The Association between PM2.5 Exposure and Hippocampal Volume: A Systematic Review
Download
Abstract
Background: Existing air quality is decreasing, as evidenced by the increase in air pollution. Air pollution does not only affect the respiratory system, but also affecting the nervous system, and furthermore causing impaired cognitive function that can be predicted through the image of the hippocampus.
Objective: This study wanted to determine the significance of the relationship between PM2.5 (Particulate Matter) pollutant exposure and hippocampal volume in adults.
Method: This research is a PRISMA 2020 based systematic study using Google Scholar, PubMed, and Proquest as databases. Research inclusion criteria were studies with subjects over 19 years old, using MRI techniques, published in English, having sufficient data for extraction.
Result: There are 5 studies from 2015 to 2020 which stated that there was no statistically significant relationship between PM2.5 pollutant exposure and hippocampal volume (n = 5) (P-value > 0.05, 0.71, 0.8, 0.32), and the study obtained significant results (n = 1) (P-value < 0.005). Discussion: Although the results of the study did not prove a significant difference in hippocampal volume, several recent theories regarding hippocampal neurogenesis in adults are able to support these results.
Conclusion: From this study, it was not proven that there was a significant relationship between PM2.5 pollutant exposure and hippocampal volume.
Introduction
According to IQAir website, there was an increase in the concentration of particulate matter (PM2.5) by more than 50% in 2018 [1]. Chronic exposure to air pollution causes multisystem disturbances [2]. Regardless of the known etiology, several studies suggest that exposure to air pollution can have a negative impact on the environment, adverse effects on the human brain, including cognitive function [2,3]. Several previous studies have strongly associated PM2.5 with decreased total cerebellar volume (TCBV), but few results have been obtained regarding PM2.5 with hippocampal volume. From the literature search, we get different results statistically, therefore it is necessary to carry out a systematic review to this topic.
Particulate Matter (PM) which categorized as ambient (outdoor) pollutants is divided into 3 types based on its size: coarse (PM10), fine (PM2.5), and ultrafine (PM0.1). Produced from coal burning, power plant waste, motor vehicle fumes, and forest fires [4]. Based on the literature search of this study, neuronal damage due to PM exposure can occur through two pathways: mediated by hypoxia and mediated by blood brain barrier damage. Theoretically, hypoxia will cause hippocampal atrophy.
From the results of research on hypoxia in neonates, it is proven that hypoxia, hippocampal atrophy, and memory impairment are one-way events that occur sequentially through activation of HIF-1α (hypoxia inducible factor 1 alpha), degradation of MAP-2 protein, formation of oxidative stress, and neuronal apoptosis [4-11]. In another study, it was explained that PM exposure caused several changes in the hippocampus of mice, such as impaired methionine-glutathione metabolism which indicated an imbalance of glutathione redox, which was characterized by the formation of oxidative stress, then hippocampal neuroinflammation, and an increase in Aβ levels [12].
Methods
This study is previously registered in PROSPERO with registration ID CRD42021264296. Primary studies used obtained through a literature search using Google Scholar, PubMed, and ProQuest databases. The keywords searched through Mesh-Term are “air pollution”, “hippocampal volume”, “magnetic resonance imaging”, and “adult” entered into the 3 databases used. We used advanced search and limitations for English and Indonesian studies, free full text, and publications in the previous 5-10 years. We selected studies based on PRISMA 2020 guidelines and pre-defined inclusion criteria, in the form of studies with subjects over 19 years of age, using MRI techniques, published in English, and sufficient data for extraction. Study screening was carried out based on the title, abstract, and full text review. To minimize bias, we used two valid instruments, CASP and AXIS based on the research method used in each study. The studies that qualified the inclusion criteria are then carried out with data extraction: population characteristics, pollutant measurement and analysis methods, as well as imaging methods and hippocampus image analysis.
Results
From the results of a literature search using the PRISMA 2020 method (Figure 1), studies using cross-sectional method (n = 3) and longitudinal cohort (n = 3) were obtained.
Figure 1. Literature Search Result Using PRISMA Flowchart 2020.
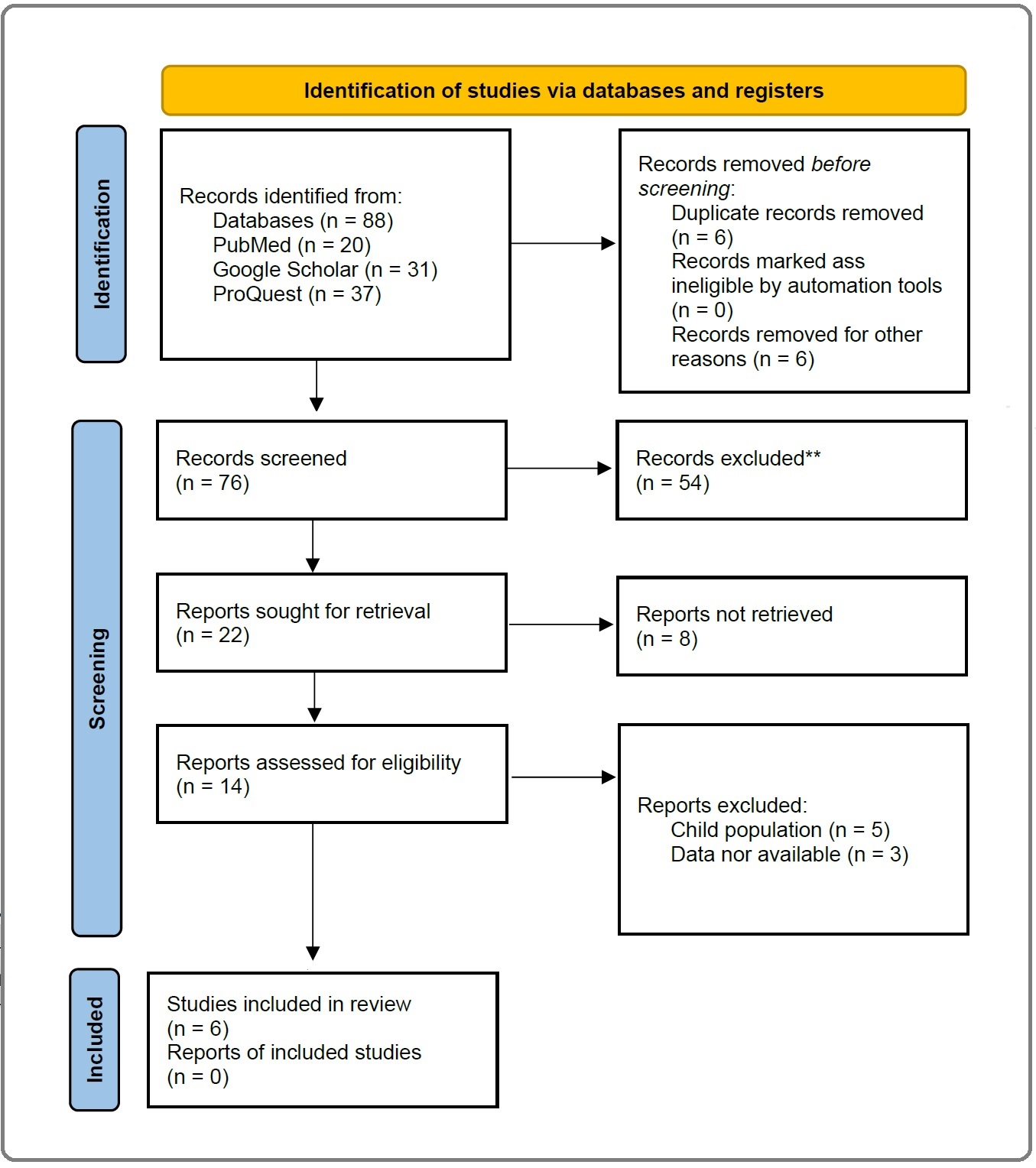
Characteristics of the studies we found are study subjects aged ≥60 years (n = 6), excluding subjects with history of neurological disease (n = 4). Measurement of pollutant concentration by BME (n = 2), spectroradiometer (n = 1), spatiotemporal statistical model (n = 1), and national prediction model (n = 1). Imaging of the hippocampus with T1-weighted MRI (n = 1), T2-weighted MRI (n = 2), and T1-weighted MPRAGE MRI (n = 3). The data obtained were then processed statistically to determine the association of the two variables. Spearman test (n = 2) and cross-validation test (n = 3) are used to determine the validity of the pollutant concentration data, while the statistical tests used to assess the correlation between PM2.5 exposure and a decrease in hippocampal volume include linear regression, logistic regression test, and chi-square test (Table 1).
| Study | Age of Subject | Inclusion Criteria | Pollutant Concentration Measurement | Hippocampal Imaging | P-value | ||
| Method | Statistical Analysis | Method | Statistical Analysis | ||||
| Chen et al [2015][13] | 65-80 | No history of dementia | BME | r = 0,90 | T1-weighted volumetric MRI scans for 7-10 years follow up | Automatic computer- based template warping | 0,8 |
| Wilker et al [2015][14] | ≥60 | No history of dementia, stroke. Attended 7 examinations. | Spectroradiometer-meter | r = -0,15 | MRI T2-weighted double spin-echo | Manual delineation | <0,005 |
| Casanova et al [2016][15] | 65-80 | Not mentioned | BME | R 2 = 0,74 | MRI T2-weighted spin echo | Regional Analysis of Volumes Examined in Normalized Space (RAVENS) | >0,05 |
| Power et al [2018][16] | Mean = 76±5 | No history of head surgery or radiation therapy, multiple sclerosis, brain tumor, and attended 5 examinations. | Spatiotemporal statistical model | R 2 = 0,50 - 0,83 | MRI T1-weighted 3D volumetric magnetization-prepared gradient echo (MPRAGE) | Semi-automatic software Freesurfer | 0,71 |
| Cho et al [2020][17] | Mean = 67,3 | No history of neurological disease such as dementia and has had imaging examination. | National prediction model | R 2 = 0,45 | MRI T1-weighted 3D MPRAGE | Semi-automatic software Freesurfer | 0,32 |
| Hedges et al [2020][18] | Mean = 62,15 | Not mentioned | Data collected from UK Biobank | MRI T1-weighted 3D MPRAGE | Automatic device | >0,05 |
Note, BME (Bayesian Maximum Entropy)
Not all studies used the same variable measurement method, and there are studies that used manual delineation method to analyze the results of MRI images (n = 1). Since the studies are not homogeneous enough, meta-analysis cannot be carried out. The results of the studies that have been qualified are statistically significant (n = 1) and not significant (n = 5).
Discussion
Based on the systematic study conducted, it was stated that there was no statistically significant relationship between PM2.5 exposure and hippocampal volume as measured by MRI results (n = 5). Mean while, another study stated that there was a significant relationship with p-value <0.05 (n = 1). Based on the theory that has been reviewed, exposure to pollutants can induce acute hypoxia, but there is still an unresolved controversy regarding the effects of acute hypoxia on hippocampal neuron cells. This theory is opposed by a study [19] which states that acute hypoxia can support hippocampal neurogenesis in adults [19] (Figure 2).
Figure 2. Adult Hippocampal Neurogenesis.
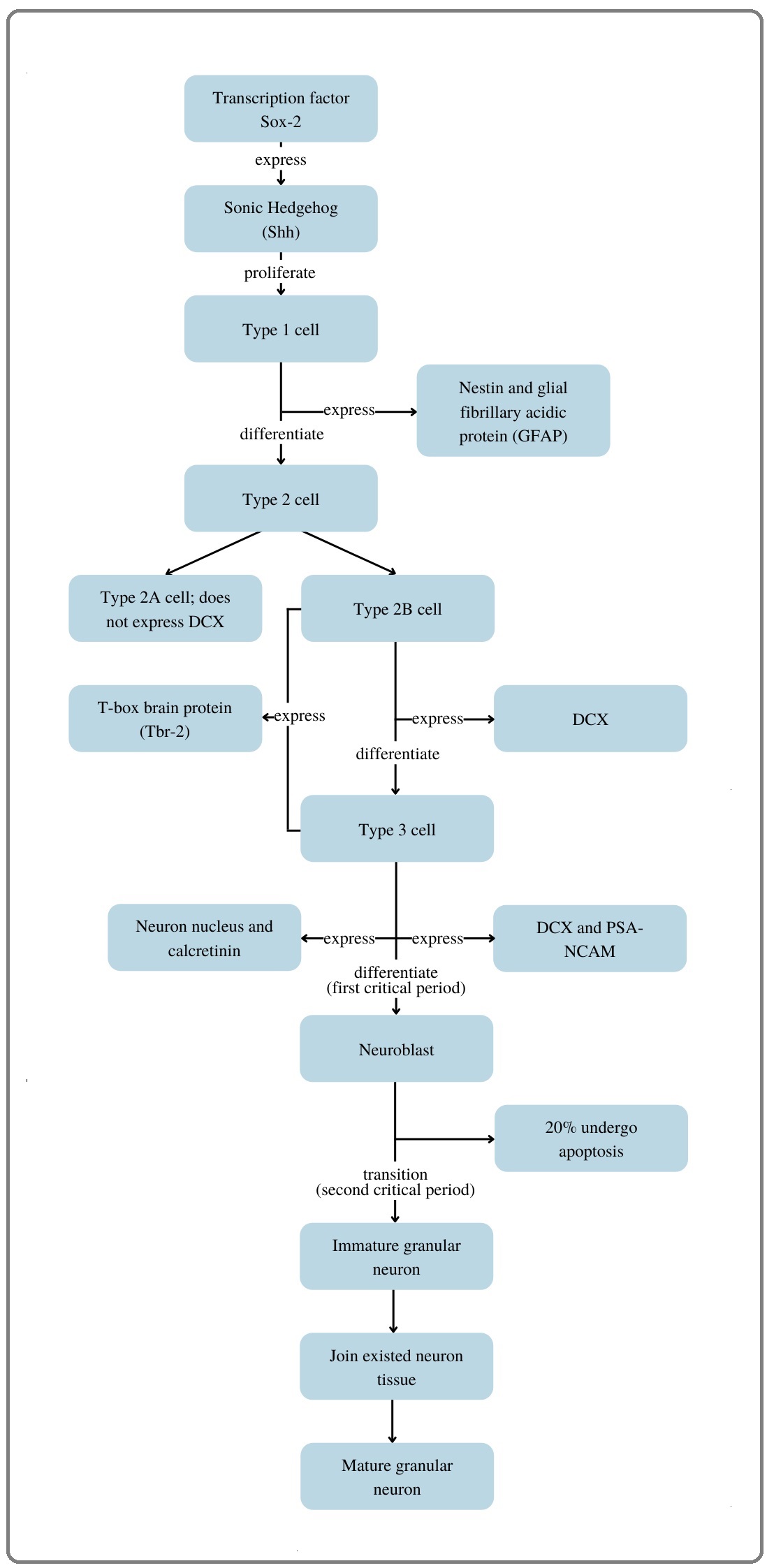
The process of hippocampal neurogenesis is regulated by the transcription factor Sox 2, expressing Sonic hedgehog (Shh) which then promotes type 1 cell proliferation. Hippocampal neurogenesis has 4 phases, namely: precursor cells, early survival phase, post-mitotic phase, and late survival phase [20]. Precursor cells are located in the sub granular zone of the hippocampus and are divided into two, neural stem cells (NSC) which consists of type 1 and type 2A cells, and intermediate neuronal progenitor (INP) which consists of type 2B and type 3 cells [21]. Precursor cells lasted for three days, during which asymmetric division of type 1 cells, which were glial cells with triangular bodies, functioned for the expression of nestin and glial fibrillary acidic protein (GFAP). Type 1 cell division will produce type 2 cells, which are also called transient cells that have the ability to migrate and proliferate, which can be further identified by the presence or absence of the expression of doublecortin (DCX). Type 2A cells do not express DCX (DCX negative), whereas type 2B cells can express DCX, which then differentiate into type 3 cells. Type 3 cells do not express nestin, however it expresses DCX and polysialylated neuronal cell adhesion molecule (PSA-NCAM). The INPs consisting of cells type 2B and 3 together then express the transcription factor T-box brain protein (Tbr2). The newly formed cells then enter the post-mitotic phase, this is characterized by the expression of the neuron nucleus (NeuN) and calretinin. Furthermore, the number of neuroblasts that have been formed will decrease due to the apoptotic process that occurs, and only 20% of neuroblasts will join the existing network of neurons [20]. There are two critical periods: the first and second critical periods. The first critical period is the transition period for Tbr2 until there is an increase in DCX expression. Neuroblasts that have been expressed will then enter the second critical period, transitioning into immature neuron granules, merging into the existing granule layer network, finally developing into mature neuron granules [19]. In addition, an experimental study in animals also found that acute hypoxia that occurs during the first critical period can increase the number of new neurons (birth-dated neurons) through HIF-1 activation which will then help neuron survival by initiating neuroprogenitor production. Further detailed mechanism of the pathway for neuroprogenitor initiation by HIF-1 is still unknown. Further research is needed [19]. By acknowledging this theory, the results of the five studies that were not significant could be explained. In conclusion, although PM2.5 is considered harmful, exposure to PM2.5 is not significantly associated with changes in hippocampal volume in adults. Further research should be focused on systematic review update and meta-analysis of smaller types of pollutants such as PM0.1.
Acknowledgements
Conflict of Interest
The author has no conflict of interest to declare.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author Contributions
The study conception and design were carried out by Yopi Simargi. Data collection, analysis, and interpretation, and first draft of the manuscript were performed and written by Elizabeth Feloni Lukito. Kevin Tandarto, Maureen Miracle Stella, Ignatius Ivan, Harvey Sudharta, Gilbert Golahi, Yuda Turana, Bryany Titi Santi, and Yanto Budiman commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- IQ-air. Jakarta Air Quality Index (AQI) and Indonesia Air Pollution | AirVisual [Internet]. 2020 Available from: https://www.iqair.com/us/indonesia/jakarta..
- Environmental and Health Impacts of Air Pollution: A Review Manisalidis Ioannis, Stavropoulou Elisavet, Stavropoulos Agathangelos, Bezirtzoglou Eugenia. Frontiers in Public Health.2020;8. CrossRef
- The role of air pollution in cognitive impairment and decline Schikowski Tamara, Altuğ Hicran. Neurochemistry International.2020;136. CrossRef
- Pathophysiological effects of particulate matter air pollution on the central nervous system Wright Joshua C., Ding Yuchuan. Environmental Disease.2016;1(3). CrossRef
- Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus Gao Huabin, Han Zhaoli, Huang Shan, Bai Ruojing, Ge Xintong, Chen Fanglian, Lei Ping. Behavioural Brain Research.2017;335. CrossRef
- Short-term effects of air pollution on oxygen saturation in a cohort of senior adults in Steubenville, Ohio Luttmann-Gibson Heike, Sarnat Stefanie Ebelt, Suh Helen H., Coull Brent A., Schwartz Joel, Zanobetti Antonella, Gold Diane R.. Journal of Occupational and Environmental Medicine.2014;56(2). CrossRef
- Air pollution and its effects on the immune system Glencross Drew A., Ho Tzer-Ren, Camiña Nuria, Hawrylowicz Catherine M., Pfeffer Paul E.. Free Radical Biology & Medicine.2020;151. CrossRef
- Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury Khan Mushfiquddin, Khan Hamza, Singh Inderjit, Singh Avtar K.. Neural Regeneration Research.2017;12(5). CrossRef
- Autophagy and the integrated stress response Kroemer Guido, Mariño Guillermo, Levine Beth. Molecular cell.2010;40(2). CrossRef
- Environmental exposures and mechanisms in allergy and asthma development Murrison Liza Bronner, Brandt Eric B., Myers Jocelyn Biagini, Hershey Gurjit K. Khurana. The Journal of Clinical Investigation.2019;129(4). CrossRef
- Effect of different levels of intermittent hypoxia on autophagy of hippocampal neurons Song Shuling, Tan Jin, Miao Yuyang, Zhang Qiang. Sleep & Breathing = Schlaf & Atmung.2017;21(3). CrossRef
- Exposure of ultrafine particulate matter causes glutathione redox imbalance in the hippocampus: A neurometabolic susceptibility to Alzheimer's pathology Park Soo Jin, Lee Jimin, Lee Seunghoon, Lim Sangchul, Noh Juhwan, Cho So Yeon, Ha Junghee, Kim Hyunjeong, Kim Changsoo, Park Sunho, Lee Do Yup, Kim Eosu. Science of The Total Environment.2020;718. CrossRef
- Ambient air pollution and neurotoxicity on brain structure: Evidence from women's health initiative memory study Chen Jiu-Chiuan, Wang Xinhui, Wellenius Gregory A., Serre Marc L., Driscoll Ira, Casanova Ramon, McArdle John J., Manson JoAnn E., Chui Helena C., Espeland Mark A.. Annals of Neurology.2015;78(3). CrossRef
- Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure Wilker Elissa H., Preis Sarah R., Beiser Alexa S., Wolf Philip A., Au Rhoda, Kloog Itai, Li Wenyuan, Schwartz Joel, Koutrakis Petros, DeCarli Charles, Seshadri Sudha, Mittleman Murray A.. Stroke; a journal of cerebral circulation.2015;46(5). CrossRef
- A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women Casanova Ramon, Wang Xinhui, Reyes Jeanette, Akita Yasuyuki, Serre Marc L., Vizuete William, Chui Helena C., Driscoll Ira, Resnick Susan M., Espeland Mark A., Chen Jiu-Chiuan. Frontiers in Human Neuroscience.2016;10. CrossRef
- The Association of Long-Term Exposure to Particulate Matter Air Pollution with Brain MRI Findings: The ARIC Study Power Melinda C., Lamichhane Archana P., Liao Duanping, Xu Xiaohui, Jack Clifford R., Gottesman Rebecca F., Mosley Thomas, Stewart James D., Yanosky Jeff D., Whitsel Eric A.. Environmental Health Perspectives.2018;126(2). CrossRef
- Long-Term Ambient Air Pollution Exposures and Brain Imaging Markers in Korean Adults: The Environmental Pollution-Induced Neurological EFfects (EPINEF) Study Cho Jaelim, Noh Young, Kim Sun Young, Sohn Jungwoo, Noh Juhwan, Kim Woojin, Cho Seong-Kyung, Seo Hwasun, Seo Gayoung, Lee Seung-Koo, Seo Seongho, Koh Sang-Baek, Oh Sung Soo, Kim Hee Jin, Seo Sang Won, Shin Dae-Seock, Kim Nakyoung, Kim Ho Hyun, Lee Jung Il, Kim Changsoo. Environmental Health Perspectives.2020;128(11). CrossRef
- Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank Hedges Dawson W., Erickson Lance D., Kunzelman Jackie, Brown Bruce L., Gale Shawn D.. Neurotoxicology.2019;74. CrossRef
- Stage-dependent effects of intermittent hypoxia influence the outcome of hippocampal adult neurogenesis Khuu Maggie A., Nallamothu Thara, Castro-Rivera Carolina I., Arias-Cavieres Alejandra, Szujewski Caroline C., Garcia Iii Alfredo J.. Scientific Reports.2021;11(1). CrossRef
- Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain Jurkowski Michal P., Bettio Luis, K Woo Emma, Patten Anna, Yau Suk-Yu, Gil-Mohapel Joana. Frontiers in Cellular Neuroscience.2020;14. CrossRef
- Tbr2 Is Essential for Hippocampal Lineage Progression from Neural Stem Cells to Intermediate Progenitors and Neurons Hodge Rebecca D., Nelson Branden R., Kahoud Robert J., Yang Roderick, Mussar Kristin E., Reiner Steven L., Hevner Robert F.. The Journal of Neuroscience.2012;32(18). CrossRef
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times