Elevated FISH Her-2/neu Amplification in ER Positive, IHC Her-2 Equivocal Invasive Breast Cancer Patients of North-East India
Download
Abstract
Background: Breast cancer management of Her-2 equivocal breast cancer patients requires assessment by fluorescent in situ hybridization (FISH) for Her-2/neu amplification. Assessment of FISH Her-2/neu amplification status in IHC Her-2 equivocal invasive breast cancer patients of North-East India.
Methods: One hundred sixty-two (n=162) IHC Her-2 equivocal (2+) breast cancer patients were analysed by FISH for Her-2/neu amplification using PathVysion Her-2/neu DNA probe kit. The interpretation of FISH Her-2/neu amplification was based on ASCO/CAP 2018 guidelines.
Results: FISH Her-2/neu amplification was detected in 59.26% (n=96) patients and 40.74% (n=66) patients had no amplification. There were 160 female and 2 male patients. ER-positive patients were 68.51% (n=111) and PR-positive were 54.93% (n=89). “ER+/PR+” status was found in 54.93% (n=89) patients whereas “ER-/PR-’’ in 31.48% (n=51) patients. Significant association was observed between histological grade and FISH Her-2/neu amplification (p=0.036). ER-positive patients had also shown significant association with FISH Her-2/neu amplification (p=0.039). ER+/PR+ patients had shown a trend for higher likelihood of FISH Her-2/neu amplification (p=0.053). There were no significant association observed between FISH Her-2/neu amplification with age (p=0.625), lymph node positivity (p=0.642), laterality (p=0.367), histopathology (p=0.226) and PR-positivity (p=0.199).
Conclusion: FISH Her-2/neu amplification in IHC Her-2 equivocal breast cancer patients of North-East India was found to be significantly associated with ER-positive status and histological grade. ER+/PR+ patients have also an enhanced likelihood of FISH Her-2/neu amplification.
Introduction
Globally among women, breast cancer (BC) remains one of the most challenging health concern in developing as well as developed countries [1]. In India, being the most common malignancy breast cancer accounts for 13.5% of all cancer cases and 10.6% of all cancer deaths [2, 3]. Age adjusted incidence rate (AAR) per 100000 population among females for breast cancer in India range from 7.0- 48.0. In Northeast India, probability of developing breast cancer over a lifetime in females is as follows: Kamrup Urban (1 in 32), Mizoram state (1 in 43), Pasighat (1 in 55), Dibrugarh district (1 in 62), Cachar district (1 in 73), West Arunachal (1 in 92), Manipur (1 in 95), Nagaland (1 in 109), Tripura state (1 in 123) and Meghalaya (1 in 142) [4].
Human epidermal growth factor receptor 2 (Her- 2), belonging to the family of epidermal growth factor receptors (EGFR), is a transmembrane tyrosine kinase receptor [5]. The protein is located on the long arm of chromosome 17 (17q12-21.32) and encoded by the Her-2 (ERBB2) gene [6]. Amplification and overexpression of this oncogene induces proliferation and antiapoptotic as well as tumorigenic growth in human mammary epithelial cells [7]. About 15-20% patients with invasive breast cancer have Her-2 protein overexpression and/or Her- 2 gene amplification [8]. The advent of Her-2 targeted therapy like Trastuzumab has dramatically improved clinical outcomes in Her-2 positive breast cancer [9]. Thus, accurate diagnostic testing for Her-2 is an important front-line investigation in individuals with invasive breast cancer. The American Society of Clinical Oncology/College of American pathologists (ASCO/CAP) guidelines 2018, recommended Her-2/neu testing by immunohistochemistry (IHC), and when equivocal (2+), the same needs to be assessed by in situ hybridization (ISH) techniques like fluorescence in situ hybridization (FISH) [10].
The ASCO/CAP 2018 guidelines define four groups of IHC results, viz., 0 (negative), 1+ (negative), 2+ (equivocal), and 3+ (positive), based on criteria for staining pattern, intensity, and the percentage of tumor cells stained [10]. Based on the Her-2/CEP17 ratio and average Her-2 copy number per cell in relation with the immunohistochemical results, the guidelines recommended criteria for FISH results to establish 5 dual probe ISH groups [11].
IHC Her-2/neu equivocal (2+) breast cancer patients with gene amplification status by FISH have shown wide range variation in various geographical regions as follows: India (14% to 71%) [12-19]; China (29% to 70%) [20-23]; Germany (14% to 26%) [24-26]; USA (8%,64%) [27, 28]; Thailand (37.5%) [29]; Egypt (18%) [30]; Hungary (38%) [31]; Netherland(24%) [32]; Denmark (48%) [33]; Taiwan (53%) [34]; Australia (0%) [35]; Iran (31.1%) [36]. Among these studies, only 7 studies have more than 100 breast cancer patients with Her-2/neu IHC Equivocal (2+) status and their corresponding FISH evaluation [13, 15, 16, 20-22, 26].
There is lack of data about Her-2/neu FISH status in Her-2/neu IHC Equivocal (2+) in breast cancer patients of North-East India. This study aims to evaluate Her-2/neu gene amplification status by FISH in IHC equivocal (2+) invasive breast cancer patients from North-East India alongwith clinicopathological characteristics.
Materials and Methods
A total of 162 patients diagnosed with invasive breast cancer and IHC Her-2/neu equivocal (2+) status were enrolled from January 2021 to June 2023 and were analysed for Her-2/neu amplification by FISH. Patients with benign tumors of the breast, other histopathological subtypes of malignant breast tumors, and incomplete clinicopathological information were excluded from the study. The patient’s demographic and clinicopathological characteristics of the tumor were recorded. This study was approved by Institutional Ethics committee (.
Immunohistochemistry (IHC): IHC staining was done on 4-5μm thickness sections of freshly cut formalin-fixed, paraffin-embedded tumor tissue from the same specimen blocks that were used for FISH Her-2/neu testing.Ventana BenchMark XT (Roche diagnostics) protocol for Her-2 IHC was followed as per manufacturer’s instruction. The details of the antibodies used on the Ventana BenchMark XT (Roche diagnostics) were as follows:
Antibodies Clone
ER SP1 (rabbit monoclonal)
PR 1E2 (rabbit monoclonal)
HER2 4B5 (rabbit monoclonal)
IHC scoring was performed by two independent pathologists as per the ASCO/CAP 2018 guidelines [11]. Fluorescence in situ hybridization (FISH): PathVysion Her-2 DNA Probe Kit (Abbott, USA) was used to detect Her-2/neu gene amplification as per the manufacturer’s protocol. In brief, the probes were pre-mixed and pre-denatured in hybridization buffer. Formalin-fixed, paraffin-embedded tissue specimens were placed on slides. The DNA was denatured to single-stranded form and then allowed to hybridize with the PathVysion probes. After that, the unbound probe was removed by series of washes and the nuclei were counterstained with DAPI (4, 6 diamidino-2-phenylindole). FISH slides were visualized at 100X using fully motorized BX61 fluorescence microscope (Olympus, Japan) with appropriate excitation and emission filters allowing visualization of the intense orange and green, fluorescent signals. Enumeration of the orange Locus Specific Identifier (LSI) Her-2/neu and green Chromosome Enumeration Probe (CEP) 17 signals was conducted by microscopic examination of the nucleus, which yielded a ratio of the Her-2/neu gene to chromosome 17 copy number. ASCO/CAP 2018 guidelines were followed for Her-2/neu FISH scoring and interpretation by two independent observers [11].
Statistical Analysis
Chi square test and Fisher’s exact test were applied to observe the association between clinicopathological features, hormone receptors and FISH Her-2/neu amplification respectively. All the statistical analyses were performed in SPSS 29.0 software (IBM Corp, NY, USA). A p-value < 0.05 was considered to be statistically significant.
Results
A total of 162 IHC Her-2 equivocal (2+) invasive BC patient’s clinicopathological data were analyzed (Figure 1). There were 160 female and 2 male patients. The median age of the BC patients was 48 years, range=23 – 83 years. 61.11% (n=99) BC patients were <50 years and 38.88% (n=63) were > 50 years. Based on the Nottingham classification, 59.87% (n=97) BC patients had histological grade II followed by 38.27% (n=62) grade III and 1.86% (n=03) grade I. Lymph node-positive outcome was detected in 35.18% (n=57) patients. 51.23% of BC patients (n=83) had cancer in the right breast and 48.77% (n=79) in the left breast. All patients (n=161) were histopathologically invasive ductal carcinoma (IDC) except one which was invasive lobular carcinoma. Hormone receptor status was as follows : 68.51% (n=111) were ER+ and 54.93% (n=89) were PR+. “ER+/PR+” status was found in 54.93% (n=89) patients whereas “ER-/ PR-’’ in 31.48% (n=51) BC patients (Table 1).
| Characteristics | Number of patients | Percentage | |
| Age | ≤50 | 99 | 61.11 |
| >50 | 63 | 38.88 | |
| Gender | Male | 2 | 1.23 |
| Female | 160 | 98.77 | |
| Grade | Grade I | 3 | 1.86 |
| Grade II | 97 | 59.87 | |
| Grade III | 62 | 38.27 | |
| Lymph node involvement | Present | 57 | 35.18 |
| Absent | 35 | 21.06 | |
| No information | 70 | 43.2 | |
| Laterality | Left | 79 | 48.77 |
| Right | 83 | 51.23 | |
| Histology | Invasive ductal carcinoma | 161 | 99.38 |
| Invasive lobular carcinoma | 1 | 0.62 | |
| ER | Positive | 111 | 68.51 |
| Negative | 51 | 31.48 | |
| PR | Positive | 89 | 54.93 |
| Negative | 73 | 45.07 | |
| Hormone receptor status | ER+PR+ | 89 | 54.93 |
| ER-PR- | 51 | 31.48 | |
| ER-PR+ | 0 | 0 | |
| ER+PR- | 22 | 13.58 |
Her-2/neu amplification by FISH was detected in 59.26% (n=96) patients whereas 40.74% (n=66) patients had no amplification (Table 2 ), (Figure 1).
| IHC HER 2 | FISH Her-2/neu | Segment of Amplified | Segment of Non-Amplified | |
| Amplified | Non-Amplified | |||
| 2+ | 96 | 66 | 59.26% | 40.74% |
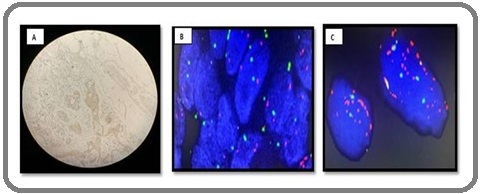
Figure 1. IHC and FISH Images of Breast Cancer Patients. A. IHC Her-2 (2+ Score); B. FISH Her-2 /neo (non amplified); C. FISH Her-2/neo (amplified).
A significant association was observed between histological grade and FISH Her-2/neu amplification (p=0.036). There was no significant association found between FISH Her-2/neu amplification status with age (p=0.625), lymph node positivity (p=0.642), laterality (p=0.367), and histopathology (p=0.226) (Table 3).
| Clinicopathological Features | FISH Her-2/neu | p-value | ||
| Amplified (%) | Non-Amplified (%) | |||
| Age | ≤ 50 | 57 (59.37) | 42 (63.63) | 0.625 |
| > 50 | 39 (40.62) | 24 (36.36) | ||
| Grade | Grade I | 00 (0.00) | 03 (4.55) | |
| Grade II | 63 (65.63) | 34 (51.52) | 0.036* | |
| Grade III | 33 (34.37) | 29 (43.93) | ||
| Lymph Node Involvement | Present | 31 (32.29) | 26 (39.40) | 0.642 |
| Absent | 22 (22.91) | 13 (19.69) | ||
| No Information | 43 (44.80) | 27 (40.91) | ||
| Laterality | Left | 44 (45.84) | 35 (53.03) | 0.367 |
| Right | 52 (54.16) | 31 (46.97) | ||
| Histology | Invasive ductal carcinoma | 96 (100) | 65 (98.49) | 0.226 |
| Invasive lobular carcinoma | 00 (00.00) | 01 (1.51) |
*Statistically Significant
ER-positive patients had shown significant association with FISH Her-2/neu amplification (p=0.039). ER and PR expression was higher in FISH Her-2/neu amplified tumors compared to non-amplified tumors. Although ER+/ PR+ patients had higher FISH Her-2/neu amplification but failed to achieve significant association (p=0.053). PR-positive cases had also not shown any significant association with FISH Her-2/neu amplification (p=0.199) (Table 4).
| Hormone Receptor | FISH Her-2/neu | p-value | ||
| Amplified | Non-Amplified | |||
| ER | Positive | 72 | 39 | |
| Negative | 24 | 27 | 0.039* | |
| PR | Positive | 57 | 32 | |
| Negative | 39 | 34 | 0.199 | |
| Hormone receptor status | ER+PR+ | 57 | 32 | |
| (Both positive/ Both Negative) | ER-PR- | 24 | 27 | 0.053 |
| Hormone receptor status | ER-PR+ | 0 | 0 | 1 |
| (Either negative/either positive) | ER+PR- | 15 | 7 |
*Statistically Significant
Discussion
Reliable laboratory data in evaluating Her-2 status is essential. Her-2 status studied at the levels of DNA using FISH and protein using IHC are the two most accessible and feasible methods used in clinical diagnosis [1]. Breast cancer is the most common malignancy among women in India. The treatment and prognosis of patients with breast cancer depend on various factors, such as grade, histology, hormone receptor status, and Her-2/neu status. Her-2/neu status determination is necessary in breast cancer patients for Her-2 targeted therapy (e.g.trastuzumab) [37, 38].
In our study of IHC Her-2 equivocal (2+) BC patients, FISH Her-2/neu amplification was detected in 59.26% (96/162) whereas it was non-amplified in 40.74% (66/162). Previous studies from India have shown higher Her-2/neu amplification in IHC Her-2 equivocal BC patients: 54% (72/134); 66.6% (24/36); 70% (7/10); 71% (20/28) [16-19]. However, other Indian studies also reported lower coincidence rate of IHC 2+/FISH Her-2/neu amplified BC cases – 14.3% (1/7); 24.4% (31/127); 25% (17/68) and 28.1 % (68/242) [12-15]. Except for three studies, rest all Indian studies have quite small sample sizes of IHC Her-2 (2+) BC patients. These studies are from various regions of India except North-East. Therefore, variations in the coincidence rate of IHC 2+/FISH Her-2/neu amplified BC patients may be attributed to the patient’s ethnic, genetic, geographical and etiological characteristics etc. We had followed the 2018 ASCO/CAP guidelines for Her-2/neu FISH scoring and interpretation whereas most of the previous studies cited by us may not have followed the 2018 guidelines and this could also have contributed to variation in FISH Her-2/neu amplification outcomes. The differences in FISH Her-2/neu may also arise due to pre-analytical condition, variation in probes and individual interpretative reporting [23, 39].
Globally FISH Her-2/neu amplification in IHC equivocal (2+) BC patients have also shown wide range variation: China (29% to 70%); Germany (14% to 26%); USA (8%, 64%); Thailand (37.5%); Egypt (18%); Hungary (38%); Netherland (24%); Denmark (48%); Taiwan (53%); Australia (0%); Iran (31.1%) [20-36].
In the Asian population, BC has a younger age at presentation i.e. 40-50 years, a decade earlier as compared to Western countries [40]. In our study, median age of breast cancer patients was 48 years. Earlier studies found the median age of Her-2/neu IHC equivocal BC patients as follows: 50 years & 52 years (India) [16,14], 42.5 years (China) [20] and 63 years (Canada) [41]. We also found that 61.11% patients (n=99) were < 50 years while the rest 38.88% (n=63) were > 50 years. The differences in the median age may be attributed to the population characteristics and variations in risk factors.
We found histological grade II in 59.87% (n=97) of the BC patients as the most prevalent histological grade. Murthy et al (44/68, 70.5%) and Zhang et al (321/478, 67.2%) had also found histological grade II as the most prominent grade in Her-2/neu IHC (2+) BC patients [14, 20]. We also observed significant association between FISH Her-2/neu amplification and histological grade (p=0.036). Panjwani et al also found a significant association between histological grading and FISH Her-2/ neu amplification [17]. However, Mostafa et al found no correlation between histological grade and FISH Her-2/ neu amplification in IHC (2+) BC patients [30]. These differences in histological grade and FISH Her-2/neu amplification may be attributed to the differences in the biological characteristics of the tumor, methods used for IHC & FISH and patient cohort size.
Our data had shown “ER+/PR+” status (54.93%) as more predominant compared to “ER-/PR- ’’ (31.48%) which is also in concordance with Zhang et al report (“ER+/PR+” = 60.2%; “ER-/PR-’’= 28.4%) [20]. We also observed that ER and PR expression were higher in FISH Her- 2/neu amplified tumors compared to FISH Her-2/neu non-amplified tumors. We found significant association between ER-positive patients and FISH Her-2/neu amplified tumors (p=0.039). However, various Indian studies reported association between Her-2/neu amplification and negative hormone receptor expression [14-17]. Her-2 amplification and ER-positive tumors co-positivity is reported to be associated with resistance to selective ER modulators like tamoxifen therapy [42]. It is postulated that Her-2 cross-talk with ER could increase the estrogen agonistic activity of tamoxifen leading to enhanced tumor growth resulting in de novo resistance to tamoxifen [43, 44]. The observed differences in hormone receptor expression status may be associated to tumor’s biological profile and antibodies used for hormone receptor expression in IHC and probes used in FISH analysis.
But, we didn’t found any significant association between age (p=0.625), lymph node involvement (p=0.642), and laterality (p=0.367) with FISH Her-2/ neu amplification status. Ji et al also noted no significant difference between Her-2/neu status with respect to age and cancer location in IHC (2+) invasive breast cancer [22]. Zhang et al found no correlation between Her-2/neu expression and age (p=0.53) but in lymph node involvement, they found trends without statistical significance (p=0.072) [20].
To the best of our knowledge, this is the first study from Northeast India analysing FISH Her- 2/neu amplification in IHC Her-2 (2+) equivocal breast cancer patients. We had also observed that ER-positive patients have direct proportional relationship with FISH Her-2/neu amplification whereas other studies have reported inverse relationship with FISH Her-2/neu amplification in IHC Her-2 (2+) equivocal breast cancer patients. Limitations of our study are that it is a single tertiary cancer care centre data. Sample size was moderate.
In conclusion, we conclude that FISH Her-2/neu amplification in IHC Her-2 equivocal (2+) breast cancer patients of North-East India was found to be significantly associated with ER-positive status and histological grade. ER/PR positive breast cancer patients have an enhanced likelihood of FISH Her-2/neu amplification. To validate our current study observation, further studies with multicentre, larger sample cohort of Her-2 equivocal (2+) breast cancer patients will be required.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Global Cancer Observatory: Cancer Today Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. , Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al . Lyon, France: International Agency for Research on Cancer.2020. Available from: https://gco.iarc.fr/today, accessed on 20 June 2023.
- Breast cancer in India: Present scenario and the challenges ahead Mehrotra R, Yadav K. World Journal of Clinical Oncology.2022;13(3). CrossRef
- ICMR-NCDIR, Profile of Cancer and Related Health Indicators in the North East Region of India, Bengaluru, India 2021. https://ncdirindia.org/All_Reports/NorthEast2021/Default.aspx.
- Untangling the ErbB signalling network Yarden Y., Sliwkowski M. X.. Nature Reviews. Molecular Cell Biology.2001;2(2). CrossRef
- Localization of the pKs gene, a raf related sequence on human chromosomes X and 7 Popescu N. C., Mark G. E.. Oncogene.1989;4(4).
- The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis Moasser M. M.. Oncogene.2007;26(45). CrossRef
- HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation Ahn S, Woo JW , Lee K, Park SY . Journal of Pathology and Translational Medicine.2020;54(1). CrossRef
- 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial Smith I, Procter M, Gelber RD , Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, et al . Lancet (London, England).2007;369(9555). CrossRef
- Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Wolff AC , Hammond MEH , AllisonKH , Harvey BE , Mangu PB , Bartlett JMS , Bilous M, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2018;36(20). CrossRef
- Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Wolff AC , Hammond MEH , Allison KH , Harvey BE , Mangu PB , Bartlett JMS , Bilous M, et al . Archives of Pathology & Laboratory Medicine.2018;142(11). CrossRef
- Breast Carcinoma - A Comparative Study of Immunohistochemistry and Fluorescence in Situ Hybridization for Her-2 Assessment and Association of ER, PR, HER-2 and Ki-67 Expression with Clinico-Pathological Parameters K S, Arumugam M, Shetty J, Shetty RA , Asani R, Shetty PD . Iranian Journal of Pathology.2022;17(4). CrossRef
- Correlation between HER2 gene amplification and protein overexpression through fluorescence in situ hybridization and immunohistochemistry in breast carcinoma patients Makroo R. N., Chowdhry M, Kumar M, Srivastava P, Tyagi R, Bhadauria P, Kaul S, et al . Indian Journal of Pathology & Microbiology.2012;55(4). CrossRef
- Assessment of HER2/Neu status by fluorescence in situ hybridization in immunohistochemistry-equivocal cases of invasive ductal carcinoma and aberrant signal patterns: a study at a tertiary cancer center Murthy SS , Sandhya D. G., Ahmed F, Goud KI , Dayal M, Suseela K., Rajappa SJ . Indian Journal of Pathology & Microbiology.2011;54(3). CrossRef
- HER2/neu Testing In 432 Consecutive Breast Cancer Cases using FISH and IHC - A Comparative Study Eswarachary V, Mohammed IG , Jayanna PK , Patilokaly GV , Nargund AR , Dhondalay GK , Prabhudesai S, Sahoo R. Journal of clinical and diagnostic research: JCDR.2017;11(4). CrossRef
- FISH and HER2/neu equivocal immunohistochemistry in breast carcinoma Patil Okaly GV , Panwar D, Lingappa KB , Kumari P, Anand A, Kumar P, Chikkalingaiah MH , Kumar RV . Indian Journal of Cancer.2019;56(2). CrossRef
- Assessment of HER-2/neu status in breast cancer using fluorescence in situ hybridization & immunohistochemistry: Experience of a tertiary cancer referral centre in India Panjwani P, Epari S, Karpate A, Shirsat H, Rajsekharan P, Basak R, Shet T, et al . The Indian Journal of Medical Research.2010;132.
- Immunohistochemical (IHC) HER-2/neu and fluorescent-in situ hybridization (FISH) gene amplification of breast cancer in Indian women Singhai R, Patil V, Patil A. Asian Pacific journal of cancer prevention: APJCP.2011;12(1).
- Evaluation of HER-2/neu status in breast cancer specimens using immunohistochemistry (IHC) & fluorescence in-situ hybridization (FISH) assay Goud KI , Dayakar S, Vijayalaxmi K, Babu SJ , Reddy PVA . The Indian Journal of Medical Research.2012;135(3).
- HER-2 gene amplification by fluorescence in situ hybridization (FISH) compared with immunohistochemistry (IHC) in breast cancer: a study of 528 equivocal cases Zhang H, Ren G, Wang X, Zhao J, Yao H, Bai Y, Bo W. Breast Cancer Research and Treatment.2012;134(2). CrossRef
- HER-2 status and its clinicopathologic significance in breast cancer in patients from southwest China: re-evaluation of correlation between results from FISH and IHC Ruan S, Liu Y, Tang X, Yang Z, Huang J, Li X, Wu N, et al . International Journal of Clinical and Experimental Pathology.2017;10(7).
- Clinicopathological variables predicting HER-2 gene status in immunohistochemistry-equivocal (2+) invasive breast cancer Ji Y, Sheng L, Du X, Qiu G, Chen B, Wang X. Journal of Thoracic Disease.2014;6(7). CrossRef
- Comparison of immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assessment for Her-2 status in breast cancer Sui W, Ou M, Chen J, Wan Y, Peng H, Qi M, Huang H, Dai Y. World Journal of Surgical Oncology.2009;7. CrossRef
- Comprehensive immunohistochemical analysis of Her-2/neu oncoprotein overexpression in breast cancer: HercepTest (Dako) for manual testing and Her-2/neuTest 4B5 (Ventana) for Ventana BenchMark automatic staining system with correlation to results of fluorescence in situ hybridization (FISH) Mayr D, Heim S, Werhan C, Zeindl-Eberhart E, Kirchner T. Virchows Archiv: An International Journal of Pathology.2009;454(3). CrossRef
- HER-2/neu analysis in breast cancer bone metastases Zustin J., Boddin K., Tsourlakis M. C., Burandt E., Mirlacher M., Jaenicke F., Izbicki J., et al . Journal of Clinical Pathology.2009;62(6). CrossRef
- Chromogenic in situ hybridization for Her-2/neu-oncogene in breast cancer: comparison of a new dual-colour chromogenic in situ hybridization with immunohistochemistry and fluorescence in situ hybridization Mayr D, Heim S, Weyrauch K, Zeindl-Eberhart E, Kunz A, Engel J, Kirchner T. Histopathology.2009;55(6). CrossRef
- Strong HER-2/neu protein overexpression by immunohistochemistry often does not predict oncogene amplification by fluorescence in situ hybridization Hammock L, Lewis M, Phillips C, Cohen C. Human Pathology.2003;34(10). CrossRef
- Accurately assessing her-2/neu status in needle core biopsies of breast cancer patients in the era of neoadjuvant therapy: emerging questions and considerations addressed D'Alfonso T, Liu Y, Monni S, Rosen PP , Shin SJ . The American Journal of Surgical Pathology.2010;34(4). CrossRef
- The concordance between IHC and ISH for HER-2 testing in breast cancer in Nakhon Pathom Hospital, Thailand, based on the ASCO/CAP 2018 guidelines: a retrospective study Thambamroong T. Ecancermedicalscience.2022;16. CrossRef
- Assessment of Her-2/neu gene amplification status in breast carcinoma with equivocal 2+ Her-2/neu immunostaining Mostafa NAE , Eissa SS , Belal DM , Shoman SH . Journal of the Egyptian National Cancer Institute.2011;23(1). CrossRef
- Detection of HER-2/neu gene amplification in breast carcinomas using quantitative real-time PCR - a comparison with immunohistochemical and FISH results Kulka J, Tôkés A, Kaposi-Novák P, Udvarhelyi N, Keller A, Schaff Z. Pathology oncology research: POR.2006;12(4). CrossRef
- HER-2/neu amplification testing in breast cancer by multiplex ligation-dependent probe amplification in comparison with immunohistochemistry and in situ hybridization Moelans CB , Weger RA , Blokland MTM , Ezendam C, Elshof S, Tilanus MGJ , Diest PJ . Cellular Oncology: The Official Journal of the International Society for Cellular Oncology.2009;31(1). CrossRef
- Comparison of HercepTest™ mAb pharmDx (Dako Omnis, GE001) with Ventana PATHWAY anti-HER-2/neu (4B5) in breast cancer: correlation with HER2 amplification and HER2 low status Rüschoff J, Friedrich M, Nagelmeier I, Kirchner M, Andresen LM , Salomon K, Portier B, et al . Virchows Archiv: An International Journal of Pathology.2022;481(5). CrossRef
- Comparison of immunohistochemical and fluorescence in situ hybridization assessment for HER-2/neu status in Taiwanese breast cancer patients Kuo S, Wang BB , Chang C, Chen T, Yeh K, Lee D, Yin P, Chen M. Taiwanese Journal of Obstetrics & Gynecology.2007;46(2). CrossRef
- Suggestions for HER-2/neu testing in breast carcinoma, based on a comparison of immunohistochemistry and fluorescence in situ hybridisation Field A. S., Chamberlain N. L., Tran D., Morey A. L.. Pathology.2001;33(3).
- Is There any Concordance between of IHC with FISH in HER2-Positive Breast Cancer Patients? Payandeh M, Sadeghi M, Sadeghi E, Janbakhsh A. International Journal of Hematology-Oncology and Stem Cell Research.2017;11(1).
- Chromogenic in situ hybridization is a reliable method for detecting HER2 gene status in breast cancer: a multicenter study using conventional scoring criteria and the new ASCO/CAP recommendations Gong Y, Sweet W, Duh Y, Greenfield L, Fang Y, Zhao J, Tarco E, et al . American Journal of Clinical Pathology.2009;131(4). CrossRef
- Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 Slamon D. J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., et al . The New England Journal of Medicine.2001;344(11). CrossRef
- HER 2 status in invasive breast cancer: immunohistochemistry, fluorescence in-situ hybridization and chromogenic in-situ hybridization Shirsat HS , Epari S, Shet T, Bagal R, Hawaldar R, Desai SB . Indian Journal of Pathology & Microbiology.2012;55(2). CrossRef
- Breast cancer in the world: incidence and mortality Curado MP . Salud Publica De Mexico.2011;53(5).
- Assessment of HER2 using the 2018 ASCO/CAP guideline update for invasive breast cancer: a critical look at cases classified as HER2 2+ by immunohistochemistry Taylor VJ , Barnes PJ , Godwin SC , Bethune GC . Virchows Archiv: An International Journal of Pathology.2021;479(1). CrossRef
- C-erbB-2 oncoprotein overexpression identifies a subgroup of estrogen receptor positive (ER+) breast cancer patients with poor prognosis Pinto A. E., André S., Pereira T., Nóbrega S., Soares J.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2001;12(4). CrossRef
- Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk Massarweh S, Schiff R. Endocrine-Related Cancer.2006;13 Suppl 1. CrossRef
- Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer Shou J, Massarweh S, Osborne CK , Wakeling AE , Ali S, Weiss H, Schiff R. Journal of the National Cancer Institute.2004;96(12). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times