Breast Cancer Treatment and Outcomes in Nigeria: A Systematic Review and Meta-analysis
Download
Abstract
Background and Objective: Nigeria has one of the highest age-standardized breast cancer (BC) mortality rates globally and the highest in Africa. Late presentation and diagnosis have been extensively studied as causes of high BC morbidity and mortality, while treatment and outcomes are underreported. We aggregated data on BC treatment and outcomes in Nigeria to identify gaps in research, challenges, and potential targets for future interventions.
Materials and Methods: We reviewed articles on female BC management in Nigeria published between 2011 and 2021 to determine the prevalence of different treatment modalities and outcomes. The meta-analytical procedure followed a random effect model.
Results: We identified 15 articles reporting on the treatment and outcomes of 3,857 BC patients. The most prevalent treatment modality was chemotherapy alone. The probability of receiving each treatment modality was 85% (95% CI 66-97) for chemotherapy, 62% (95% CI 51-73) for surgery, and 31% (95% CI 8-59) for radiotherapy. Multimodality treatment, including chemotherapy, surgery, and radiation, was administered to 24% (95% CI 10-43) of patients. In studies with available data, nearly half of patients who initiated chemotherapy did not complete the recommended number of doses or received treatments at irregular intervals. The utilization of radiotherapy was five times higher when patients received treatment in centers with radiation facilities. Overall survival estimates were 80% at one year, 43% at two years, and 32% at five years. Patients with early-stage (AJCC I/II) disease survived longer, with a 5-year survival difference of 32% compared to patients with late-stage (AJCC III/IV) disease. Patients receiving multimodality therapy also had longer survival. The three-year survival for patients who received chemotherapy, surgery, and radiotherapy was 68%, whereas it was 43% for patients who received chemotherapy and surgery only.
Conclusion: Improving access to complete systemic therapy, surgery, and radiation for breast cancer patients in Nigeria is imperative and should be the target of future interventions.
Introduction
The burden of breast cancer (BC) is rising in Nigeria. The International Agency Research on Cancer (IARC) recorded 28,380 new BC cases in Nigeria in 2020, representing 22.7% of new cancers and accounting for the highest proportion of all cancer types [1]. According to a 2013 study [2], BC accounts for 45.5% and 38.2% of cancers among women younger than 45 years and women aged 45 years or older, respectively, in the Ibadan population-based registry, and 55.6% and 45.7% among women younger than 45 years and women aged 45 years and older in the Abuja population-based cancer registry. The same study reported a four-fold increase in the age-standardized rate from 13.7 per 100 000 in 1998-1999 to 54.3 per 100 000 in 2009-2010.
Nigeria has one of the world’s highest age-standardized mortality rates of BC and the highest in Africa [3]. GLOBOCAN 2020 reported BC as the most common cause of cancer-related death in Nigeria, accounting for 14,274 (18.1%) of all cancer deaths [4]. A recent sub-Saharan African (SSA) multinational study by McCormack et al. found that BC patients in Nigeria had the lowest three-year survival rate of six countries evaluated: 36% in Nigeria, compared to 44% in Uganda, 47% in Zambia, 56% for Black women in Namibia and 59% for Black women in South Africa [5].
Prior research has identified delayed presentation, late-stage diagnosis, and inadequate treatment as challenges linked to poor BC outcomes in Nigeria and most SSA settings. Many reports have focused on patient-related and health system challenges from symptom development through diagnosis. In contrast, treatment, which is also a critical determinant of BC outcomes, has been under-reported. McCormack et al. [5] predicted a remarkable absolute survival gain of up to 12% by improving BC treatment alone, similar to the expected survival gain by early diagnosis.
This review aimed to aggregate data on treatment (surgery, chemotherapy, radiotherapy, hormonal and targeted therapy) and outcomes of BC in Nigeria to identify gaps in research, challenges, and potential targets for future intervention.
Methods
This review was conducted according to the Preferred Reported Items for Systematic Reviews and Meta-analysis (PRISMA) recommendations. The needs assessment and preliminary literature review [in PubMed, African Journal Online (AJOL), Cochrane library, and Prospero [ID CRD42021257958] confirmed no similar meta-analysis was ongoing or previously conducted. The primary literature search used the criteria “Management or Outcome AND Breast Cancer AND Nigeria” in PubMed. gov, with extensive snow-balling search in Pubmed Central, Google, Google Scholar, Researchgate, and AJOL. The article search opened on May 5, 2021, and closed on May 26, 2021.
Article screening and data extraction
The full-text screening was based on the predetermined Population, Intervention, Control, Outcome, Time, Study design (PICOTS) criteria in Table 1.
| Participants/Population | Freely available articles found on electronic search or request to corresponding authors, reporting on female breast cancer treatment outcomes in Nigeria. The article must report on the total number of female breast cancer patients; the male representation must be less than 5%. Articles reporting on a subpopulation of breast cancer such as recurrence, young females, rare cases, metastatic, or early BC alone were excluded. We included articles reporting on LABC alone and premenopausal women since they constitute Nigeria's largest proportion of presentations. |
| Multinational studies where Nigerian data could be extracted were included. | |
| Intervention | Not applicable |
| Control | Not applicable |
| Outcomes | The outcomes of interest were the distribution of treatment modalities (surgery, chemotherapy, radiotherapy, hormonal and targeted therapy, the pattern of response to chemotherapy in LABC, pattern of 1, 2, 3, and 5-year survival, and pattern of disease recurrence or progression |
| Time | We included articles published between January 2011 and May 2021. Articles published in the specified period but including data earlier than January 2000 were excluded. |
| Study design | The study design was not a strict exclusion criterion provided there was extractable data on treatment and or outcome variables of interest. The language was also not an exclusion criterion. We included only original articles with an extractable sample size of 10 subjects treated or followed for at least one of our outcomes of interest. |
Author AO performed the article title and abstract screening while AO and AI performed the full article review and data extraction independently using specially designed forms. The authors discussed resolving any disagreement.
Quality assessment
The Newcastle-Ottawa, quality assessment tool, was modified (supplementary file) for the quality assessment. The quality score did not influence the meta-analytical weighing.
Statistical analysis
The outcomes were summary estimates of each variable defined in our PICOTS criteria. We conducted the meta-analytical procedure in MetaXL (www.epigear. com) add-in for Microsoft Excel. A random-effect model was implemented to obtain summary estimates using the double arcsine transformation to avoid overweighting studies with values close to 0 or 100%. I-squared (I2) values above 75% indicated high heterogeneity. We conducted subgroup analysis based on treatment types or combinations and treatment availability.
We analyzed the variables as proportions of the total in each publication (n/N). To assess each treatment modality’s overall prevalence, we pooled all treatments ‘N’ and found the proportion accounted for by each treatment mortality ‘n.’ The denominator (N) was adjusted for loss to follow up before analysis.
We performed the meta-analytical procedure on the outcome variables contributed by at least two studies. Otherwise, we narrated the reported prevalence or proportions. Using only articles with nonpurposive sampling, we found the probability of receiving each treatment modality by finding the summary estimate of the proportion in each article, where ‘N’ is the number eligible for the treatment modality in the article, and ‘n’ is the number receiving the treatment modality.
Results
Fifteen articles met the eligibility criteria (Figure 1).
Figure 1. PRISMA Flow Chart, Showing the Article Selection Process.
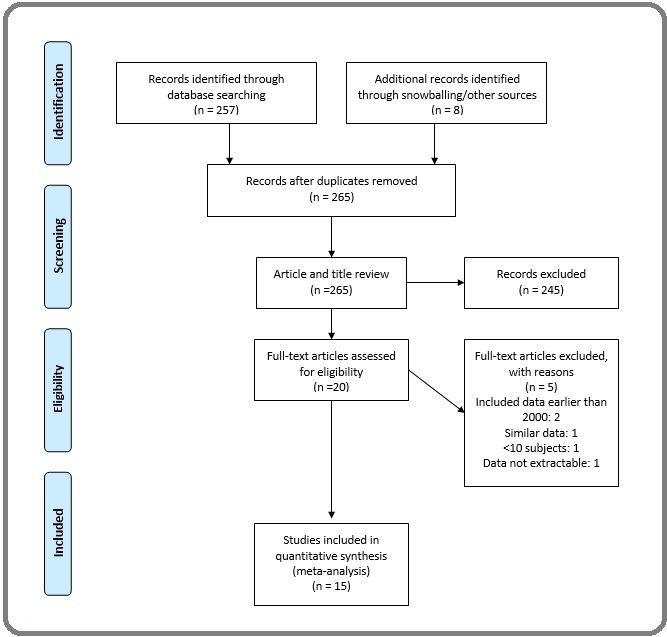
The full electronic search found 257 articles; 11 of the 257 were eligible for full article review following title and abstract screening. Four were excluded after the full review— one used similar data as an already included article [6], two included data earlier than 2000 [7, 8], and data were not extractable in one [9]. An extensive snow-balling search found eight more articles [10-17]. One of the 15 eligible articles was multinational research [5]. Attempts to clarify incomplete or obscured data on overall survival by stage in three eligible articles [5,18,19] via email communications with authors were successful in two [18, 19].
As shown in Table 2, the data were from three [Southwest-9, Northcentral-4, and Southeast-2] of the country’s six geopolitical zones, with the Southwest contributing 60%. The total number of subjects was 3857. The minimum number of subjects in a study was 44 [20], and the maximum was 1141 [21].
| First author | Period | Publication year | Sample | Center | Age (Range/Mean/ Median) | Design | Diagnostic Criteria | Staging Modality | Population |
| Adejumo (26) | 2013-2018 | 2019 | 199 | Federal Medical Center, Keffi/ NC | 20-60/N/N | Retro | Histo/IHC | N | All patients |
| Adeniji (13) | 2003-2007 | 2016 | 203 | University of Ilorin Teaching Hospital, Ilorin/NC | 21-92/49.2/N | Pros | Histo/IHC | N | All patients |
| Ali-Gombe (23) | 2004-2008 | 2021 | 378 | University College Hospital, Ibadan/SW | 22-84/47.6/N | Retro | Histo | N | Completed Surgery, Chemo, Radio |
| Ayoade (17) | 2004-2008 | 2014 | 139 | Olabisi Onabanjo University Teaching Hospital, Sagamu/ SW | 24-84/48.1/45 | Pros | Histo | Xray, US | All patients |
| Egwuongu (24) | 2009-2010 | 2013 | 44 | Nnamdi Azikiwe University Teaching Hospital, Nnewi/SE | 26-51/42.1/N | Pros | Histo/Cyto | Xray, US, LFT | Premenopausal LABC |
| Makanjuola (12) | 2005-2008 | 2014 | 224 | Lagos State University Teaching Hospital, Lagos/SW | 28-85/N/N | Retro | Histo | Xray, US | All patients |
| McWormack (5) | 2014-2017 | 2020 | 385 | Multicenter | N/45/N | Pros | Histo/Cyto/ IHC/Clinical | Xray, US, CT, MRI, bone scan | All patients |
| Olaogun (19) | 2011-2015 | 2020 | 82 | Ekiti State University Teaching Hospital, Ado-Ekiti/ SW | 26-95/48.9/47.5 | Retro | Histo/Cyto | Xray, US | At least one treatment |
| Olaogun2 (10) | 2016-2018 | 2021 | 78 | Ekiti State University Teaching Hospital, Ado-Ekiti/ SW | 24-94/50.1/50 | Retro | Histo | N | All patients |
| Olasehinde (20) | 2010-2018 | 2020 | 607 | Obafemi Awolowo University Teaching Hospital, Ile Ife/SW | N/49.8/N | Bidirectional | Histo/IHC | Xray, US | All patients |
| Olatoke (25) | 2013-2015 | 2018 | 57 | University of Ilorin Teaching Hospital, Ilorin/NC | N/47.9/N | Retro | Histo/Cyto | N | LABC |
| Olatunji (14) | 2004-2013 | 2019 | 1141 | Lagos State University Teaching Hospital, Lagos/SW | N/48/ | Retro | Histo/Cyto | N | All patients |
| Popoola (11) | 2005-2010 | 2012 | 176 | Lagos State University Teaching Hospital, Lagos/SW | 23-80/48.7/N | Pros | Histo | Xray, US | All patients |
| Titiloye (16) | 2004-2006 | 2013 | 89 | Obafemi Awolowo University Teaching Hospital, Ile Ife/SW | 22-82/N/N | Retro | Histo/Cyto | N | All patients |
| Umoke (15) | 2016-2017 | 2019 | 55 | University of Abuja Teaching Hospital, Gwagwalada/NC | 25-70/49.2/N | Retro | Histo | N | All patients |
Four study designs were prospective, one bidirectional, while all others were retrospective. Diagnostic criteria included histology or cytology in all reports except one that included clinical evaluation [5]. One study included patients who received surgery, chemotherapy, and radiotherapy [22]. Two included LABC only [23, 24]. Others included all patients managed in the study period. All eligible articles reported on patients receiving treatment in tertiary care referral centers. The quality assessment score ranged between 4 and 9. (Supplementary File). The overall summary estimates showed marked heterogeneity.
Stage distribution
Eight articles, including 2441 subjects, contributed data on staging. Stage distribution data were not extractable in three articles [5, 13, 15], three included only LABC [10, 23, 24], and one was a purposive sampling of patients receiving complete multimodal therapy [22]. Two articles used Manchester staging, while all others used the AJCC system. The predominant staging modalities were chest x-ray and abdominal ultrasound scanning. Only the multinational research [5] included CT scan, magnetic resonance imaging, and bone scan as first-line staging modalities in some patients. Fifty-five percent (n-1220, 95% CI 44-64, I2 95%) of patients were stage III, 27% (n-795, 95%CI 18-36) were stage IV, 13% (n-343,95% CI 7-21) were stage II and 5% (n-93, 95%CI 1-9) were stage I. (See supplementary file).
Treatment modalities
Articles reported surgery, chemotherapy, radiation therapy, hormonal therapy, and HER2-targeted therapy. All patients received at least one treatment modality in five reports [11, 15, 18, 22, 24]. In two reports, the uniform treatment was hormonal therapy alone [15, 18]. In one report, all patients received hormonal and chemotherapy [11]. Two reports purposively sampled patients receiving neoadjuvant systemic therapy: one included premenopausal patients only [23], and the second included all patients who received neoadjuvant systemic therapy [24]. Another study purposively sampled patients who combined surgery, chemotherapy, and radiotherapy [22]. To assess the overall prevalence of each treatment modality, we pooled all treatments ‘N’ and found the proportion accounted for by each treatment modality ‘n. Nine studies [10-12, 14, 15, 17, 18, 19, 22], including 5744 instances of treatment, contributed to the treatment modality’s prevalence analysis. The most prevalent modality was chemotherapy (41%, 95% CI 19-54, n-2044), followed by surgery (31%, 95% CI 13-45, n 1671), hormonal therapy 18 (4-31, n-777), radiotherapy 10% (95% CI 1-23, n-1246) and HER2 targetted therapy (0% (95% CI 0-3, n-6) (See Supplementary file).
Using only articles with nonpurposive sampling, it was possible to estimated the probability of receiving each of the three main modalities of treatment [Chemotherapy, Surgery and Radiotherapy] by finding the summary estimate of the proportion in each article. Where N is the number eligible for the treatment modality in the article, and ‘n’ is the number that received the treatment modality. The overall probability of receiving chemotherapy was 85% (95% CI66-97 ) contributed by seven articles [10, 12, 14, 15, 17, 18, 19] with 2308 subjects. The overall probability of receiving surgery was 62% (95% CI 51-73), contributed by eight articles [10-12, 14, 15, 17, 18, 19] with 1293 subjects. The overall probability of receiving radiation therapy was 31% (95% CI 8-59) contributed by seven articles [10-12, 14, 15, 17, 19] with 1884 subjects. (See supplementary file).
Chemotherapy
The indication for chemotherapy was explicitly documented as neoadjuvant or adjuvant, and there was no report on salvage therapy. The utilization rate of neoadjuvant and adjuvant chemotherapy was extractable in Olaogun et al. [18]. In this study, 36 of 82 (44%) patients who qualified for neoadjuvant chemotherapy received it, while 9 of 45 (20%) patients who qualified for adjuvant received it. The number eligible specifically for neoadjuvant or adjuvant chemotherapy was not extractable in other articles.
In four articles [10], 766 subjects contributed to data on the type of chemotherapy. Anthracycline-based therapy was the most frequent regimen, accounting for 78%, followed by taxane-based 12%. All available reports on chemotherapeutic regimens described intravenous cycles of six doses every three weeks, with no dose-dense or metronomic therapies and no oral therapies. The prevalence of non-completion or haphazard use of chemotherapy was extracted from four articles [10, 15, 18, 23], including 319 subjects. In this cohort, 156 (48%, 95% CI 35-60) subjects did not complete the recommended number of chemotherapy doses or received it at irregular intervals. Four studies [14, 23-25], including 433 subjects, contributed data on response to neoadjuvant chemotherapy. Overall, 85% of patients who received neoadjuvant chemotherapy experienced a clinical benefit: 26% had a complete response, 41% had a partial response, and and 18 had stable disease. Fifteen percent had tumor progression.
Surgery
Overall, 6 articles [10, 17, 18, 19, 22, 25] with 1010 subjects contributed to data on the type of surgery. Modified radical mastectomy (MRM) accounted for 90% of surgeries. Only two percent had breast-conserving surgery; the others received simple or toilet mastectomy.
Hormonal and HER2-targeted systemic therapy
Four articles reported on hormonal therapy. The use of hormonal therapy based on immunohistochemistry (IHC) testing was extractable in Olasehinde et al. [19] only, where 39 of 53 (74%, 95% CI 60-85) patients with hormone-receptor-positive tumors received hormonal therapy. Three other articles reported routine use of hormonal therapy without IHC testing [11, 17, 18]. HER2- targeted treatment data were extractable in Olasehinde et al. only, where 6 of 43 (14%, 95% CI 5.3-30) HER2- positive patients received HER2-targeted therapy [19].
Radiation
One in ten patients utilized radiation therapy in the overall analysis. Sensitivity analysis showed a heterogeneous distribution of radiation therapy used across the country. One in five patients received radiation therapy in centers/states with radiation oncology services compared with one in 20 patients in centers/states without radiation oncology services.
Multimodality treatment
Among studies with nonpurposive sampling, three articles [10, 12, 14], and 983 subjects contributed data on multimodal therapy. The prevalence of receiving multimodality treatment with surgery and chemotherapy was 56% (95% CI 46-68), and the prevalence of receiving surgery, chemotherapy, and radiation therapy was 24% (10 -43). In this cohort, the stage distribution was 3%, 14%, 46%, and 37% for stages I, II, III, and IV, respectively.
Survival
Overall, the one-year survival estimate was 80%, declining sharply to 43% at the end of the second year and 32% at five years. The overall survival estimates based on tumor biology were similar for triple-negative and hormone receptor-positive diseases. After surgery, the overall prevalence of recurrence was 28% (95% CI 11-48) from data in 4 articles [10, 13, 16, 19] with 484 subjects. Two of the studies [Titiloloye et al [16] 7% (0-20%), Olaogun [10], 61% (95% CI 48-73) recorded recurrence in 3 years. Titiloye et al. l included all cases whereas Olaogun included LABC cases only and less than 10% received radiotherapy], one [Adeniji e t al [13], 33% (95% CI 24-42)] recorded in recurrence in 5 years and one, [Olasehinde [19], 19% (95% CI 15-24)] recorded in 9 years. Olasehinde et al [19] reported the recurrence pattern as 70% locoregional, 11% contralateral breast, and 19% distant. The summary estimate of the loss to follow- up was 45% in 3-5 years.
The survival gains in early [AJCC stages I & II] versus advanced disease increased with time elapsed. At two and 3-5 years, there was a 30% (95% CI 5-54) and 32% (95% CI 23-40) survival difference, respectively, in favor of early disease. A single study of 196 subjects, Adejumo et al [25]., recorded data with estimated six-month and 1-year survival differences of 23% (95% CI 16-30) and 53% (95% CI 41-65), respectively, in favor of early disease.
The summary for survival estimates appeared higher among patients receiving multimodal therapy. In Ali-Gombe et al. [22]., completing the multimodal treatment of surgery-chemotherapy-radiotherapy achieved 2-year disease-free survival of 67% (95% CI 61-71). The three-year survival estimate was 68% among the surgery-chemotherapy subgroup whereas the overall 3-year survival was 43%. In Makanjuola et al. [12]., the five-year survival difference between those receiving surgery-chemotherapy-radiotherapy compared to surgery-chemotherapy alone was 30% (95% CI 18-41).
Discussion
This first meta-analytical review on the treatment and outcomes of BC in Nigeria found a low prevalence of multimodality treatment with the combination of surgery, chemotherapy, and radiation therapy. Most patients received single modality treatment or a combination of surgery and chemotherapy. Most mortality occurred between the first and second year of therapy, and receiving the multimodal combination of chemotherapy, surgery, and radiotherapy or presenting at an earlier stage was associated with longer survival.
Research on BC in Nigeria focuses largely on breast health awareness, early detection, and diagnosis; hence, it is not surprising to find only 15 eligible articles, with fewer than 4000 total subjects, reporting on BC treatment and outcomes. Evidence-based BC treatment is necessary to reduce morbidity, improve quality of life and prolong survival, particularly in a setting such as Nigeria, where most patients present with late-stage disease.
BC treatment varies across centers throughout Nigeria. The disparity is worst in radiation therapy, with patients treated in centers with radiation services having a 64% probability of receiving radiotherapy compared to just 12% in centers without radiation services. Most countries in Africa lack access to radiotherapy or have a deficit of radiation services [26], and the number of cancer patients is predicted to increase from 844 279 in 2012 to over 1.5 million in 2030 [27]. Samie [26] documented Africa was short of radiotherapy megavoltage by over 5000 units in a 2013 report, noting that Europe had 17 times as many radiotherapy units as were available in Africa per million inhabitants. According to the International Atomic Energy Agency (IAEA), 60% of African cancer patients need radiotherapy at least once in their treatment [26]. Ordinarily, each radiotherapy machine has the capacity to treat approximately 500 persons per year, yet there were over 124,000 new cancers diagnosed in Nigeria in 2020 [4], and only ten radiotherapy centers [28]. The gap for megavoltage units in Africa is estimated as 1018 megavoltage units deficit from the number needed to serve more than 1.1 million patients with cancer in 2020 [27]. In Nigeria, our findings that 21% of breast cancer is diagnosed at an early stage, and 26% of those receiving neoadjuvant chemotherapy had a complete response., show that availability of radiation services could allow for more breast-conserving therapy (BCT), hence improving patient adherence to surgical treatment recommendations. BCT could be offered by synchronizing treatment with centers that have radiation facilities. Patients planned for BCT might have separate bookings to ensure scheduled treatment.
Included studies reported using recommended chemotherapeutic agents. However, utilizing appropriate drugs is not the only prerequisite for improving cancer treatment outcomes. Optimal dosing and scheduling as well as compliance with treatment recommendations are necessary to achieve the maximum benefit from chemotherapy. One study [18] reported haphazard scheduling and a high non-completion rate of close to 50%. With the 26% complete clinical response in patients receiving neoadjuvant chemotherapy found in this review, ensuring appropriate planning of treatment regimens, having medication available to mitigate side effects, and encouraging patient compliance can help improve treatment completion and outcome.
Considering dose-dense or metronomic therapy where available and ensuring regular scheduling might prolong survival or time to recurrence and progression- free survival [29-31] in advanced BC. Also, studies show potential clinical benefits of metronomic therapy in the primary palliative setting and salvage therapy [32-34]. While the dose-dense treatment is resource-intensive and demands more supportive care, metronomic chemotherapy is more tolerable [30].
We found high mortality rates during BC treatment and shortly thereafter. This is likely due to under-staging, as the most common staging work-up in the studies reviewed was a chest x-ray and abdominal ultrasound. Chest x-ray and abdominal ultrasound is the recommended metastatic work-up in resource-limited settings where advanced imaging is largely unavailable [35]. However, plain chest radiograph has low sensitivity for pulmonary metastasis, missing lesions smaller than 1.6cm, and abdominal ultrasound is less than 70% sensitive for hepatic metastasis [36]. Accurate staging of disease at presentation is crucial for effective BC management, sparing patients unnecessary side effects from treatment unlikely to change outcomes, and appropriate resource allocation. Notwithstanding, recommending high-resolution imaging routinely to stage BC is not practical in resource-poor settings. Innovative, resource-appropriate solutions are desperately needed.
The complementary role of biochemical prognostic markers for the initial diagnostic workup is relatively inexpensive and virtually unexplored in resource-limited settings. Tumor markers could potentially be used as a pre-staging tool to select patients to undergo more costly investigations. A study evaluating serum markers in BC showed that CEA and CA 15-3 rose by 41.3% and 80.3%, respectively, in metastatic disease. CEA and CA15-3 rose in 30 and 70% of patients with recurrence [37]. Other studies similarly found increased levels of CA15-3 as tumor burden increased [37, 38], with CA15-3 elevation above 30 units/ml in 12% of stage I, 22% of stage II, 36% of stage III, and 80% of stage IV disease [37]. At the time of this review, in early 2022, CA15-3 and CEA cost 10USD- 25USD in Nigeria, compared to$100-$120 USD for a CT scan.
The survival gaps between early- and late-stage diseases are widely known. For example, a Ghanaian study recorded over 90% 5-year survival for stage 0 and I BC, compared to approximately 15% 5-year survival in stage IV disease [39]. However, the time-related widening of survival gaps between early- and late-stage disease are not as commonly reported and might be another representation of under-staging. The elevated short-term mortality rate seen in stage I and II disease likely existed until most erroneously under-staged patients died.
There are several limitations associated with this review. Several of the studies included used purposive sampling, which has the potential to skew the analysis. To minimize bias, these studies were excluded in sensitivity analysis as appropriate. Importantly, while the disease stage and treatment modalities received both influenced disease survival, the available data made it impossible to describe the interaction between these two variables. Reasons for not receiving treatment or for not completing it are beyond the scope of this study. Additionally, there was significant heterogeneity in the results because of the reporting pattern in the available report. Deliberately designing studies with uniform or standard reporting patterns in Nigeria will improve data quality and reduce heterogeneity in subsequent reviews.
In conclusion, the utilization of multimodality treatment with the combination of chemotherapy, surgery, and radiation therapy is low in Nigeria. Receipt of complete chemotherapy and access to radiation are inadequate. Survival is poor, with the highest mortality occurring in the first year with short-term mortality in early-stage BC is paradoxically elevated, likely due to under-staging. Receiving the multimodal combination of chemotherapy, surgery, and radiotherapy or presenting at an earlier stage is associated with longer survival. Improving BC staging and access to complete chemotherapy, surgery, and radiation should be targets of future interventions.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Cancer incidence in Nigeria: a report from population-based cancer registries Jedy-Agba E, Curado MP , Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, Osubor G, et al . Cancer Epidemiology.2012;36(5). CrossRef
- Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review Azubuike SO , Muirhead C, Hayes L, McNally R. World Journal of Surgical Oncology.2018;16(1). CrossRef
- IARC. Nigeria-Global Cancer Observatory. https://gcoiarcfr/today/data/factsheets/populations/566-nigeria-fact-sheetspdf. 2020 .
- Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C, Anele A, et al . The Lancet. Global Health.2020;8(9). CrossRef
- Neoadjuvant chemotherapy for locally advanced premenopausal breast cancer in Nigerian women: early experience Anyanwu SNG , Nwose P, Ihekwoaba E, Mbaeri AT , Chukwuanukwu TO . Nigerian Journal of Clinical Practice.2010;13(2).
- Mastectomy for management of breast cancer in Ibadan, Nigeria Ogundiran TO , Ayandipo OO , Ademola AF , Adebamowo CA . BMC surgery.2013;13. CrossRef
- The impact of neoadjuvant chemotherapy on patients with locally advanced breast cancer in a Nigerian semiurban teaching hospital: a single-center descriptive study Arowolo OA , Akinkuolie AA , Lawal OO , Alatise OI , Salako AA , Adisa AO . World Journal of Surgery.2010;34(8). CrossRef
- Treatment Of Breast Cancer: Imo State Nigeria Versus Indiana, Usa Women - Comparative Analytic Study Anele AA , Bowling M, Eckert GJ , Gonzalez E, Kipfer H, Sauder C. Journal of the West African College of Surgeons.2014;4(4).
- Management of Locally Advanced Breast Cancer: Challenges and Treatment Outcomes in an Emerging Tertiary Hospital in South-Western Nigeria Olaogun J, Agodirin O, Etonyeaku A, Omonisi A, Joseph O. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH.2021;15. CrossRef
- Five Year Survival of Patients with Breast Cancer at the Lagos State University Teaching Hospital, Nigeria Ao P, Ogunleye O, Ibrahim N, Fo O, Ai I. J Med Med Sci Res.2012;1.
- Radiation therapy: a major factor in the five-year survival analysis of women with breast cancer in Lagos, Nigeria Makanjuola SBL , Popoola AO , Oludara MA . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2014;111(2). CrossRef
- Survivorship patterns of histopathological variants and molecular subtypes of breast cancer in a teaching hospital in Nigeria Adeniji KA , Hugo D, Rahman GA , Akande TM , Olatoke SA , Akande HJ , Durowade KA , et al . East African Medical Journal.2016;93(9). CrossRef
- Sociodemographic correlates and management of breast cancer in Radiotherapy Department, Lagos University Teaching Hospital: A 10-year review Olatunji T, Sowunmi A, Ketiku K, Campbell O. Journal of Clinical Sciences.2019;16. CrossRef
- Breast cancer in North-Central Nigeria: challenges to good management outcome Umoke I, Garba E. International Surgery Journal.2019;6. CrossRef
- Breast Cancer in a Nigerian Cohort: Histopathology, Immunohistochemical Profile and Survival Titiloye N, Omoniyi-Esan G, Adisa A, Komolafe A, Afolabi O, Adelusola K. PMJG.2013;2(2).
- Clinical Characteristics and Survival Outcome of Breast Cancer in Southwest Nigerian Women Ayoade B, Agboola A, Olatunji A, Tade A, Salami B, Adekoya A. J Afr Cancer.2014.
- Socio-demographic, pattern of presentation and management outcome of breast cancer in a semi-urban tertiary health institution Olaogun JG , Omotayo JA , Ige JT , Omonisi AE , Akute OO , Aduayi OS . The Pan African Medical Journal.2020;36. CrossRef
- Contemporary management of breast cancer in Nigeria: Insights from an institutional database Olasehinde O, Alatise O, Omisore A, Wuraola F, Odujoko O, Romanoff A, Akinkuolie A, et al . International Journal of Cancer.2021;148(12). CrossRef
- Epidemiology and surgical management of breast cancer in gynecological department of Douala General Hospital Nguefack CT , Biwole ME , Massom A, Kamgaing JT , Njamen TN , Ekane GH , Obinchemti TE , Priso EB . The Pan African Medical Journal.2012;13.
- [Breast cancer in Cameroon, histo-epidemiological profile: about 3044 cases] Engbang JPN , Essome H, Koh VM , Simo G, Essam JDS , Mouelle AS , Essame JLO . The Pan African Medical Journal.2015;21. CrossRef
- Pattern of survival of breast cancer patients in a tertiary hospital in South West Nigeria Ali-Gombe M, Mustapha MI , Folasire A, Ntekim A, Campbell OB . Ecancermedicalscience.2021;15. CrossRef
- Efficacy of neoadjuvant chemotherapy in down staging locally advanced pre-menopausal breast cancer in Eastern Nigeria: is four courses adequate? Egwuonwu OA , Anyanwu SN , Nwofor AM . Journal of Cancer Research and Therapeutics.2013;9(4). CrossRef
- Relationship between tumour size and response to neoadjuvant chemotherapy among breast cancer patients in a tertiary center in Nigeria Samuel O, Olayide A, Ganiyu R, Olufemi H, Halimat A. Malawi Medical Journal: The Journal of Medical Association of Malawi.2018;30(1). CrossRef
- Epidemiology and Challenges of Managing Breast Cancer in Keffi, North-Central Nigeria: A Preliminary Report Adejumo AA , Ajamu OJ , Akanbi OO , Onwukwe JC , Adeosun OA , Omoregie PO , Amos A, et al . Nigerian Medical Journal: Journal of the Nigeria Medical Association.2019;60(4). CrossRef
- Challenges of making radiotherapy accessible in Developing countreis Cancer Control Massoud S. ;:85-94.
- Radiotherapy resources in Africa: an International Atomic Energy Agency update and analysis of projected needs Elmore SNG , Polo A, Bourque JM , Pynda Y, Merwe D, Grover S, Hopkins K, Zubizarreta E, Abdel-Wahab M. The Lancet. Oncology.2021;22(9). CrossRef
- Challenges and Prospects for Providing Radiation Oncology Services in Africa Balogun O, Rodin D, Ngwa W, Grover S, Longo J. Seminars in Radiation Oncology.2017;27(2). CrossRef
- Extending the duration of first-line chemotherapy in metastatic breast cancer: a perspective review Gennari A, D'amico M, Corradengo D. Therapeutic Advances in Medical Oncology.2011;3(5). CrossRef
- Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer Stockler MR , Harvey VJ , Francis PA , Byrne MJ , Ackland SP , Fitzharris B, Van Hazel G, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2011;29(34). CrossRef
- Dose density in breast cancer: a simple message? Lin NU , Gelman R, Winer EP . Journal of the National Cancer Institute.2005;97(23). CrossRef
- Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience Simsek C, Esin E, Yalcin S. Journal of Oncology.2019;2019. CrossRef
- Efficacy and Toxicity of Metronomic Chemotherapy in Metastatic Breast Cancer: Egyptian Experience Hussein MM , Gaafar RM , Abdel-Warith AM , Ahmed WA , Allahloubi NMA , Salem SE , Abdel-Salam IM . Clinical Breast Cancer.2017;17(8). CrossRef
- Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer Fedele P, Marino A, Orlando L, Schiavone P, Nacci A, Sponziello F, Rizzo P, et al . European Journal of Cancer (Oxford, England: 1990).2012;48(1). CrossRef
- Sub-Saharan Africa Harmonized Breast Cancer Guidelines s. National Comprehensive Cancer Network 2021;https://www.nccn.org/global/what-we-do/harmonized-guidelines .
- Diagnostic Imaging method in Metastatic Disease Milosevic Z, Gajic-Dobrosavljevic M, Stevanovic J. Archive of Oncology.2006;14(Suppl 1):73-74.
- A re-evaluation of carcinoembryonic antigen (CEA) as a serum marker for breast cancer: a prospective longitudinal study Guadagni F, Ferroni P, Carlini S, Mariotti S, Spila A, Aloe S, D'Alessandro R, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2001;7(8).
- Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer Shao Y, Sun X, He Y, Liu C, Liu H. PloS One.2015;10(7). CrossRef
- Survival Outcomes of Breast Cancer in Ghana: An Analyiss of Clinicopathologic Features Mensah A, Yarney J, Kaku Nokoe S, Opoku S, Clegg-Lamptey J. Open Acess Library Journal.2016;3:e2145.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times