore, no statistically significant differences were observed in alcohol consumption, mean age, weight, height, body mass index, waist, and hip circumference between the two groups (Table 1).
Liver Function and Viral Load in HBV-infected Patients
The HBV-infected patients’ serum HBV DNA, bilirubin, and serum liver enzymes are summarized in Table 2.
| Median (range) | Mean ±SD | Ref. values | Elevated level | ||
| n | % | ||||
| Serum HBV DNA (IU/mL) | 21.0 (0-25492) | 1.92±0.3 | <2000 | 2 | 5 |
| Serum ALT (IU/L) | 24.0 (8-53) | 26.9±14.3 | 0.0-40.0 | 10 | 25 |
| Serum GGT (IU/L) | 28.9 (17-68) | 32.8±13.4 | 7.0-50.0 | 5 | 12.5 |
| Serum AST (IU/L) | 25.0 (16.9-40) | 25.0±5.4 | 0.0-37.0 | 2 | 5 |
| Total serum bilirubin (mg/dL) | 0.6 (0.2-1.5) | 0.6±0.3 | 0.2-1.0 | 7 | 17.5 |
| Conj. serum bilirubin (mg/dL) | 0.3 (0.1-0.6) | 0.3±0.2 | 0.0-0.4 | 7 | 17.5 |
| Total serum protein (g/dL) | 7.6 (0.4-8.6) | 7.2±1.5 | 6.0-8.0 | 4 | 12 |
| Serum albumin (g/dL) | 4.3 (3.5-5.3) | 4.4±0.3 | 3.0-5.0 | 5 | 5 |
Abbreviations, HBV-DNA; Hepatitis B virus, ALT; Alanine Amino Transferase, GGT; Gamma-glutamyl Transferase AST; Aspartate Transaminase Min, Minimum; Max, Maximum; SD, Standard Deviation
The HBV DNA levels in serum varied from 0 to 25,492 IU/mL, with a median of 21.0 IU/mL. Notably, only two patients (5.0%) exhibited elevated HBV DNA levels. The table also presents the median, mean, and count of patients with elevated levels of ALT, GTT, AST, bilirubin, protein, and albumin.
Levels of 4-Hydroxynonenal, 8-Hydroxydeoxyguanosine, and Selenium
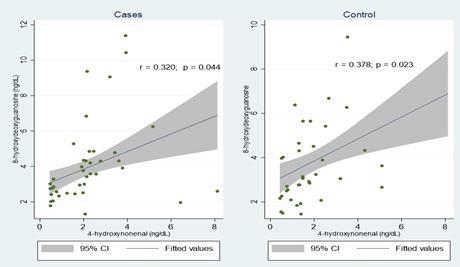
Comparative analysis revealed no significant difference between HBV-infected and uninfected groups in the mean levels of 4-HNE (2.2±1.6 ng/mL for infected vs. 1.8±1.3 ng/mL for uninfected) and 8-OHdG (4.1±2.4 ng/mL for infected vs. 3.6±1.8 ng/mL for uninfected). However, the plasma selenium levels were significantly higher in the HBV-infected group (78.4±25.4 μg/dL) compared to the HBV-uninfected group (35.6±11.4 μg/dL). There was a notable positive correlation between 8-OHdG and 4-HNE in both HBV-infected (r = 0.320; p = 0.044) and uninfected groups (r = 0.378; p = 0.023). A similar positive correlation was found between 8-OHdG and selenium in the HBV- uninfected group (r = 0.388; p = 0.019), but this correlation was absent in the HBV-infected group. Conversely, a significant negative correlation was observed between 8-OHdG and weight in the HBV-uninfected group (r = -0.341; p = 0.041) (Table 3).
| Study parameters | HBV-infected | HBV-uninfected | ||
| r | p | r | p | |
| 4-hydroxynonenal | 0.32 | 0.044 | 0.378 | 0.023 |
| Selenium | -0.026 | 0.872 | 0.388 | 0.019 |
| Weight | -0.154 | 0.342 | -0.341 | 0.041 |
| Height | 0.01 | 0.95 | -0.327 | 0.051 |
| Waist circumference | -0.233 | 0.146 | -0.121 | 0.481 |
| Hip circumference | -0.121 | 0.458 | -0.161 | 0.348 |
| Body mass index | -0.15 | 0.354 | -0.191 | 0.265 |
| Waist-to-hip ratio | -0.195 | 0.226 | -0.034 | 0.843 |
Results are correlation coefficient (r) and corresponding p values. Significant correlations were p < 0.05. Abbreviations: HBV – Hepatitis B Virus
The graphical representation of these correlations, including lines of best-fit and 95% confidence intervals, is illustrated in Figure 1.
Figure 1. Scatter Plots Showing the Correlation between 8-hydroxydeoxyguanosine and 4-hydroxynonenal in Cases and Controls.
Discussion
Hepatitis B virus (HBV) infection continues to pose a significant public health challenge, particularly in developing regions like Nigeria. This study investigated the plasma levels of oxidative stress markers (8-hydroxydeoxyguanosine [8-OHdG] and 4-hydroxynonenal [4-HNE]) and selenium in Nigerian adults diagnosed with HBV. Contrary to initial hypotheses, our findings revealed no significant difference in the levels of 8-OHdG and 4-HNE between the HBV-infected and uninfected groups. However, we noted a significant elevation in selenium levels among the HBV-infected participants, offering important biochemical insights for HBV management strategies.
The comparable levels of 8-OHdG and 4-HNE in both groups challenge the prevailing notion that HBV infection exacerbates oxidative DNA damage or lipid peroxidation, as suggested by existing literature [7, 16]. The increased selenium levels in HBV-infected subjects may indicate an adaptive mechanism to counterbalance oxidative stress, aligning with selenium’s recognized antioxidative properties [8]. This rise in selenium levels, especially in the HBV-infected group, resonates with prior research indicating an inverse relationship between serum selenium levels and HBV infection [11]. The lack of disparity in oxidative stress markers could reflect a homeostatic response, potentially facilitated by the upregulation of selenium, a crucial element in the antioxidant enzyme GPx. These findings suggest a complex interplay between HBV infection and oxidative stress pathways.
The composition of our study participants, encompassing both HBV-infected and uninfected individuals, enhances the reliability of our findings. The consistency in demographic variables such as age and lifestyle factors, including alcohol consumption, minimizes potential confounding biases, allowing for a more precise evaluation of the primary biomarkers. While other studies have reported heightened oxidative stress in HBV-infected individuals, the lack of significant differences in our study might be attributed to our limited sample size and the unique demographic and environmental characteristics of our study population. For instance, Ha, Shin [16] discussed the association of HBV infection with oxidative stress, leading to cellular damage in lipids, proteins, and DNA [16]. Additionally, Loureiro, Tout [17] explored the impact of HBV on mitochondrial oxidative stress and DNA damage in the context of advanced fibrosis or cirrhosis [17]. These studies highlight the intricate relationship between HBV infection and oxidative stress pathways.
The increase in selenium levels within the HBV-infected group supports the hypothesis of selenium’s protective role against HBV-associated oxidative stress [11]. This suggests the potential applicability of selenium in HBV therapeutic strategies, especially in regions with selenium deficiency. Theoretically, our study adds to the understanding of the intricate relationship between HBV infection and oxidative stress mechanisms. Clinically, the elevated selenium levels in HBV-infected patients highlight the possibility of incorporating selenium supplementation into HBV treatment protocols. Additionally, our findings corroborate the existing literature on the correlation between oxidative DNA damage and lipid peroxidation in HBV-related liver disease [18, 19].
Acknowledging the limitations of our study, including its cross-sectional design and relatively small sample size, we note that these factors may limit the generalizability of our findings and the ability to draw causal inferences. Additionally, the study did not account for potential confounding factors like antiviral medication usage and variations in HBV DNA levels among infected participants.
In summary, this research offers a comprehensive examination of the relationship between HBV infection and oxidative stress markers in adults in Ibadan, Nigeria. While no significant differences were observed in the levels of 4-HNE and 8-OHdG, the elevated selenium levels in HBV-infected individuals open new avenues for investigating metabolic and molecular pathways in HBV infection. Future research should focus on longitudinal studies to monitor oxidative stress markers throughout the course of HBV infection and assess the therapeutic potential of selenium supplementation in conjunction with antiviral therapy for HBV management.
Acknowledgements
We express our sincere gratitude to all the participants who willingly took part in this study, contributing significantly to the advancement of scientific knowledge in this area.
Conflicts of interest
No conflict of interest is declared.
Authors Contribution
BEO conceived and designed the study; acquired, analysed, and interpreted the data; drafted the manuscript; approved the final version to be published. UOD Assisted in the study design; conducted the data collection; contributed to data analysis and interpretation; revised the manuscript critically for important intellectual content; approved the final version to be published. KOA managed the study participants and provided material support; contributed to the analysis and interpretation of data; participated in drafting the manuscript; approved the final version to be published. JIA contributed to the conception and design of the work; supervised the research project; revised the manuscript critically; gave final approval of the version to be published.
Data Availability
Not applicable
Study Registration
Not applicable
References
- Epidemiology of hepatitis B in Europe and worldwide Alter MJ . Journal of hepatology.2003;39 Suppl 1. CrossRef
- Hepatitis B infection in sub-Saharan Africa. The African Regional Study Group Kiire C. F.. Vaccine.1990;8 Suppl. CrossRef
- Seroprevalence of Hepatitis B Infection in Nigeria: A National Survey Olayinka AT , Oyemakinde A, Balogun MS , Ajudua A, Nguku P, Aderinola M, Egwuenu-Oladejo A, et al . The American Journal of Tropical Medicine and Hygiene.2016;95(4). CrossRef
- Statins and metformin for chemoprevention of hepatocellular carcinoma Choi J, Roberts LR . Clinical Liver Disease.2016;8(2). CrossRef
- Reactive oxygen species promote heat shock protein 90-mediated HBV capsid assembly Kim YS , Seo HW , Jung G. Biochemical and Biophysical Research Communications.2015;457(3). CrossRef
- Effect of Hepatitis Viruses on the Nrf2/Keap1-Signaling Pathway and Its Impact on Viral Replication and Pathogenesis Bender D, Hildt E. International journal of molecular sciences.2019;20(18). CrossRef
- Reactive oxygen species modulates the intracellular level of HBx viral oncoprotein Wang J, Yun C, Kim S, Lee J, Yoon G, Lee M, Cho H. Biochemical and Biophysical Research Communications.2003;310(1). CrossRef
- Selenoproteins: Antioxidant selenoenzymes and beyond Steinbrenner H, Speckmann B, Klotz L. Archives of Biochemistry and Biophysics.2016;595. CrossRef
- Selenium alleviates chromium toxicity by preventing oxidative stress in cabbage (Brassica campestris L. ssp. Pekinensis) leaves Qing X, Zhao X, Hu C, Wang P, Zhang Y, Zhang X, Wang P, Shi H, Jia F, Qu C. Ecotoxicology and Environmental Safety.2015;114. CrossRef
- The Functions of Selenium and Selenoproteins Relating to the Liver Diseases Shang N, Wang X, Shu Q, Wang H, Zhao L. Journal of Nanoscience and Nanotechnology.2019;19(4). CrossRef
- Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection Yu M. W., Horng I. S., Hsu K. H., Chiang Y. C., Liaw Y. F., Chen C. J.. American Journal of Epidemiology.1999;150(4). CrossRef
- Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania Kupka R, Msamanga GI , Spiegelman D, Morris S, Mugusi F, Hunter DJ , Fawzi WW . The Journal of Nutrition.2004;134(10). CrossRef
- Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis Shimoda R., Nagashima M., Sakamoto M., Yamaguchi N., Hirohashi S., Yokota J., Kasai H.. Cancer Research.1994;54(12). CrossRef
- Anthropometric Measurement. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: John Kiel declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023 Casadei K , Kiel J . .
- Plasma selenium levels of the general population of an area in northern Italy Sesana G., Baj A., Toffoletto F., Sega R., Ghezzi L.. The Science of the Total Environment.1992;120(1-2). CrossRef
- Oxidative stress and antioxidants in hepatic pathogenesis Ha H, Shin H, Feitelson MA , Yu D. World Journal of Gastroenterology.2010;16(48). CrossRef
- Mitochondrial stress in advanced fibrosis and cirrhosis associated with chronic hepatitis B, chronic hepatitis C, or nonalcoholic steatohepatitis Loureiro D, Tout I, Narguet S, Bed CM , Roinard M, Sleiman A, Boyer N, et al . Hepatology (Baltimore, Md.).2023;77(4). CrossRef
- Dietary iron overload induces visceral adipose tissue insulin resistance Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, Girelli D, et al . The American Journal of Pathology.2013;182(6). CrossRef
- [Fulminant hepatitis B] Dokić M, Begović V, Rajić-Dimitrijević R, Aleksić R, Popović S, Hristović D. Vojnosanitetski Pregled.2003;60(3). CrossRef
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times