Diagnostic Lymphocytosis as a Favorable Prognostic Factor in Childhood Acute Myeloblastic Leukemia
Download
Abstract
Objective: Childhood acute myeloblastic leukemia (AML) has been associated with an unfavorable prognosis, particularly in lower-middle-income countries (LMICs). The limited resources for cytogenetic-based risk stratification in many LMICs constitute the contributing factors. To improve the outcomes of childhood AML, a better understanding of its pathogenesis, which further leads to the development of applicable risk stratification criteria, is required. Studies on adult AML linked lymphocytosis due to increased regulatory T-cells (Tregs) in the bone marrow stroma with a higher risk of remission failure and an early relapse. Since Treg population is influenced by age-dependent bacterial colonization, conducting a study on children with AML is reasonable.
Methods: A retrospective cohort study involved 60 patients younger than 18 years with non-M3 AML, 25 of whom had diagnostic absolute lymphocyte counts (ALC0) of less than 4.7 x 109 cells/L and 35 of whom had ALC0 of more than 4.7 x 109 cells/L. These patients were observed for the occurrence of events, which consisted of remission failure, relapse, and death, within five years of treatment.
Results: Patients with ALC0 of more than 4.7 x 109 cells/L had the higher five-year event-free (EFS, 24% vs. 0%; p = 0.01) and overall survivals (OS, 31% vs. 10%; p = 0.02). Cox regression analysis demonstrated that ALC0 of less than 4.7 x 109 cells/L was an independent prognostic factor for the lower five-year EFS (hazard ratio [HR], 3.5; 95% confidence interval [95% CI], 1.7 – 7.5; p < 0.01) and OS (HR, 2.1; 95% CI, 1.1 – 4.0; p = 0.03).
Conclusion: In contrast to studies in adults, our study showed a correlation between diagnostic lymphocytosis and higher five-year EFS and OS.
Introduction
Acute myeloblastic leukemia (AML) is the second most prevalent childhood hematopoietic neoplasm throughout the world. Several studies in lower-middle- (LMICs) and high-income countries (HICs) reported annual incidences of 7.1 – 10.9 per million from 1993 to 2010 [1-4] with an average global increment of 3.2% per year [5]. Despite their comparable number of new cases each year, survival gaps between LMICs and HICs remain challenging. A study in the United States, which applied allogeneic hematopoietic stem cell transplantation (HSCT) in selected patients, revealed a five-year event-free survival (EFS), overall survival (OS), and treatment-related mortality (TRM) of 62%, 71%, and 15%, respectively [6]. Nevertheless, in the absence of allogeneic HSCT, our previous study disclosed a higher TRM of 52% in the second year of treatment, which resulted in a lower EFS of 9% and OS of 16% (unpublished data). It is hypothesized that the absence of a risk-adapted treatment protocol, as a result of the lack of applicable karyotype-based risk stratification criteria, constitutes one of the contributing factors to the unfavorable outcomes of childhood AML in several LMICs.
The interaction of leukemic myeloblasts with the surrounding bone marrow stroma and their role in the pathogenesis of AML have widely been described. The leukemic myeloblasts and bone marrow mesenchymal stem cells (MSCs) secrete indoleamine 2,3-dioxygenase (IDO) and arginase, which induce the maturation of CD4+ CD25- into the active CD4+ CD25+ regulatory T-cells (Tregs) and proliferation of myeloid-derived suppressor cells [7]. Moreover, CD8+ cytotoxic T-cells (TC) of patients with AML overexpress the inhibitory programmed cell death 1 (PD1), T-cell immunoglobulin and mucin-domain containing-3 (TIM3), and lymphocyte-activating gene 3 (LAG3) proteins [8, 9]. The simultaneous proliferation of Tregs and other suppressor cells and inhibition of TC-mediated cytotoxicity indicate the contribution of immune evasion strategies, which are demonstrated by the leukemic myeloblasts and their microenvironment, to developing progressive disease.
The dysregulation of effector and suppressor cells may affect the number of circulating lymphocytes and provide insight into the application of lymphocyte counts in risk stratification. A study on 54 children with AML in the United States associated the peripheral blood absolute lymphocyte counts on day 15 of treatment (ALC15) of less than 0.4 x 109 cells/L with the lower five-year relapse-free survival (RFS) and OS [10]. This study, however, did not anticipate the occurrence of death within the first 15 days of treatment as a result of severe malnutrition [11] and infection [12, 13]. A question on the prognostic role of diagnostic ALC (ALC0) is raised to address these issues. The studies of Bar et al. [14] and Le Jeune et al. [15], which involved adults with AML in the United States and France, respectively, found an association between ALC0 of more than 4.5 – 4.8 x 109 cells/L and unfavorable outcomes in terms of remission failure, five-year RFS, and OS. Since childhood AML shows distinctive biological features compared to adult AML [16] and the Treg population is influenced by age-dependent bacterial colonization [17], it is obligatory to validate these findings in the population of pediatric patients.
Materials and Methods
Study design
A medical record-based retrospective cohort study was conducted on a population of patients younger than 18 years with non-M3 AML according to the French American British (FAB) classification system. These patients were admitted to the Pediatric Hematology-Oncology Division of Dr. Sardjito General Hospital from 2011 – 2019. The diagnosis of AML was confirmed by the presence of myeloid-lineage blasts, which were stained with Sudan Black B and expressed cytoplasmic myeloperoxidase (MPO), in more than 20% of bone marrow nucleated cells [18]. Patients with Down syndrome, secondary AML, and mixed phenotypic leukemia were excluded from our study. Our study protocol has been approved by the Medical and Health Research Ethics Committee of Universitas Gadjah Mada and Dr. Sardjito General Hospital.
Basic hematological examination
The diagnostic white blood cell (WBC), peripheral blood lymphocyte, and blast counts were obtained during the initial hospital admission. An XN-1000 (Sysmex, Kobe, Japan) and a Cell-Dyn Ruby (Abbott, Chicago, IL) hematology analyzers were used to assess the complete blood counts, while microscopic examination of the Wright-stained (Merck, Darmstadt, Germany) peripheral blood smears was performed to confirm the percentage of lymphocytes and blasts. ALC and the absolute blast counts were determined by multiplying WBC with the percentage of lymphocytes and blasts, respectively.
Treatment protocols
Patients with a confirmed diagnosis of non-M3 AML received the National Pilot Protocol of Indonesian Childhood AML (2011 – 2016), the modified low-intensity protocol (2016 – 2018), and the modified International Society of Pediatric Oncology – Pediatric Oncology in Developing Countries Committee (SIOP PODC) protocol (2018 – 2019). The first remission-induction phase of these protocols consisted of intravenous daunorubicin (National Pilot Protocol and modified low-intensity protocol, 50 mg/m2/day on days 1, 3, and 5; modified SIOP PODC protocol, 25 mg/m2/day on days 1, 3, and 5) and cytarabine (National Pilot Protocol, 100 mg/ m2/12 hours on days 1 – 5; modified low-intensity and SIOP PODC protocols, 10 mg/m2/12 hours on days 1 –10). The age-adjusted dose of intrathecal methotrexate was administered on the first day of the National Pilot Protocol. In the absence of hyperleukocytosis, subcutaneous granulocyte-colony stimulating factor (5 μg/kg/day on days 1 – 10) was given by the modified low-intensity and SIOP PODC protocols. The modified SIOP PODC protocol also inserted a pre-induction phase of intravenous etoposide (37.5 mg/m2/day on days 1 – 7), oral 6-mercaptopurine (50 mg/m2/day on days 1 – 21), and prednisone (40 mg/m2/day on days 1 – 14).
The second remission-induction phase with the same regimens was commenced on day 29 in patients receiving the National Pilot Protocol and the modified low-intensity protocol, irrespective of their remission status. In contrast, patients in the modified SIOP PODC protocol were stratified at the end of the first remission-induction phase. Patients showing complete (CR) or partial remission (PR) status received the same regimens. At the same time, the higher dose of daunorubicin (50 mg/m2/day on days 1, 3, and 5) and cytarabine (100 mg/m2/12 hours on days 1 – 7) were given to those who failed to achieve CR or PR. The high-dose cytarabine (National Pilot Protocol, 1 g/m2/day; modified low-intensity protocol, 2 – 3 g/m2/12 hours; modified SIOP PODC protocol, 1.5 g/m2/12 hours) constituted the backbone of the consolidation phase.
Treatment outcomes and statistical analysis
Each eligible patient was observed for events that consisted of remission failure, relapse, and death within five years of treatment. CR was defined as reducing bone marrow myeloid-lineage blasts toward less than 5% at the end of the first remission-induction phase. In comparison, patients with bone marrow blast counts of 6 – 15% were classified as PR and considered as achieving remission by the study of Fleming et al. [19]. Patients with CR and PR status later showed bone marrow blast counts of more than 20% were classified as relapse.
0 A linear regression analysis was performed to determine whether ALC0 correlates with the diagnostic blast counts and WBC. Kaplan-Meier survival analysis and log-rank test were performed to compare the EFS and OS between patients with ALC0 of more than 4.7 x 109 cells/L and those with ALC of less than 4.7 x 109 cells/L. Cox regression analysis was then performed to determine the hazard ratio (HR) of remission failure, relapse, and death within five years of treatment in patients with ALC0 of less than 4.7 x 109 cells/L. All collected data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 27 software (IBM, Armonk, NY).
Results
Patient characteristics
Sixty-nine patients with accessible medical record data were enrolled in our study. Nine patients were excluded because of the previous diagnosis of myelodysplastic syndrome (n = 1), chronic myeloid leukemia (n = 1), and the presence of the morphological, cytochemical, and immunophenotypic features of mixed phenotypic leukemia (n = 7). Our study, therefore, analyzed 60 patients consisting of 25 patients with ALC0 of less than 0 4.7 x 109 cells/L and 35 patients with ALC of more than 4.7 x 109 cells/L. Of these 60 patients, 34 (57%) were male, 25 (42%) were older than ten years, and 18 (30%) were morphologically classified as AML-M4. In terms of treatment protocols, 51 (85%) patients received intravenous daunorubicin and cytarabine at the beginning of treatment (National Pilot Protocol, n = 45; the modified low-intensity protocol, n = 6), while nine (15%) patients received the pre-induction cytostatics according to the modified SIOP PODC protocol (Table 1). Ten patients were classified as drop-outs in the pre-induction and induction phases (n = 5) and after the achievement of remission (n = 5). These patients were included in the analysis and considered as experiencing no event until the last date of contact. There was no significant difference between patients with CR and PR on the five-year EFS (p = 0.88; data not shown) and OS (p = 0.57; data not shown).
Correlation of ALC0 with the diagnostic blast counts and WBC
The descriptive analysis of diagnostic complete blood counts revealed the median blast counts and WBC of 12.9 (interquartile range [IQR], 3.5 – 43.9) and 30.2 (IQR, 13.5 – 70.0) x 109 cells/L, respectively. Twenty-four (40%) patients were classified as having blast counts of more than 19 x 109 cells/L, while 21 (35%) patients had a WBC of more than 50 x 109 cells/L (Table 1).
| Characteristics | ALC 0 <4.7 x 10 cells/L 9 | ALC 0 ≥ 4.7 x 10 cells/L 9 | Total |
| n = 25 | n = 35 | n = 60 | |
| Sex | |||
| Male (%) | 14 (56.0) | 20 (57.1) | 34 (56.7) |
| Female (%) | 11 (44.0) | 15 (42.9) | 26 (43.3) |
| Median age at diagnosis in years (IQR) | 9.9 (6.7 – 14.2) | 7.6 (2.2 – 11.8) | 8.8 (3.4 – 13.0) |
| Age < 10 years (%) | 13 (52.0) | 22 (62.9) | 35 (58.3) |
| Age ≥ 10 years (%) | 12 (48.0) | 13 (37.1) | 25 (41.7) |
| FAB morphological subtypes | |||
| M0 (%) | 1 (4.0) | 2 (5.7) | 3 (5.0) |
| M1 (%) | 4 (16.0) | 5 (14.3) | 9 (15.0) |
| M2 (%) | 5 (20.0) | 7 (20.0) | 12 (20.0) |
| M4 (%) | 7 (28.0) | 11 (31.4) | 18 (30.0) |
| M5 (%) | 3 (12.0) | 6 (17.1) | 9 (15.0) |
| M6 (%) | 1 (4.0) | 1 (2.9) | 2 (3.3) |
| M7 (%) | 4 (16.0) | 3 (8.6) | 7 (11.7) |
| Median diagnostic blast counts in 10 9 cells/L (IQR) | 7.9 (0.5 – 23.7) | 20.2 (8.4 – 91.2) | 12.9 (3.5 – 43.9) |
| Blasts < 19 x 10 9 cells/L (%) | 19 (76.0) | 17 (48.6) | 36 (60.0) |
| Blasts ≥ 19 x 10 9 cells/L (%) | 6 (24.0) | 18 (51.4) | 24 (40.0) |
| Median diagnostic WBC in 10 9 cells/L (IQR) | 13.8 (5.3 – 36.5) | 46.6 (26.5 – 133.6) | 30.2 (13.5 – 70.0) |
| WBC < 50 x 10 9 cells/L (%) | 21 (84.0) | 18 (51.4) | 39 (65.0) |
| WBC ≥ 50 x 10 9 cells/L (%) | 4 (16.0) | 17 (48.6) | 21 (35.0) |
| Treatment protocols | |||
| National Pilot Protocol (%) | 19 (76.0) | 26 (74.3) | 45 (75.0) |
| Modified low intensity (%) | 2 (8.0) | 4 (11.4) | 6 (10.0) |
| Modified SIOP PODC (%) | 4 (16.0) | 5 (14.3) | 9 (15.0) |
ALC0, diagnostic absolute lymphocyte counts; FAB, French American British; IQR, interquartile range; SIOP PODC, International Society of Pediatric Oncology – Pediatric Oncology in Developing Countries Committee; WBC, white blood cell counts
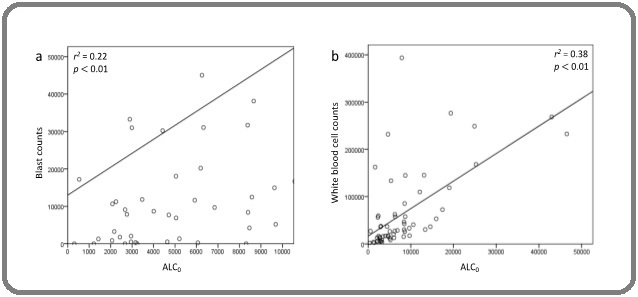
Linear regression analysis demonstrated a weak correlation of ALC0 with the blast counts (r2 = 0.22; p < 0.01; Figure 1a) and WBC (r2 = 0.38; p < 0.01; Figure 1b).
Figure 1. Correlation of ALC0 with the Diagnostic Blast Counts (a) and WBC (b).
ALC0-based treatment outcomes
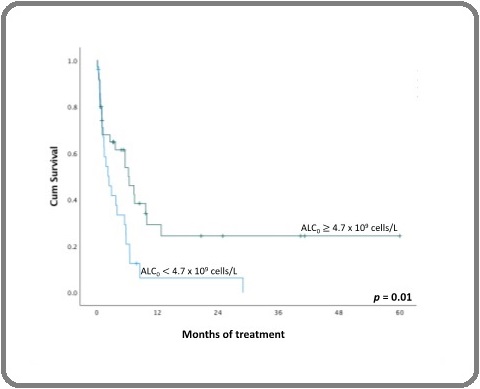
Forty-five (75%) of 60 patients experienced their first events within five years of treatment. Four (7%) patients failed to achieve remission, 17 (28%) patients developed a relapsed disease, and 24 (40%) patients died. In a survival analysis, patients with ALC of more than 4.7 x 109 cells/L had a higher EFS (24% vs. 0%; p = 0.01; Figure 2).
Figure 2. Five-year EFS of Patients with ALC0 of more than 4.7 x 109 Cells/L (green line) and Less than 4.7 x 109 Cells/L (blue line). ALC , Diagnostic Absolute Lymphocyte Counts.
Moreover, a univariate Cox regression analysis showed that patients with ALC0 of less than 4.7 x 109 cells/L had a higher risk of remission failure, relapse, and death (HR, 2.1; 95% confidence interval [CI], 1.2 – 3.8; Table 2). The multivariate analysis, which also involved the diagnostic blast counts and treatment protocols, revealed the role of ALC0 of less than 4.7 x 109 cells/L as an independent prognostic factor for these events (HR, 3.5; 95% CI, 1.7 – 7.5; Table 2).
| Variables | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p |
| ALC 0 | ||||
| < 4.7 x 10 9 cells/L | 2.1 (1.2 – 3.8) | 0.02 | 3.5 (1.7 – 7.5) | 0.01 |
| ≥ 4.7 x 10 9 cells/L | 1 | 1 | ||
| Sex | ||||
| Male | 0.7 (0.4 – 1.3) | 0.32 | ||
| Female | 1 | |||
| Age at diagnosis | ||||
| < 10 years | 1 | |||
| ≥ 10 years | 1.1 (0.6 – 2.0) | 0.77 | ||
| Diagnostic blast counts | ||||
| < 19 x 10 9 cells/L | 1 | 1 | ||
| ≥ 19 x 10 9 cells/L | 1.5 (0.8 – 2.6) | 0.21 | 2.7 (1.3 – 5.7) | 0.01 |
| Diagnostic WBC | ||||
| <50 x 10 9 cells/L | 1 | |||
| ≥ 50 x 10 9 cells/L | 1.0 (0.6 – 1.9) | 0.91 | ||
| Treatment protocols | ||||
| National Pilot Protocol | 1 | 1 | ||
| Modified low intensity | 1.0 (0.3 – 2.7) | 0.96 | 1.4 (0.5 – 4.1) | 0.54 |
| Modified SIOP PODC | 1.7 (0.7 – 3.8) | 0.23 | 1.6 (0.7 – 3.6) | 0.3 |
ALC0, diagnostic absolute lymphocyte counts; CI, confidence interval; HR, hazard ratio; SIOP PODC, International Society of Pediatric Oncology – Pediatric Oncology in Developing Countries Committee; WBC, white blood cell counts
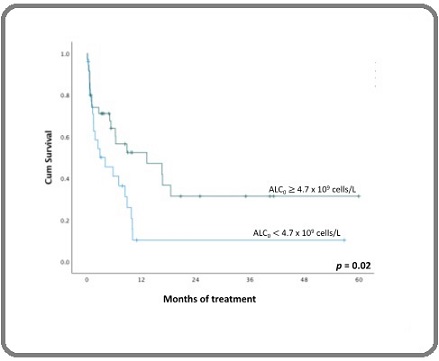
Thirty-nine (65%) of 60 patients died of progressive disease and the adverse effects of cytostatics within five years of treatment, irrespective of their remission and relapse status. The descriptive analysis revealed the median time of death of 2.4 (IQR, 0.6 – 8.5) months after the initiation of treatment. Kaplan-Meier survival analysis and log-rank test showed that 90% of patients with ALC0 of less than 4.7 x 109 cells/L and 69% of patients with ALC of more than 4.7 x 109 cells/L died during these five years (p = 0.02; Figure 3).
Figure 3. Five-year OS of Patients with ALC0 of more than 4.7 x 109 Cells/L (green line) and Less than 4.7 x 109 Cells/L (blue line). ALC , Diagnostic Absolute Lymphocyte Counts.
A univariate Cox regression analysis showed an increased risk of death in patients with ALC0 of less than 4.7 x 109 cells/L (HR, 2.1; 95% CI, 1.1 – 4.0; Table 3).
| Variables | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p |
| ALC 0 | ||||
| < 4.7 x 10 9 cells/L | 2.1 (1.1 – 4.0) | 0.03 | 2.1 (1.1 – 4.0) | 0.03 |
| ≥ 4.7 x 10 9 cells/L | 1 | 1 | ||
| Sex | ||||
| Male | 0.7 (0.4 – 1.3) | 0.23 | 0.8 (0.4 – 1.5) | 0.46 |
| Female | 1 | 1 | ||
| Age at diagnosis | ||||
| 10 years | 1 | |||
| 10 years | 0.9 (0.5 – 1.8) | 0.81 | ||
| Diagnostic blast counts | ||||
| < 19 x 10 9 cells/L | 1 | |||
| ≥ 19 x 10 9 cells/L | 1.4 (0.7 – 2.6) | 0.32 | ||
| Diagnostic WBC | ||||
| < 50 x 10 9 cells/L | 1 | |||
| ≥ 50 x 10 9 cells/L | 0.7 (0.4 – 1.5) | 0.38 | ||
| Treatment protocols | ||||
| National Pilot Protocol | 1 | 1 | ||
| Modified low intensity | 0.6 (0.2 – 2.1) | 0.47 | 0.6 (0.2 – 2.1) | 0.46 |
| Modified SIOP PODC | 2.4 (1.0 – 5.5) | 0.05 | 2.0 (0.8 – 5.0) | 0.13 |
ALC0, diagnostic absolute lymphocyte counts; CI, confidence interval; HR, hazard ratio; SIOP PODC, International Society of Pediatric Oncology – Pediatric Oncology in Developing Countries Committee; WBC, white blood cell counts
This association also reached a statistical significance when the multivariate analysis was performed (HR, 2.4; 95% CI, 1.1 – 5.2; Table 3).
Discussion
Our study found a weak but statistically significant correlation of ALC0 with the diagnostic blast counts and WBC. There was also an association of ALC0 of more than 4.7 x 109 cells/L with the higher five-year EFS and OS. Our study, therefore, showed the conflicting roles of lymphocytes in the pathogenesis of childhood AML, in which lymphocytosis was linked to an increasing leukemic cell burden and favorable outcomes. While there are limited reports on the correlation of ALC0 with blast counts and WBC, our findings contrasted with those of adult AML, which associated remission failure, an earlier onset of relapse, the lower five-year RFS, and OS with ALC0 of more than 4.5 – 4.8 x 109 cells/L [14, 15]. Despite different cut-off points of ALC and time of examination, the results of our study were similar to those involving children with cancers. In a study involving 54 children with AML in the United States, patients with ALC15 of less than 0.4 x 109 cells/L had a higher risk of relapse and death within five years [10], while another study on 100 Peruvian children with musculoskeletal tumors reported that patients with ALC0 of less than 1 x 109 cells/L and ALC15 of less than 0.8 x 109 cells/L showed a lower five-year OS [20].
The association of lymphocytosis with the higher blast counts and WBC indicated the predominant role of Tregs at the time of diagnosis. Several previous studies correlated an increasing number of these suppressor cells with the colonization of nasopharyngeal otopathogens [17] that was more prevalent in children [21]. Our findings, however, conflicted with the previous reports on IDO-induced immune suppression. Folgiero et al. [22] reported that none of 37 children with AML constitutively expressed IDO protein and 49% of patients did not upregulate IDO expression in response to interferon (IFN)-γ. The low expression levels of IDO in children with AML might be specific to certain cytogenetic features of leukemic blasts [22], which were not routinely examined in our patients. Our findings and others [23] might indicate the predominant role of arginase, which was secreted by the leukemic blasts, irrespective of the ages and cytogenetic features. Nevertheless, since the observed events (remission failure, relapse, and death) occurred after the initiation of treatment protocols, our findings might also provide insight into the synergistic effects of TC- and natural killer (NK) cell-mediated cytotoxicity and cytostatic treatment in childhood AML. In a population of children with acute lymphoblastic leukemia (ALL), Bhattacharya et al. [24] reported the cytostatic-induced suppression of forkhead box P3 (FoxP3) expression and interleukin (IL)-10 synthesis by Tregs, which might further augment the TC- and NK cell-mediated cytotoxicity [25]. Our study found that 40% of patients experienced death as their first event, with a median of nine weeks after the initiation of treatment protocols. Despite the application of three different treatment protocols, our study showed that these patients died after achieving CR, which might indicate the treatment-related causes of death. Infection constitutes one of the significant challenges in the management of children with cancers in several LMICs. Our previous studies revealed that 43% of deaths in children with ALL [26] and 52% of deaths in children with AML (unpublished data) were preceded by clinically and microbiologically documented infections. Our results were similar to those reported in several studies which involved adults with AML. In those studies, the administration of high-dose cytarabine during the consolidation phase of treatment increased the risk of bacteremia and fungemia [27–29]. Despite lacked supportive data, it is reasonable to correlate ALC0 of less than 4.7 x 109 0 cells/L with a higher risk of infection Several studies involving children [30] and adults [31–33] with solid tumors revealed that patients with grade III lymphopenia had a higher risk of febrile neutropenia during treatment than those with grade I – II lymphopenia.
As a conclusion, our study showed the association of ALC0 of more than 4.7 x 109 cells/L with the higher five-year EFS and OS. These findings provide insight into the application of diagnostic lymphocyte counts in the risk stratification of childhood AML in pediatric oncology centers with limited resources, where cytogenetic and molecular analyses are not routinely performed. The heterogeneous nature of treatment protocols applied and treatment adherence might affect the Kaplan-Meier survival analysis. Nevertheless, the multivariate Cox regression analysis, which involved treatment protocols, demonstrated the independent role of ALC0 as a prognostic factor of childhood AML. Further studies are required to confirm the dysregulation of immune effector and regulatory cells.
Acknowledgements
General: The authors wish to thank Dr. Tri Ratnaningsih for providing hematologic data and Dr. Pudjo H. Widjajanto, Dr. Sri Mulatsih, Dr. Alexandra
W.S. Pangarso, Mr. Ignatius Purwanto, and Mrs. Naufal F. Azizah for their assistance in performing survival analysis.
Funding Statement
Not applicable
Approval
Not applicable
Conflict of Interest
Nothing to declare
Ethical declaration
The protocol of this study has been approved by the Medical and Health Research Ethics Committee of Universitas Gadjah Mada and Dr. Sardjito General Hospital.
Authors’ contribution
BA, RT, and SS designed the study, analyzed the data, and prepared the manuscript; CC and IA collected the data; ES performed survival analysis.
References
- Incidence of childhood leukemia in Yogyakarta, Indonesia, 1998-2009 Supriyadi E, Widjajanto PH , Purwanto I, Cloos J, Veerman AJP , Sutaryo S. Pediatric Blood & Cancer.2011;57(4). CrossRef
- Incidence of cancer in children aged 0-14 years in Taiwan, 1996-2010 Liu Y, Lo W, Chiang C, Yang Y, Lu M, Hsu W, Ho W, et al . Cancer Epidemiology.2015;39(1). CrossRef
- Incidence and survival of childhood leukemia in Recife, Brazil: A population-based analysis Lins MM , Santos MDO , AlbuquerqueMDFPM , Castro CCL , Mello MLG , Camargo B. Pediatric Blood & Cancer.2017;64(8). CrossRef
- Childhood cancer incidence and survival in Japan and England: A population-based study (1993-2010) Nakata K, Ito Y, Magadi W, Bonaventure A, Stiller CA , Katanoda K, Matsuda T, et al . Cancer Science.2018;109(2). CrossRef
- The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017 Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. Journal of Hematology & Oncology.2020;13(1). CrossRef
- Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials Alexander TB , Wang L, Inaba H, Triplett BM , Pounds S, Ribeiro RC , Pui C, Rubnitz JE . Cancer.2017;123(19). CrossRef
- The role of the immunosuppressive microenvironment in acute myeloid leukemia development and treatment Isidori A, Salvestrini V, Ciciarello M, Loscocco F, Visani G, Parisi S, Lecciso M, et al . Expert Review of Hematology.2014;7(6). CrossRef
- Assessment of the presence and anti-tumor potential of tumor-infiltrating lymphocytes in patients with acute myeloid leukemia Wei L, Wang Z, Zhang Z, Li Y, Fan S, Zhao Y, Liu Z, et al . Cancer Management and Research.2019;11. CrossRef
- The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia Williams P, Basu S, Garcia-Manero G, Hourigan CS , Oetjen KA , Cortes JE , Ravandi F, et al . Cancer.2019;125(9). CrossRef
- Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies De Angulo G, Yuen C, Palla SL , Anderson PM , Zweidler-McKay PA . Cancer.2008;112(2). CrossRef
- Causes of early death and treatment-related death in newly diagnosed pediatric acute myeloid leukemia: Recent experiences of the Dutch Childhood Oncology Group Klein K, Litsenburg RRL , Haas V, Dors N, Heuvel-Eibrink MM , Knops RRG , Tissing WJE , et al . Pediatric Blood & Cancer.2020;67(4). CrossRef
- Early deaths in pediatric acute leukemia: a population-based study Cheng S, Pole JD , Sung L. Leukemia & Lymphoma.2014;55(7). CrossRef
- Early deaths in pediatric acute leukemia: A major challenge in developing countries Hafez HA , Soliaman RM , Bilal D, Hashem M, Shalaby LM . J Pediatr Hematol Oncol.2019;41(4):261-266. CrossRef
- Elevated lymphocyte count at time of acute myeloid leukemia diagnosis is associated with shorter remission Bar M, Othus M, Park HM , Sandhu V, Chen X, Wood BL , Estey E. Leukemia & Lymphoma.2015;56(11). CrossRef
- Initial absolute lymphocyte count as a prognostic factor for outcome in acute myeloid leukemia Le Jeune C, Bertoli S, Elhamri M, Vergez F, Borel C, Huguet F, Michallet M, et al . Leukemia & Lymphoma.2014;55(4). CrossRef
- Acute myeloid leukemia in adolescents and young adults: challenging aspects Ofran Y, Rowe JM . Acta Haematologica.2014;132(3-4). CrossRef
- Regulatory T lymphocytes are associated with increased nasopharyngeal colonization in children Browne JJ , Matthews EH , Taylor-Robinson AW , Kyd JN . International Journal of Pediatric Otorhinolaryngology.2019;120. CrossRef
- Immunophenotypic patterns of childhood acute leukemias in Indonesia Supriyadi E, Widjajanto PH , Veerman AJ , Purwanto I, Nency YM , Gunawan S, Nafianti S, et al . Asian Pacific journal of cancer prevention: APJCP.2011;12(12).
- Partial response after induction chemotherapy has clinical relevance in acute myeloid leukaemia Fleming S, Ong DM , Jackson K, Avery S, Mollee P, Marlton P, Kennedy G, Wei AH . British Journal of Haematology.2017;177(2). CrossRef
- Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas Vasquez L, León E, Beltran B, Maza I, Oscanoa M, Geronimo J. Journal of Pediatric Hematology/Oncology.2017;39(7). CrossRef
- Impact of age on pneumococcal colonization of the nasopharynx and oral cavity: an ecological perspective Miellet WR , Mariman R, Veldhuizen J, Badoux P, Wijmenga-Monsuur AJ , Litt D, Bosch T, et al . ISME communications.2024;4(1). CrossRef
- Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia Folgiero V, Goffredo BM , Filippini P, Masetti R, Bonanno G, Caruso R, Bertaina V, et al . Oncotarget.2014;5(8). CrossRef
- Arginine dependence of acute myeloid leukemia blast proliferation: a novel therapeutic target Mussai F, Egan S, Higginbotham-Jones J, Perry T, Beggs A, Odintsova E, Loke J, et al . Blood.2015;125(15). CrossRef
- Critical stoichiometric ratio of CD4(+) CD25(+) FoxP3(+) regulatory T cells and CD4(+) CD25(-) responder T cells influence immunosuppression in patients with B-cell acute lymphoblastic leukaemia Bhattacharya K, Chandra S, Mandal C. Immunology.2014;142(1). CrossRef
- Regulatory T Cells in Kidney Transplantation: New Directions? Braza F., Durand M., Degauque N., Brouard S.. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons.2015;15(9). CrossRef
- Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia Widjajanto PH , Sumadiono S, Cloos J, Purwanto I, Sutaryo S, Veerman AJ . Journal of Blood Medicine.2013;4. CrossRef
- Infectious complications in adults undergoing intensive chemotherapy for acute myeloid leukemia in 2001-2005 using the Japan Adult Leukemia Study Group AML201 protocols Kato H, Fujita H, Akiyama N, Kimura S, Hiramoto N, Hosono N, Takahashi T, et al . Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2018;26(12). CrossRef
- Influence of chemotherapy courses on the rate of bloodstream infections during neutropenia in adult acute myeloid leukaemia Kinnunen U, Koistinen P, Ohtonen P, Koskela M, Syrjälä H. Scandinavian Journal of Infectious Diseases.2008;40(8). CrossRef
- A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, Sakura T, et al . Blood.2011;117(8). CrossRef
- Which one is a risk factor for chemotherapy-induced febrile neutropenia in childhood solid tumors: early lymphopenia or monocytopenia? Oguz A, Karadeniz C, Ckitak EC , Cil V. Pediatric Hematology and Oncology.2006;23(2). CrossRef
- Early lymphopenia as a risk factor for chemotherapy-induced febrile neutropenia Choi CW , Sung HJ , Park KH , Yoon SY , Kim SJ , Oh SC , Seo JH , et al . American Journal of Hematology.2003;73(4). CrossRef
- Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy Ray-Coquard I., Borg C., Bachelot Th, Sebban C., Philip I., Clapisson G., Le Cesne A., et al . British Journal of Cancer.2003;88(2). CrossRef
- Risk factors for febrile neutropenia in patients receiving docetaxel chemotherapy for castration-resistant prostate cancer Shiota M, Yokomizo A, Takeuchi A, Kiyoshima K, Inokuchi J, Tatsugami K, Naito S. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer.2014;22(12). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times