Promise and Pitfalls of Human Papilloma Virus Vaccine: An Updated Review
Download
Abstract
The human papilloma virus (HPV) is a primary cause of cervical cancer which is a global health concern. Even though there is a lot of evidence that HPV vaccinations prevent diseases linked to HPV, there are also many unanswered questions about the vaccines. Moreover, the high incidence of HPV-related malignancies highlights serious gaps in the vaccination coverage and education. The unequal distribution of vaccination programs is one of the main reasons why the prevalence of HPV-related malignancies remains high. Many low- and middle-income nations find it difficult to establish and maintain HPV vaccination programs, despite the fact that certain high-income countries have successfully reduced the prevalence of HPV infections via intense vaccination efforts. Due to lack of infrastructure, logistics, and resources, as well as restricted access to healthcare, vaccination rates in poor developing countries continue to fall considerably short of what is required to establish herd immunity. The issue is made worse by inadequate healthcare systems, such as the absence of school-based immunization programs and challenges in reaching rural communities. However, both men and women are at risk of acquiring avoidable malignancies in nations with low vaccination rates, contributing to the global health imbalance. To increase the efficacy of the HPV vaccination, suggestions are made for raising public awareness, expanding accessibility, and doing long-term research.
Introduction
A family of more than 200 viruses known as the Human Papilloma virus (HPV) affects both men and women’s mouths, throats, and genital regions. Over 40 of them are known to spread during intercourse. Based on the virus’s propensity to cause cancer, it is divided into high-risk and low-risk varieties. Cervical cancer, the fourth most frequent disease in women worldwide, is most closely associated with high-risk HPV strains, especially HPV 16 and HPV 18. HPV is linked to malignancies of the anus, oropharynx, vulva, vagina, and penis in addition to cervical cancer [1]. Research indicates that almost all occurrences of cervical cancer are caused by HPV infection, with 70% of cases occurring worldwide [2]. Because HPV is linked to cancer, preventing it has become a top public health goal.
An important advancement in cancer prevention has been made with the release of the HPV vaccine. High-risk HPV strains are the main focus of HPV vaccinations, which prevent infection with the virus before exposure. Gardasil, Cervarix, and Gardasil 9 are among the vaccines designed to guard against the most prevalent forms of HPV that cause cancer. Since these vaccinations can stop the first infection that leads to cancer, they work best when given before to the start of sexual activity [3]. Therefore, HPV vaccination offers a proactive method to lower the incidence of virus-related malignancies, especially cervical cancer, in situations where standard screening programs may not be available or effective.
There is a significant global burden of HPV-related malignancies. More than 300,000 women die from cervical cancer alone each year; most of these fatalities take place in low- and middle-income nations where screening and treatment are scarce [4]. Because HPV causes a large number of anal, oropharyngeal, and other cancers, it also adds to the worldwide cancer burden. Widespread HPV vaccination has already demonstrated promise in lowering the incidence of HPV infections and related precancerous lesions in high-income nations, especially among young women [5]. Notwithstanding these successes, there is still a vaccine coverage gap in the world, with many areas finding it difficult to reach sufficient immunization rates because of logistical, cultural, and financial obstacles.
Burden of Human Papilloma Virus
Given these difficulties, the importance of HPV vaccination in preventing cancer cannot be emphasized. As part of its worldwide goal to eradicate cervical cancer, the World Health Organization (WHO) has urged swift action to increase HPV vaccination [6]. Increased vaccine coverage can significantly lower the incidence of HPV-related malignancies, improving public health for coming generations. With an emphasis on its methods, effectiveness, and implications for public health policy, this study aims to investigate the role of HPV vaccine in cancer prevention.
The worldwide burden of malignancies linked to the Human Papilloma virus (HPV) is still rather high, despite the availability of very effective vaccinations. Every year, over 500,000 women are affected with cervical cancer, which is mostly caused by a persistent HPV infection. More than 300,000 people die from it globally, most of them in low- and middle-income nations [4]. Anal, oropharyngeal, and penile cancers are among the several malignancies for which HPV is a leading cause and a contributor to the worldwide cancer burden. Even though the development of HPV vaccinations has significantly improved public health, a sizable portion of the world’s population is still unvaccinated. This illness is significant because, although the majority of HPV-related malignancies may be prevented by vaccination, they nonetheless happen often, highlighting the obstacles that still prevent universal vaccination adoption.
The unequal distribution of vaccination programs is one of the main reasons why the prevalence of HPV- related malignancies remains continuously high. Many low- and middle-income nations find it difficult to establish and maintain HPV vaccination programs, despite the fact that certain high-income countries have successfully reduced the prevalence of HPV infections via intense vaccination efforts [3]. Due to a lack of infrastructure, logistics, and resources, as well as restricted access to healthcare, vaccination rates in these areas continue to fall considerably short of what is required to establish herd immunity. The issue is made worse by inadequate healthcare systems, such as the absence of school-based immunization programs and challenges in reaching rural communities. However, both men and women are at risk of acquiring avoidable malignancies in nations with low vaccination rates, contributing to the global health imbalance.
The general lack of knowledge and awareness of HPV and its vaccinations is another significant issue. The adoption of HPV vaccination has been hampered in many communities by misconceptions regarding the efficacy and safety of vaccinations, which are fostered by vaccine hesitancy and false information. Cultural taboos around sexual health in many regions also hinder the spread of proper information regarding HPV transmission and the advantages of vaccination [7]. Low vaccination rates are a result of this disinformation, especially among groups whose public health education has not included awareness- raising initiatives. For instance, whereas HPV vaccination rates in certain Western nations are over 70%, rates as low as 10% to 20% have been reported in other regions, including sub-Saharan Africa and portions of Asia, leaving a sizable population vulnerable [8]. These differences show how urgently tailored education programs are needed to raise knowledge and support for HPV vaccinations throughout the world.
Furthermore, the persistence of HPV-related malignancies is also impacted by gender differences in vaccination. Since teenage girls are the population most at risk of getting cervical cancer, they have historically been the focus of HPV vaccination efforts. But males can also get HPV, and infections can result in anal, penile, and oropharyngeal malignancies. In many countries, there is a substantial gap in population-level protection since there are no initiatives designed specifically to vaccinate males [2]. In addition to having an impact on men’s health, this imbalance lessens the overall efficacy of vaccination efforts in fostering herd immunity. Lowering the prevalence of HPV-related malignancies in the general population will require extending vaccination programs to include boys and stepping up awareness initiatives aimed at both sexes.
Unveiling HPV and Cancer
Human papilloma virus (HPV), one of the most prevalent viral infections worldwide, is mostly transmitted by direct skin-to-skin contact, with sexual activity being the most frequent mode of transmission. Based on their propensity to cause cancer, the virus’s more than 200 strains are divided into high-risk and low-risk categories. Benign disorders like genital warts are usually linked to low-risk HPV strains, such HPV 6 and HPV 11. Nonetheless, it is evident that high-risk HPV strains, including HPV 16 and HPV 18, are connected to the emergence of cancer [9]. Prolonged infections from these high-risk strains can result in cellular alterations and the formation of precancerous lesions, which can eventually evolve into cancer, if treatment is not administered. Although most of these infections are asymptomatic and resolve on their own within two years, persistent infections with high-risk strains of HPV constitute a significant public health problem (Figure 1).
Figure 1. Diagram Showing the HPV Virus.
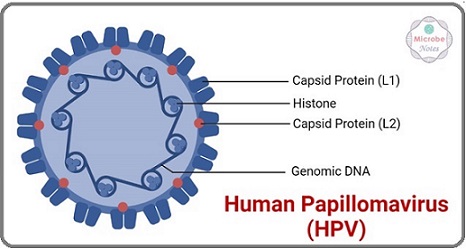
Of the malignancies linked to HPV that afflict individuals worldwide, cervical cancer is the most common and most researched. Cervical cancer, the fourth most frequent illness in women globally, results in around 500,000 new cases and 300,000 fatalities annually. Most of these deaths occur in low- and middle- income nations with inadequate access to prevention treatments [2]. About 70% of cases of cervical cancer are attributed to chronic infection with high-risk HPV strains, including HPV 16 and 18, according to [4]. HPV is linked to malignancies of the anus, oropharynx, penis, vulva, and vagina in addition to cervical cancer. Among these, oropharyngeal cancer is a major health issue that is on the rise, especially in affluent nations, and affects males today [10]. The prevalence of these illnesses emphasizes how important it is to implement efficient preventative strategies, such immunization and screening programs, in order to lower the incidence and death rate of malignancies linked to HPV.
People in impoverished nations, where cervical cancer screening and treatment programs are frequently either nonexistent or poorly administered, bear a disproportionate share of the worldwide burden of HPV-related malignancies. Cervical cancer continues to be among the top causes of cancer-related mortality for women in regions of South Asia, Latin America, and sub-Saharan Africa [8]. Cancers linked to HPV have significant social and economic repercussions, especially in areas with little resources where healthcare systems are already having difficulty. Additionally, women in their prime are frequently affected by malignant tumors, which increases the risk of premature deaths and exacerbates gender disparities in health outcomes. Therefore, preventing HPV infections by vaccination and public health initiatives is essential to lowering the incidence of cancer worldwide.
HPV vaccination: history and development
One of the biggest developments in cancer prevention in recent decades has been the introduction of HPV vaccinations. When researchers discovered a connection between chronic HPV infections and cervical cancer in the 1990s, the concept of cancer prevention by vaccination was born. Gardasil, the first HPV vaccination, received a license for use in 2006 following years of extensive research and clinical testing [11]. Gardasil was created to guard against genital warts caused by HPV 6 and 11 and high-risk HPV 16 and 18, which cause most cervical malignancies. Cervarix, a new vaccination that targets HPV 16 and 18, was created in response to the success of Gardasil. A more sophisticated Gardasil version, Gardasil 9, was released in 2014. By providing protection against nine different HPV strains (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58), this vaccination provides more thorough protection against a broader variety of malignancies and genital warts [3] (Figure 2).
Figure 2. Diagram of the Mechanism of Action of the Vaccine.
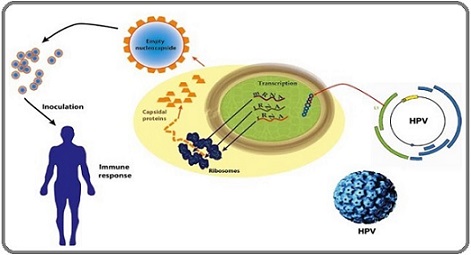
Since its main goal is to prevent infections before exposure, especially before they begin having sex, teenagers are the best candidates for HPV vaccinations. Since girls between the ages of 9 and 14 are most likely to develop cervical cancer later in life, the World Health Organization (WHO) advises regular immunization [2]. Since males in the same age group are equally susceptible to HPV-related malignancies, such as anal and oropharyngeal cancers, and contribute to the virus’s transmission, several nations also have vaccination programs for them [12]. Wider protection for all populations has resulted from high-income nations’ growing acceptance of gender- neutral immunization regimens.
The ideal age for vaccination is crucial since HPV vaccinations are most effective when given before virus exposure. However, it is advised that young adults and older adolescents who missed the first vaccination window receive a catch-up shot, usually until the age of 26 and in certain circumstances until the age of 45 [6]. There are still obstacles in the way of reaching high coverage rates worldwide, even with the proven safety and effectiveness of HPV vaccinations. The broad adoption of HPV vaccination in low-income countries is hampered by a lack of infrastructure to support vaccination campaigns, financial limitations, and vaccine hesitancy [7]. If the HPV vaccination is to fulfill its potential of averting millions of cancer cases globally, these issues must be resolved.
Cancer prevention through vaccination
Vaccine-based cancer vaccination is a novel strategy to disease prevention, particularly when considering malignancies brought on by viruses such as the Human Papilloma virus (HPV). Cancer-preventive vaccines function by enhancing the immune system’s capacity to identify and eliminate virus particles or infected cells before they have a chance to develop into cancer. Immunizations against HPV target specific high-risk strains of the virus that cause cancer, including those that cause malignancies of the cervical, anal, oropharyngeal, penile, and vulvar regions [13]. Vaccines are one of the most effective cancer prevention methods now available because they protect against infection with carcinogenic strains of HPV, which greatly lowers the chance of getting numerous forms of cancer. Because HPV vaccinations are preventive, they do not cure an illness; rather, they stop it before it begins. The vaccinations are not infectious because they are made of virus-like particles (VLPs), which resemble the HPV virus’s outer shell but lack viral DNA. By encouraging the production of neutralizing antibodies by the immune system, these VLPs can efficiently target HPV in the event that a person is exposed to the virus later in life [11]. High-risk HPV strains like HPV 16 and HPV 18, which cause around 70% of cervical cancer cases worldwide, are the main targets of the vaccinations. Additionally, depending on the vaccination, they target various strains, offering broader protection against malignancies linked to HPV.
Since HPV vaccinations can prevent the first infection, which is the earliest stage of the oncogenic process, they are successful in avoiding cancer. Normal cell cycle management may be disrupted by infection with a high- risk strain of HPV that can integrate into the host’s cellular DNA. If treatment is not given, this disturbance may ultimately lead to unchecked cell proliferation, which may cause precancerous lesions to form and, ultimately, cancer [9]. HPV vaccinations successfully halt the oncogenesis process before it starts by preventing the first infection. HPV vaccinations can lower the frequency of HPV infections, the development of precancerous cervical lesions, and the consequent risk of cervical cancer by up to 90%, according to clinical studies and empirical evidence [3].
Although research on vaccines’ impact on vulvar, oropharyngeal, and anal cancers is still ongoing, they can help prevent other HPV-related malignancies. Further study is required to completely comprehend the long-term advantages of immunization in avoiding certain tumors. For example, HPV is associated to a rise in oropharyngeal cancer in males, and although gender-neutral vaccination initiatives are helping to halt the virus’s spread [12]. Herd immunity, which happens when a sizable section of the population is inoculated, is another benefit of mass vaccination. It lowers the general viral circulation and protects individuals who cannot or will not get the immunization.
Even though HPV vaccinations have been quite successful in lowering the incidence of HPV-related malignancies, there are still a number of obstacles standing in the way of worldwide vaccination-based cancer prevention. Ensuring equal access to vaccinations is the main obstacle, especially in low- and middle-income nations where cervical cancer is most prevalent and immunization programs are frequently deficient or inadequately supported [2]. Overcoming vaccine reluctance and disinformation, which can impede vaccination efforts even in locations where vaccines are accessible, is another difficulty. To fully achieve the potential of cancer prevention by vaccination, these issues must be addressed by international health efforts, public awareness campaigns, and legislative reforms.
Previous studies on HPV vaccination and cancer rates
In nations with high vaccination rates, the frequency of precancerous lesions and HPV-associated illnesses has decreased since the introduction of HPV vaccinations, which has resulted in a notable drop in cancer rates. The effects of HPV vaccination programs at the community level have been the subject of several studies, all of which have consistently demonstrated that widespread vaccination has greatly enhanced public health, especially with regard to lowering the incidence of cervical cancer. Drolet et al. [3] examined the impact of HPV vaccination programs in 14 high-income nations in a comprehensive systematic review and meta-analysis. According to the study, HPV 16 and 18, the two main high-risk strains that cause the majority of instances of cervical cancer, are now far less prevalent. The incidence of these HPV strains dropped by up to 83% in adolescent girls and 66% in women in their early twenties in nations with high vaccination rates. The significant decrease in genital wart frequencies in vaccinated populations which are brought on by low-risk HPV strains further illustrated the vaccination’s wider preventative benefits.
Australia, which was one of the first nations to introduce a nationwide HPV vaccination program in 2007, is among the most notable instances of how much the HPV vaccine lowers cancer rates. Australia’s program first targeted school-aged girls before extending to include males in order to achieve gender-neutral coverage. The program has led to a notable decrease in the prevalence of cervical precancers (CIN2 and CIN3), which are reliable predictors of future cervical cancer risk [7]. According to the research, women between the ages of 18 and 24 showed a 77% decrease in high-grade cervical lesions; the age group that had the vaccination earlier saw the largest decreases. According to these results, Australia is on course to eradicate cervical cancer as a public health issue by 2035, provided present trends continue.
Notable reductions in HPV infections and associated illnesses have also been observed in Sweden, Scotland, and other high-vaccination nations like Australia. Lei et al. [14] investigated the effect of HPV vaccination on the incidence of cervical cancer in a Swedish cohort research that included over 1.6 million women and girls. According to the study, women who had their vaccinations prior to the age of 17 were 88% less likely to get cervical cancer than those who did not. The 53% risk decrease for women who had vaccinations between the ages of 17 and 30 was noteworthy even if it was smaller. This emphasizes how crucial it is to vaccinate people before to their exposure to the virus, as early vaccination increases the vaccine’s ability to prevent cancer. Palmer et al. [15], another significant study from Scotland, assessed how the national HPV vaccination program affected the prevalence of cervical cancer. Data from women who received vaccinations under the national program and those who did not were compared by the researchers. The efficacy of the vaccine in avoiding the precursors of cervical cancer was further demonstrated by the 71% decrease in cervical intraepithelial neoplasia (CIN3) among vaccinated women. Because CIN3 rates dropped even among women who were not vaccinated, the study further demonstrated the importance of herd immunity. This was probably caused by less HPV being spread in the general population. This result demonstrates the wider influence that mass immunizations have on public health.
Cervical cancer has received a lot of attention, but other HPV-related diseases have also decreased in nations with high vaccination rates. For example, as more nations implement gender-neutral vaccination programs, the incidence of anal and oropharyngeal cancers which are associated with high-risk HPV strains is anticipated to decline [10]. However, because these malignancies sometimes appear later in life than cervical cancer, the whole impact might not be apparent at first.
In summary, an increasing amount of data shows that HPV vaccinations are successful in lowering the prevalence of HPV infections, precancerous lesions, and, more recently, cancer rates. Cervical cancer precursors have been reduced in nations with strong vaccination programs, and significant worldwide reductions in HPV-related malignancies are anticipated as long-term implications. Notwithstanding these achievements, more has to be done to increase vaccination rates, especially in low- and middle-income nations where cervical cancer remains the most common illness.
Gaps in the Literature
Even while there is a lot of evidence that HPV vaccinations can help prevent diseases linked to HPV, there are also a lot of unanswered questions. The effects of HPV vaccination in men are among the most important topics that require more investigation. Even though HPV vaccination efforts were first aimed at women because to the high rate of cervical cancer, HPV is responsible for a considerable number of malignancies in males, including anal, penile, and oropharyngeal cancers. There is currently little information on the long-term effects of HPV vaccination in lowering the incidence of cancer in men, particularly non-genital cancers, despite some studies demonstrating encouraging results regarding the reduction of HPV infections and precancerous lesions in vaccinated males [10]. To completely comprehend the vaccine’s potential to lower HPV-related malignancies in both sexes, further population-level research concentrating on male vaccination and its wider public health effects is required. Additionally, there is conflicting evidence about the effectiveness and safety of HPV vaccination in older individuals. Assuming that vaccination is most effective when given before sexual contact exposure to HPV, current immunization guidelines largely target preadolescence and young adults. However, older persons, especially those aged 26 and up, who might not have been eligible for vaccination during the first roll out, are expressing a greater desire to get vaccinated. The long-term effectiveness and cost-effectiveness of this approach are yet unknown, despite some data that shows immunizing older adults may help prevent cancer, particularly in those who are more likely to have a persistent HPV infection [16]. To find out if widespread HPV vaccination of this population can dramatically lower cancer rates and if older persons can receive the same degree of protection from these vaccinations as younger ones, more study is required. Immunological mechanisms of HPV vaccination By focusing on the viral particles before they have a chance to create a chronic infection, human papilloma virus (HPV) vaccines aim to produce a strong and durable immune response that stops infection. The main way that HPV vaccines function is through virus-like particles (VLPs), which resemble the virus but do not have its DNA and are thus not contagious. The L1 protein, an essential HPV capsid protein, makes up these VLPs. These VLPs function as antigens when given, which causes the immune system to produce neutralizing antibodies that target HPV specifically [13]. Vaccines like Cervarix, Gardasil, and Gardasil 9 encourage the immune system to identify and react to the L1 protein, which makes up the virus’s outer shell. B cells play a crucial role in mediating this response because they generate antibodies that attach to the HPV virus during repeated exposure and neutralize it before it can infect host cells [11]. Previous report by Giuliano claimed that the vaccination produces extremely specific antibodies that can stop infection by the HPV strains it contains, such as HPV 16 and 18, which cause the majority of malignancies linked to HPV [17].
High titers of neutralizing antibodies, which are many times more than those produced by a normal infection, are a hallmark of the immune response to the HPV vaccination. Long-term protection against HPV infection is thought to depend on this enhanced immune response. Crucially, research indicates that the vaccine may provide protection for decades, and these antibodies remain heightened for years after immunization [13]. Activated T helper cells (CD4+) work in tandem with B cells to maintain the immune response and aid in the generation of antibodies [11]. The ability of HPV vaccinations to produce memory B cells is a crucial component of their immunological mechanism. When the virus is re-exposed, these cells can swiftly develop an immune response since they remain in the body for a considerable amount of time after vaccination. Even if an individual contracts HPV years after getting the vaccination, this process guarantees that they will be protected against the virus for the rest of their lives [18]. Last but not least, adding L1 VLPs to the vaccination has been very effective in producing a strong and long-lasting immune response, preventing HPV infection, and lowering the risk of later developing cancer.
Prevention of oncogenesis
Because HPV vaccines target the early phases of the viral infection before the virus can integrate into the host genome and produce cellular alterations that lead to cancer, they are essential in avoiding the malignant transformation of HPV-infected cells. HPV infection is the main cause of cervical cancer and a major risk factor for other malignancies such anal and oropharyngeal cancers. High-risk HPV strains, especially HPV 16 and 18, infiltrate epithelial cells to initiate oncogenesis, or the conversion of healthy cells into cancerous cells. Precancerous lesions and unchecked cell proliferation result from these strains’ production of viral oncoproteins (E6 and E7) that disrupt the tumor suppressor proteins p53 and retinoblastoma (Rb) [19]. Vaccines interrupt the entire carcinogenic process by preventing early infection with high-risk strains of HPV. In the absence of infection, the virus cannot manufacture the E6 and E7 oncoproteins because its genome is not integrated into the host cells. By doing this, the cells are kept from developing into malignant ones by delaying the breakdown of p53 and Rb [17]. Numerous clinical trials have shown how efficient the immunizations are at preventing HPV 16 and 18 infection. Persistent HPV infection, which is significantly less prevalent in individuals who receive the immunizations, is a major risk factor for the development of high-grade cervical intraepithelial neoplasia (CIN2/3), a precursor to invasive cervical cancer [7].
In addition to avoiding initial infection, HPV vaccinations lower the chance of viral persistence, another important determinant in the development of cancer. The immune system usually eliminates HPV infections on its own, but in certain people, the virus might linger for a long time, raising the risk of oncogenesis. By priming the immune system to eliminate the virus upon entrance, vaccination stops the virus from avoiding immunological detection and living in the host, hence preventing a chronic infection [16]. Precancerous lesions are known to be prevented by HPV vaccinations. According to clinical research, vaccination significantly lowers the incidence of CIN2/3 and adenocarcinoma in situ (AIS), two conditions that are direct precursors to invasive cervical cancer, in addition to lowering the frequency of chronic infection [16]. The decrease in high-grade lesions in vaccinated populations has led to a decrease in cervical cancer incidence, highlighting the vaccines’ ability to stop infection from developing into malignancy.
Duration of immunity and long-term protection
The length of immunity that the injections produce and the long-term protection they provide against HPV infections and associated malignancies are important factors to take into account when assessing the efficacy of HPV vaccination. Examples of HPV vaccinations that seek to produce robust immune responses that offer sustained protection against high-risk HPV strains, especially HPV 16 and 18, which cause the majority of HPV-related malignancies, are Cervarix, Gardasil, and Gardasil 9 [11]. Neutralizing antibodies, which attach to the virus and stop it from infecting host cells, are produced as a result of these vaccinations. Nonetheless, research is presently underway to determine how long these antibodies last as well as how long vaccine-induced immunity lasts overall. According to recent research, HPV vaccinations produce a strong immunological response that last for a number of years. Vaccinated people had high levels of neutralizing antibodies for at least 10–12 years following vaccination, with no indications of declining immunity during this time, according to long-term follow-up studies [20]. According to one research, almost all Cervarix vaccine recipients exhibited measurable antibody levels against HPV 16 and 18, and there were no documented incidences of chronic infection or associated precancerous lesions [14]. These results imply that the protection provided by HPV vaccinations is robust and durable, offering a significant defense against HPV infection and the emergence of associated malignancies.
The age at which vaccination takes place and an individual’s immunological response seem to have an impact on how long immunity lasts. Younger people, especially those who receive vaccinations between the ages of 9 and 14, frequently produce stronger and more persistent antibody levels than older people. Due to their more sensitive immune systems, preadolescents and teens are probably more immunogenic than adults [13]. Therefore, early adolescent HPV vaccination is seen to be the best way to provide long-term protection, particularly if given prior to any viral exposure linked to sexual activity. Whether further injections will be required to sustain long-term protection is one of the primary concerns regarding the durability of HPV vaccine-induced immunity. Based on existing data, booster doses are not necessary because antibody levels increase progressively over time without further immunizations. Furthermore, studies have shown that HPV vaccination significantly increases the generation of neutralizing antibodies compared to spontaneous infection, which may explain why vaccinated individuals exhibit long-lasting protection [16]. However, to determine if protection is effective after 10–12 years and to assess the potential for future booster doses, ongoing monitoring and long-term follow-up study are crucial.
Both the durability of antibodies and the clinical efficacy of HPV vaccines in preventing HPV-related cancers have been evaluated in long-term trials. Communities with high vaccination coverage have shown a significant reduction in the prevalence of cervical cancer, genital warts, and high-grade cervical intraepithelial neoplasia (CIN2/3). According to a Swedish study, for example, women who got the Gardasil vaccine had a much lower risk of developing invasive cervical cancer than those who did not, and the protective effect persisted for up to 11 years after vaccination [14]. According to statistics from Australia, where HPV vaccination programs have been widely implemented, the prevalence of genital warts and high-grade cervical lesions has significantly decreased among vaccinated individuals [7].
A key element of long-term protection is the vaccines’ ability to prevent infections caused by non-targeted HPV strains. For instance, Gardasil 9 provides protection against nine other HPV strains, including five additional variations outside of HPV 16 and 18 that are associated with a decreased incidence of HPV-related cancers. Even while its long-term efficacy is still being evaluated, preliminary evidence suggests that Gardasil 9 provides extensive protection against these additional strains, thereby reducing the overall burden of HPV-related disorders [16]. Continuous monitoring will be necessary to verify the long-term benefits of this enhanced protection.
Clinical Trials and real-World effectiveness
Numerous clinical studies have thoroughly examined the effectiveness of HPV vaccinations, and actual data from nations with substantial immunization programs has confirmed this. The foundation for our understanding of how HPV vaccinations, specifically Cervarix and Gardasil, protect against high-risk HPV strains and associated disorders was established by two of the most important clinical investigations, the FUTURE and PATRICIA trials. Gardasil, a quadrivalent HPV vaccine that targets HPV strains 6, 11, 16, and 18, has been shown to be efficacious in the FUTURE trials (Females United to Unilaterally Reduce Endo/Ectocervical Disease), which comprise FUTURE I and FUTURE II. While FUTURE II only looked at the vaccine’s capacity to prevent high-grade cervical intraepithelial neoplasia (CIN2/3), which is a precursor to cervical cancer, FUTURE I concentrated on preventing vulvar and vaginal lesions, genital warts, and cervical intraepithelial neoplasia (CIN). Both studies included sizable cohorts of 16–26-year-old women. The outcomes were remarkable: in women who had never been exposed to HPV 16 or 18, Gardasil was almost 100% effective in avoiding CIN2/3, which is linked to these virus types [20]. Additionally, the vaccination demonstrated robust protection against HPV types 6 and 11-induced genital warts, underscoring its potential to lower the prevalence of HPV-related disorders that are both carcinogenic and non-cancerous.
The effectiveness of Cervarix, a bivalent vaccination that targets HPV strains 16 and 18, in preventing cervical precancerous lesions was assessed in the PATRICIA trial, a phase III clinical investigation (Papilloma Prevention against Cancer in Young Adults). This research was one of the biggest HPV vaccination initiatives to date, with over 18,000 women from various nations participating. In women who were HPV-naïve, Cervarix was up to 93% effective in avoiding CIN2/3 lesions linked to HPV 16 and 18 [21]. Cervarix’s importance in preventing cancer was further supported by the experiment’s noteworthy findings, which demonstrated that the medication offered partial cross-protection against additional high-risk HPV strains, such as HPV 31 and 45. Although clinical trials offer crucial proof of vaccination effectiveness under carefully monitored conditions, real-world effectiveness statistics reveal how vaccines function in situations involving a larger population. Notable reductions in HPV-related illnesses and diseases have been observed in nations that have instituted nationwide HPV immunization programs. For example, genital warts and high-grade cervical lesions have significantly decreased in Australia, one of the first nations to implement a nationwide HPV vaccination program in 2007. By 2018, genital warts had been almost eliminated in vaccinated cohorts, and high-grade cervical abnormalities in women under 25 had decreased by 77% in Australia [7]. When given at the population level, HPV vaccinations are very successful in preventing non- cancerous HPV-related illnesses and precancerous lesions, according to real-world research.
According to long-term statistics collected after the HPV vaccine was introduced, Sweden has seen a considerable decline in the incidence of cervical cancer among vaccinated women. Women who had an HPV vaccine before the age of 17 had an 88% reduced incidence of invasive cervical cancer than those who did not, according to a 2020 population-based study [14]. This notable decrease in the incidence of cervical cancer emphasizes the value of getting vaccinated before being exposed to HPV and the vaccine’s capacity to offer sustained protection against one of the most prevalent diseases linked to HPV.
When the national HPV vaccination program was implemented, researchers in Scotland saw an 89% decrease in CIN3 lesions and an almost complete eradication of HPV 16 and 18 infections in young women [22]. These studies offer more proof of the effectiveness of HPV vaccination in reducing community-level precursors of cervical cancer. These practical results are important because they show that the protection provided by HPV vaccinations is long-lasting and in line with clinical trial results.
HPV vaccinations have been demonstrated to be successful in lowering the incidence of anal, oropharyngeal, and vulvar cancers in addition to guarding against cervical cancer. According to US study, the frequency of precancerous anal and oropharyngeal lesions has decreased in tandem with a decrease in the incidence of HPV infections among vaccinated persons [23]. As more cohorts of vaccinated persons age into risk periods for these cancers, it is anticipated that the widespread use of Gardasil 9, which protects against nine HPV strains (6, 11, 16, 18, 31, 33, 45, 52, and 58), would considerably reduce the incidence of HPV-related cancers worldwide. All things considered, clinical studies and empirical evidence unequivocally show that HPV vaccinations are effective in preventing HPV-related infections, precancerous lesions, and invasive malignancies. The findings’ stability across various demographics and healthcare environments, particularly in regions where HPV-related cancers are prevalent, emphasizes the necessity of ongoing efforts to raise vaccination rates. The worldwide burden of illnesses like cervical cancer is predicted to continue to decrease as more nations introduce or broaden their HPV vaccination programs.
Vaccine safety profile
Clinical studies and post-marketing surveillance have been used to thoroughly examine the safety profile of HPV vaccinations. In addition to proving the effectiveness of HPV vaccinations, clinical trials like the FUTURE and PATRICIA studies have also yielded important information about their safety. Common adverse events in these studies were headaches, moderate fever, and injection site responses (pain, swelling, and redness); these were often temporary and self-limiting [20, 21]. Severe side effects were uncommon and didn’t seem to be caused by the vaccines. The safety of the vaccinations has been further validated by post-marketing surveillance. According to extensive research by the Vaccine Adverse Event Reporting System (VAERS) and the European Medicines Agency (EMA), the HPV vaccines have an acceptable safety record. For instance, a thorough examination of post-marketing data showed that there was no evidence to support claims that HPV vaccines significantly increase the risk of autoimmune diseases, infertility, or other long-term health issues, and that the incidence of serious adverse events was low [7].
Numerous health agencies, including as the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), have confirmed these findings and continue to believe that the advantages of the HPV vaccine much exceed the dangers.
There are still misunderstandings and disputes about the safety of the HPV vaccination, even with the comforting safety data. In response to anecdotal evidence rather than definitive scientific proof, several people and organizations have voiced worries about the vaccine’s potential to cause a range of health problems. To dispel these myths, public health campaigns and educational programs have emphasized the stringent testing and surveillance procedures that vaccinations must pass before receiving a license. To promote vaccination uptake, it is essential to address misconceptions and worries, especially in settings where reluctance may be caused by social or cultural factors [23].
Factors influencing vaccine eficacy
The timing of the vaccination, the number of doses administered, and the demographics of the vaccinated population are some variables that may affect how effective HPV vaccinations are. Time is the most important element to consider because studies show that the immunization works best when given before sexual activity begins. Higher antibody levels and longer- lasting protection are the outcomes of stronger immune responses in preadolescence and teens [16]. Although vaccination can start as early as age 9, the Advisory Committee on Immunization Practices (ACIP) advises regular vaccination for preteens between the ages of 11 and 12 [23]. One important factor influencing the effectiveness of vaccinations is the quantity of doses. Recent research indicates that a two-dose schedule may offer enough protection, especially for those who receive the injections before the age of 15 [24]. Originally, the HPV vaccine schedule called for three doses. Given that better completion rates might be attained with fewer doses, this has implications for expanding immunization coverage. However, the protective advantages of the vaccination may be greatly diminished if people do not receive all of the recommended doses. Some of the obstacles to vaccine uptake that result in inadequate vaccination include lack of access, financial limitations, and disinformation, underscoring the necessity of focused public health initiatives to address these issues.
Age, gender, and socioeconomic position are some of the demographic variables that affect how effective vaccines are. For instance, studies show that girls are more likely than boys to get vaccinated, which may have an effect on initiatives to prevent cancer and increase herd immunity in general [20]. The disparities in vaccination rates may increase if people from lower socioeconomic backgrounds find it more difficult to get healthcare services like immunizations. To improve vaccination effectiveness and provide population-wide protection against HPV-related malignancies, these gaps must be addressed by community engagement, education, and easily available healthcare facilities.
HPV vaccination coverage and disparities
For a number of social, cultural, and pragmatic reasons, HPV vaccination coverage varies greatly by country and area around the world. According to the WHO, many LMICs fall short of even 20% coverage, but some high-income nations have coverage rates of 80% [25]. Given the high incidence of HPV-related malignancies in LMICs, where cervical cancer continues to be the primary cause of cancer-related death for women, this discrepancy is especially worrisome. In many locations, vaccination efforts are severely hampered by socioeconomic constraints, such as lack of health insurance, high expenditures connected with immunization programs, and restricted access to healthcare. There are cultural considerations as well; in certain cultures, societal norms and attitudes around vaccinations might cause reluctance and resistance to vaccination, especially among parents of young females. To dispel misconceptions and promote acceptance, educational initiatives that incorporate cultural sensitivity and offer concise, fact-based information on the advantages of HPV vaccination are crucial [7].
Efforts to increase vaccination coverage are complicated by logistical concerns such as a lack of educated healthcare personnel, a lack of suitable healthcare facilities, and supply chain problems. Missed vaccination chances may arise from the fact that many LMICs lack the infrastructure required for successful immunization programs [23]. To overcome these obstacles, governments, non-governmental groups, and international health organizations must collaborate. Initiatives like school-based immunization programs that combine HPV vaccination with pre-existing medical services have been effective in increasing coverage in a variety of contexts [24]. In conclusion, differences in HPV vaccination coverage are caused by a complex interaction of logistical, cultural, and financial variables. Multi-modal strategies that take into account local circumstances, encourage education and awareness, and improve access to immunization services are needed to address these inequities. Global efforts to lessen the burden of these diseases can be greatly aided by increasing HPV vaccine coverage, especially in areas where HPV-related malignancies are most prevalent.
Implications for public health policy and future research
Future research objectives and public health regulations are significantly impacted by the effectiveness of HPV vaccination campaigns. Policymakers must give the HPV vaccination top priority as a crucial part of cancer prevention programs because the incidence of cancers associated with HPV is still a significant problem. Increasing vaccine coverage should be a top priority for effective public health policy, especially for underprivileged groups and in areas with low immunization rates. The actions listed below can be usedto accomplish this:
Educational Campaigns: Raising knowledge and comprehension of HPV and the dangers it poses should be the goal of public health initiatives. By debunking misconceptions regarding the effectiveness and safety of vaccines via comprehensive education initiatives aimed at parents, teenagers, and medical professionals, vaccination rates can be increased. Engagement and acceptance may be increased by crafting messages that speak to cultural and communal values.
Accessibility and Affordability: It is imperative that policymakers guarantee that HPV vaccinations are reasonably priced and available to all groups, particularly in nations with low and moderate incomes. This might entail lowering vaccine costs, integrating HPV vaccination into current medical services, and establishing school- based immunization campaigns. Partnerships with non- governmental groups can also help outreach initiatives in populations that are difficult to reach.
Monitoring and Surveillance: To assess the success of public health campaigns, it is crucial to conduct continuous surveillance of HPV vaccination coverage and its relationship to cancer rates. Health officials will be able to track adverse events, spot vaccine uptake gaps, and evaluate the long-term effects of vaccination on the prevalence of HPV-related malignancies by putting in place reliable data gathering tools.
Policy Integration: The delivery of healthcare can be improved by combining HPV vaccination with other medical interventions, such as regular gynecological examinations and sexual health education. By encouraging a thorough understanding of sexual health and disease prevention, this all-encompassing strategy helps to establish a culture that normalizes and supports immunization.
Future research should focus on numerous critical areas to increase the efficacy of HPV vaccination. First, long-term studies are needed to examine the durability and efficiency of vaccine-induced immunity in preventing HPV-related malignancies in a range of groups. Second, studies on the efficiency of the HPV vaccination should be extended to include males as they are also susceptible to HPV-related diseases, such as oropharyngeal and anal cancers. To pinpoint and comprehend the precise obstacles to HPV vaccine uptake, particularly in underprivileged regions, more research is required.
In conclusion, the consistently high incidence rates of HPV-related malignancies, particularly in low- and middle-income nations, underscore serious gaps in vaccination coverage and awareness despite the established safety and effectiveness of HPV vaccinations. It is widely established how HPV vaccines provide protection, and the introduction of L1 virus-like particles to elicit immune responses is essential to preventing the neoplastic transformation of infected cells. Nonetheless, there are still obstacles in the way of guaranteeing universal access to these vaccinations, and notable inequalities have been seen worldwide. Achieving high coverage rates is made more difficult by a number of variables, including socioeconomic constraints, insufficient immunization regimens, and vaccine timing.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data are available from the corresponding author upon request.
Competing interests
The authors declare no conflict of interest.
Funding
None
Author’s contributions
SMG and SGY conceptualized and write the original draft edited the manuscript as well as performed the critical literature search, JOA and BW supervised the manuscript. All authors read and approved the final manuscript.
References
- Epidemiology and burden of HPV-related disease Serrano B, Brotons M, Bosch FX , Bruni L. Best Practice & Research. Clinical Obstetrics & Gynaecology.2018;47. CrossRef
- ICO/IARC information centre on HPV and cancer (HPV information centre) Bruni, L., L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX , de Sanjosé S. Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019.2020.
- Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis Drolet M, Bénard E, Pérez N, Brisson M. Lancet (London, England).2019;394(10197). CrossRef
- HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019 Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS , Afsar OZ , et al . Preventive Medicine.2021;144. CrossRef
- Current Epidemiology and Trends of HPV Infections in Europe and the Role of National HPV Vaccination Programs Harder T, Wichmann O, Klug SJ . Acta Cytologica.2021;65(6):541-552.
- World Health Organization (WHO).Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization 2020.
- Impact and safety of human papillomavirus vaccines: a 10-year review of post-licensure data on vaccine safet Garland SM , Kjaer SK , Muñoz N, Block SL , Brown DR , DiNubile MJ , Luxembourg A. International Journal of Cancer.2020;147(3):684-698.
- Examining the Challenges and Strategies for Improving Cervical Cancer Screening in Nigeria Gamde SM , Omotola OS , Avwioro GO , Adisa JO . International Journal of Human and Health Sciences (IJHHS).2024;8(1). CrossRef
- Human papillomavirus and cervical cancer Burd EM . Clinical Microbiology Reviews.2003;16(1). CrossRef
- Prevalence of oral HPV infection in unvaccinated men and women in the US, 2009–2016 Chaturvedi AK , Graubard BI , Broutian T, Pickard RK , Tong ZY , Xiao W, Gillison ML . JAMA Oncology.2021;7(3):490-492.
- Explanations for the high potency of HPV prophylactic vaccines Schiller JT , Lowy DR . Vaccine.2020;38(28):4327-4335.
- Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. BMC cancer.2012;12. CrossRef
- HPV vaccination: What do we know about the population-level impact? Stanley M. Journal of Clinical Virology.2019;117:16-21.
- HPV Vaccination and the Risk of Invasive Cervical Cancer Lei J, Ploner A, Elfström KM , Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. The New England Journal of Medicine.2020;383(14). CrossRef
- Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12-13 in Scotland: retrospective population study Palmer T, Wallace L, Pollock KG , Cuschieri K, Robertson C, Kavanagh K, Cruickshank M. BMJ (Clinical research ed.).2019;365. CrossRef
- A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women Joura EA , Giuliano AR , Iversen O, Bouchard C, Mao C, Mehlsen J, Moreira ED , et al . The New England Journal of Medicine.2015;372(8). CrossRef
- Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study Giuliano AR , Lee J, Fulp W, Villa LL , Lazcano E, Papenfuss MR , Abrahamsen M, et al . Lancet (London, England).2011;377(9769). CrossRef
- Next generation prophylactic human papillomavirus vaccines Schiller JT , Müller M. The Lancet. Oncology.2015;16(5). CrossRef
- The biology and life-cycle of human papillomaviruses Doorbar J, Quint W, Banks L, Bravo IG , Stoler M, Broker TR , Stanley MA . Vaccine.2012;30 Suppl 5. CrossRef
- Human papillomavirus and cervical cancer Okunade KS . Journal of Obstetrics and Gynaecology: The Journal of the Institute of Obstetrics and Gynaecology.2020;40(5). CrossRef
- Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: Final analysis of a long-term follow-up study up to 9.4 years post-vaccination Naud PS , Roteli-Martins CM , De Carvalho NS , Teixeira JC , De Borba PC , Sanchez N, Harper DM . Human Vaccines & Immunotherapeutics.2020;17(2):467-477.
- Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland Pollock KG , Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, Palmer TJ . British Journal of Cancer.2019;120(11):1171-1176.
- Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices Meites E, Szilagyi PG , Chesson HW , Unger ER , Romero JR , Markowitz LE . MMWR. Morbidity and mortality weekly report.2019;68(32). CrossRef
- Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study Simms KT , Steinberg J, Caruana M, Smith MA , Lew J, Soerjomataram I, Castle PE , et al . The Lancet. Oncology.2019;20(3). CrossRef
- Cervical Cancer Study Guide. Let’s Talk Medicine. Retrieved from: https://www.letstalkmed.com/cervical-cancer (Accessed November 4, 2023) .
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times