The Role of Circadian Rhythm Disruption in Cancer Progression: Molecular Mechanisms, Impact on Tumor Biology, and Therapeutic Insights
Download
Abstract
The circadian clock is an innate oscillator that synchronizes a number of biological processes into an approximately 24-hour cycle. Circadian rhythm disruption significantly affects the cancer progression by interfering with cellular processes such as angiogenesis, metabolism, immune surveillance, DNA repair, and cell cycle regulation. CLOCK, BMAL1, PER, and CRY are essential clock genes that are involved in interlocked transcription–translation feedback loops that sustain the circadian clock at the molecular level. When these processes are disrupted by environmental factors, lifestyle modifications, or genetic changes, they lead to immune evasion, unchecked cell growth, and metabolic and vascular remodeling that supports tumors. Furthermore, by altering the chromatin remodeling and gene expression, disturbance of the circadian rhythm might change the epigenetic landscape and further promote carcinogenesis. The molecular connections between cancer biology and circadian disruption are summarized in this review, which also focuses on new chronotherapeutic approaches that try to maximize effectiveness by coordinating cancer therapy with the body’s natural biological clock.
Introduction
Circadian rhythm is an endogenous, 24-hour cycle that controls a wide range of biological functions, such as sleep-wake cycles, hormone release, and metabolic function. Circadian rhythm is controlled by a master clock in the suprachiasmatic nucleus of the hypothalamus and several peripheral clocks, and they are synchronized to environmental signals, primarily the light-dark cycle [1]. It is important to understand how disturbances in the circadian rhythm lead to cancer. This review aims to describe and list the mechanisms by which cancer is caused and affected by disruptions in circadian rhythms.
The molecular mechanism of circadian rhythm
The molecular basis of the circadian clock is regulated by a transcription-translation feedback loop that controls physiological and behavioral rhythms with a cycle of about 24 hours [2]. The core of this mechanism consists of two major transcription factors: Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK). These transcriptional factors constitute a heterodimer that binds to the E-box element to activate the transcription of clock-controlled genes, such as Period (PER1, PER2) and Cryptochrome (CRY1, CRY2). Transcription of these genes results in the cytoplasmic accumulation of PER and CRY proteins, which subsequently form complexes and move into the nucleus. After entering the nucleus, the PER/CRY complexes suppress the transcriptional activity of the BMAL1/CLOCK heterodimer, thus repressing their own expression in a negative feedback loop, allowing for a new cycle of transcription [3-5]. Besides this central loop, secondary feedback loops with other transcriptional factors like REV-ERBα and RORα provide for the stability of circadian cycles by controlling expression of Bmal. Interlocked feedback loops provide for sharp timing and synchrony of cell processes in tissues [6].
Light is an important environmental stimulus controlling circadian rhythms. Photosensitive retinal ganglion cells (RGCs) with the photopigment melanopsin perceive light and convey messages to the suprachiasmatic nucleus (SCN) to synchronize the internal clock with the external environment. Other photoreceptors, like rods and cones, also help by perceiving light, further helping circadian regulation [7, 8].
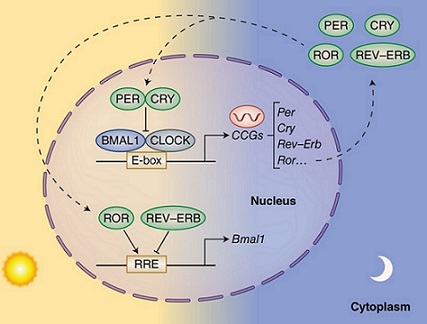
In addition to light, various internal factors also impact the biological clock. These factors include the flow of intracellular calcium, which is important for cellular signaling; membrane depolarization, which influences neuron activity; and cyclic AMP signaling, a pathway that regulates gene expression. These processes work together with the core molecular clock to maintain the proper functioning of circadian rhythms (Figure 1) [9, 10].
Figure 1. Molecular Mechanism of Circadian Rhythm. The transcriptional activator complex CLOCK/BMAL1 and its repressors (PER/CRY, REV-ERBα) or activators (RORα) combine to generate an autoregulatory feedback loop that functions as a molecular clock oscillator.
Mechanisms linking
Following are mechanisms that explain how disruption of circadian rhythm results in and affects cancer.
1. Disruption of cell cycle control: The disrupted circadian rhythm altered the expression of cell cycle regulatory proteins, leading to uncontrolled cell proliferation and potentially cancer [11-13]. Furthermore, it damages the DNA repair systems, leading to a build- up of genetic mutations that support the development of cancer [14].
2. Angiogenesis promotion: The circadian rhythm components control the expression of pro-angiogenic factors such as Vascular Endothelial Growth Factor (VEGF), Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), and Hypoxia-Inducible Factor 1-alpha (HIF-1α), resulting in the growth and spread of tumors [15].
3. Immune cells modulation: The activity, distribution, and development of immune cells are all affected by a wide range of circadian rhythm-related variables such as genes, cytokines, adhesion molecules, etc. Gene expression associated with the disrupted circadian rhythm is linked to cancer risk, and the disruption of the circadian rhythm has changed the tumor microenvironment (TME) [16].
4. Metabolic reprograming: Circadian disruption changed the major metabolic pathways, with consequent altered glucose, lipid, and amino acid metabolism. This dysregulation affects cellular energy homeostasis and promotes pro-oncogenic microenvironment through enhancing of anabolic activity, oxidative stress, and DNA damage [17]. Furthermore, core clock gene disruption can result in aberrant expression of metabolic enzymes and signaling proteins like mTOR and AMPK, contributing to abnormal cell growth and tumorigenesis [18].
5. Epigenetic Reprogramming: Circadian rhythm disruption can cause epigenetic reprogramming through the modification of expression and activity of chromatin- modifying enzymes, including histone acetyltransferases, deacetylases, and DNA methyltransferases. This leads to abnormal histone modifications and DNA methylation patterns, which in turn influence the rhythmic expression of genes that control cell cycle, metabolism, and DNA repair [19]. These epigenetic changes provide an environment for oncogene activation and silencing of tumor suppressors, eventually leading to cancer initiation and progression [20].
Disruption of cell cycle control
Components of the circadian rhythm also act as cell cycle regulators that coordinate the timing of cell division by triggering the rhythmic production of important cell cycle checkpoint proteins [21].
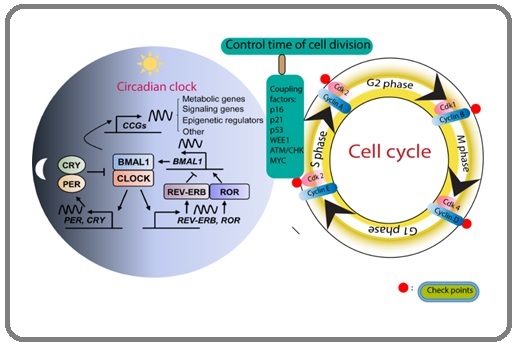
CLOCK: BMAL1 heterodimer activates the transcription of several cell cycle regulatory proteins by attaching to the E box element of the respective gene. This includes cyclin-dependent kinases (CDKs) and their subunit cyclins (e.g., Cyclin D, Cyclin A, Cyclin B), which control the cell cycle through cell cycle phases G1/S and G2/M transitions [22]. CLOCK: BMAL1 also stimulates the production of WEE1, a G2/M checkpoint kinase that inhibits CDK1 and prevents premature mitotic entry [23]. The other components of circadian rhythm such as CRY1,REV-ERBα also synchronizes each stage of the cell cycle by interacting with checkpoints (such as p16, p21, p53, and ATM/CHK), as shown in the following diagram [24].
It has been demonstrated that PER2, in particular, interacts with and stabilizes tumor suppressor protein p53, promoting cell cycle arrest and apoptosis in response to DNA damage [25]. This defense system is upset when PER2 is lost, which results in less DNA damage detection and makes it easier for c-MYC overexpression, a recognized oncogene that speeds up the cell cycle [26, 27]. Further connecting the DNA damage response with circadian rhythm is the regulation of DNA repair pathways, including ATR-CHK1,is also regulated by CRY proteins [28].
In addition to this, REV-ERBα, in particular, can indirectly control p53 activity by suppressing BMAL1 and interfering with disregulating circadian transcriptional activity, which leads to abnormal cell cycle dynamics (Figure 2) [21].
Figure 2. Connection between the Circadian Clock Components and the Cell Cycle Regulation. Diagram showing that the circadian clock components and cell cycle regulators are coupled. A single cell contains both the CDK/cyclin complex-regulators and the circadian oscillators. Cell division timing is set by the interaction of both oscillators, defined by particular coupling factors.
Angiogenesis promotion
Angiogenesis associated with cancer is affected by circadian rhythm disruption through a number of interconnected mechanisms [29].
The overexpression of important pro-angiogenic factors like VEGF results from the dysregulation of essential clock genes, such as PER2 and CLOCK, which modifies cellular signaling [30]. The activation of hypoxia-inducible factor 1-alpha (HIF-1α), especially in the hypoxic areas of tumors, further aggravates this process. A pseudo-hypoxic environment is frequently produced by metabolic changes brought on by circadian disturbance, which stabilizes HIF-1α even when there isn’t actual hypoxia. In turn, HIF-1α promotes the transcription of VEGF and other angiogenic genes, which increases vascular development and aids in the formation of tumors [31]. Moreover, when melatonin and cortisol levels are disturbed, it exacerbates tumor vascularization. Together, these mechanisms greatly accelerate the development of tumors [32]. It has been discovered that angiogenic factors, including as Epidermal Growth Factor (EGF) and Insulin Like Growth Factor Binding Protein (IGFBP) in breast cancer patients, swing in a circadian manner, with maximum plasma concentrations typically occurring throughout the day and minimal levels at night [33].
Immune cells modulation
Recent research has provided additional knowledge into the relationship between immunological escape and the circadian clock components. T-cell activation/ differentiation indicators, T-cell exhaustion markers, Programmed Cell Death Proten-1 (PD-1), Cytotoxic T-lymphocyte-Associated Protein 4 (CTLA4) and Programmed Death-Ligand 1 (PD-L1) are all regulated by BMAL1 in metastatic melanomas. Additional research on the possible functions of circadian clock genes has revealed that immune-related pathways are associated with the circadian clock molecular mechanism. These pathways in cancer includes the CTLA4 and PD-1 checkpoint route, the PD-L1 expression pathway,
TNF signaling system and the route for T cell receptor signaling. This support the hypothesis that the components of circadian clock plays an important role in regulating immune response [33]. Two essential circadian clock genes, PER1 and CRY2, have been linked to CD4+T cell expression [32]. Tumor growth is impacted by circadian modulation of the Tumor Microenvironment (TME), as evidenced by the deletion of clock genes in different immune cell types, such as neutrophils, endothelial cells, macrophages, T cells, and B cells as shown in Figure 3 [30].
Figure 3. The Function of Clock Elements in Immune cells Response in Cancer. The RORy, PER1, CRY2, and BMAL1 components of the circadian clock adversely influence the production of PD-1 in effector T cells. Furthermore, in effector T cells, BMAL1 inhibits the production of CTLA4 and PD-L1. PER1 and BMAL1 can increase NK cells' production of perforin, granzyme B, and IFN-γ. When cancer cells die, blood is exposed to necrotic substances and antigens from cancerous cells.
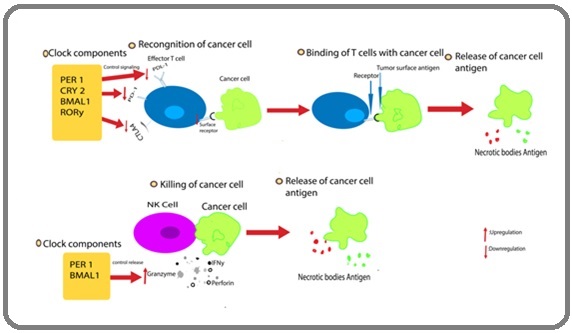
In a melanoma model, the researchers discovered that the time of day has a significant impact on the effectiveness of an immune response to tumor cells. Their results indicate that circadian clock components regulates the activation and function of CD8+ T cells, which thereby plays role in inhibition of tumors at specific times of the day. BMAL1 directly controls the production of CD80, that codes for a receptor on the cell surface of DCs and serves as an essential co-stimulatory protein for CD8+ T cells. Thus, circadian rhythms directly control CD80 signaling between CD8+ and DCs T cells, which in turn mediates the tumor-suppressive actions of the adaptive immune system (Figure 3) [14].
Metabolic reprograming
When circadian rhythm is disrupted, it leads to the activation of glycolysis (the Warburg effect), increases lipogenesis, and decreases oxidative phosphorylation [34]. This metabolic shift promote the tumor growth and proliferation [35]. It has been studied that BMAL1 and CLOCK upregulate glycolytic genes such HK2 and LDHA and increases the expression of c-MYC, a proto- oncogene that alter cellular metabolism to promote tumor growth [36, 37].
Furthermore, BMAL1 loss affects mitochondrial oxidative phosphorylation, which raises reactive oxygen species ROS levels and causes oxidative DNA damage [38]. NF-κB and other pro-survival pathways are favored by this change, which may also inhibit apoptosis by downregulating p53 [39].
Lipid metabolism is also regulated by circadian rhythm through nuclear receptors including RORγ and REV-ERBα,that is transcriptionally regulated by CLOCK:BMAL1. REV-ERBα suppresses lipogenic enzymes, such as FASN and SCD1, and this inhibition may encourage the accumulation of lipids in tumors [9].
This growing understanding of circadian metabolic crosstalk positions the circadian machinery as a promising therapeutic target in cancer chronotherapy and metabolism-based interventions.
Epigenetic Reprogramming
The disturbance of the circadian rhythm contributes to cancer through epigenetic reprogramming, which modifies patterns of gene expression essential for preserving cellular homeostasis [40].
Core clock components, especially CLOCK and BMAL1, have histone acetyltransferase activity and can remodel chromatin and control rhythmic transcription of genes of cell proliferation, DNA repair, and apoptosis. When circadian rhythm is disrupted, expression and function of these clock proteins are affected, resulting in loss of histone modification, including acetylation and methylation [41]. This dysregulation promotes oncogenic pathways but represses tumor suppressor genes by aberrantly recruiting chromatin-modifying enzymes such as histone deacetylases (HDACs) [42]. Further, interference with nuclear receptors like REV-ERBs and RORs, which regulate epigenetic landscapes, impacts chromatin remodeling and inflammatory gene expression [43, 44]. Experimental models, such as PER1/PER2 knockout mice, have proven global changes in chromatin structure and transcriptomic instability to confirm the function of circadian-regulated epigenetic mechanisms in tumorigenesis [45]. Therefore, circadian misalignment not only disrupts the temporal regulation of gene expression but also remodels the epigenetic landscape in a manner that promotes cancer development and progression.
Chronotherapy: Targeting circadian rhythm disruption in cancer treatment
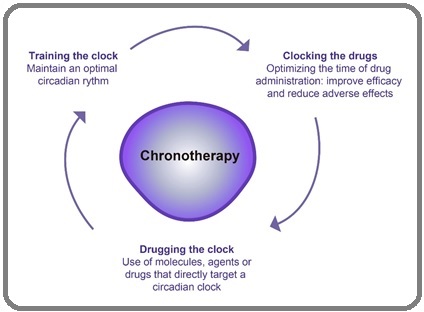
Now a days, there is increasing interest in understanding how circadian rhythms can be used to enhance the treatment outcome of various diseases. To achieve this, chronotherapeutic techniques that can be used alone or in combination are explained. The first includes any measures that promote or maintain an optimal circadian rhythm, such as “training the clock.” The second one involves the use of molecules or drugs that “drug” a circadian clock gene (“drugging the clock”). The third one concentrates on the timing and effectiveness of administering a drug; it aims to minimize undesirable side effects (“clocking the drugs”). Therefore, the two approaches, chronotherapy and clinical chronopharmacology, focus on the monitoring and control of how the treatment is being administered for its timed response to the circadian rhythm of the patient. They seek to optimize the drug’s action by maximizing health benefits while minimizing adverse effects on the patient. Consequently, chronotherapy involves administering a drug or applying a therapeutic intervention responsive to circadian rhythms (Figure 4) [46].
Figure 4. Chronobiological Approaches to the Treatment and Prevention of Cancer.
Effect of chronotherapy in chemotherapy
Certain drugs, including antimetabolites, antimitotic agents, intercalants, and alkylators, often achieve the most effective result when they are administered at the time best suited for them [47].
Aprime example illustrating the safety and effectiveness of the drug oxaliplatin along with chronopharmacology concluded that the most effective way to administrate oxaliplatin was with the help of a chronomodulated delivery system that reached its peak at 16:00 hours. The clinical effectiveness of oxaliplatin was confirmed in a large phase trial for colorectal cancer with this type of delivery and afterward confirmed in randomized Phase trials [48, 49]. According to preclinical research, patients with ovarian cancer who received doxorubicin in the morning (06:00) and cisplatin in the evening (16:00–20:00), when both medications are less toxic and the tumor response is greater, experienced fewer side effects and problems. In fact, patients who followed this schedule had a 44% chance of surviving at five years [46, 50]. Furthermore, individuals with advanced or recurrent endometrial cancer responded effectively to this doxorubicin and cisplatin regimen, with a 60% response rate. With an exceptional quality of life and little toxicity, this circadian-timed combination chemotherapy also produced a clinically complete response in the majority of patients with metastatic bladder cancer. According to current clinical research, the best time to start treatment may reduce medication toxicity and boost effectiveness, enabling a more effective yet dose-intensive course of treatment. Chemotherapy and chronotherapy together, therefore, seem like a promising treatment combination [50].
Effect of chronotherapy in radiotherapy
The use of radiotherapy at various times of the day hasn’t been well studied.Certain circadian genes contribute to the development of rhythmicity in mechanism induced by ionizing radiation like DNA repair or apoptosis, which increases a cell’s susceptibility to radiotherapeutic treatments during specific hours of the day [51]. According to a study on brain metastases in patients with non-small cell lung cancer, individuals who got radiotherapy in the morning, before 12:30, had a much higher survival rate than those who received treatment in the evening [52].The sex independency in radiatiotherapy is similar to that in chronomodulated chemotherapy. The full or partial response was greater in females who received radiation between 11:00 and 14:00 in bone metastases cases. Another study found that individuals with rectal cancer who underwent radiation therapy after midday had a greater tumor response, whereas women appeared to have a worse response [53, 54]. According to a research by Fuzisakki et al., individuals with breast cancer who had radiotherapy in the afternoon show less skin damage than those who got it in the morning.Thus, it’s critical to emphasize that each person’s biological clock plays a significant part in the treatment outcome [55].
Challenges and future of chronotherapy
The International Agency for Research on Cancer (IARC) and other studies have shown that when there is a disruption in the circadian rhythm, the chance of acquiring cancer, including breast cancer, intestinal cancer, GBM, Prostate cancer and lung cancer increases dramatically [11, 56, 57]. The majority of tissues and metabolic activities follow a 24-hour rhythm that is in line with circadian cycles, as shown by several research on circadian clock mechanism [58]. Furthermore, those who exhibit a disturbed circadian rhythm, such as shift workers, have an elevated chance of developing cancer [59]. Cancer patients with disrupted circadian rhythms had a worse prognosis, according to clinical research [60]. Treatment schedules can be more effectively administered, lessen adverse effects, and accentuate a patient’s quality of life overall by matching with their body’s natural circadian rhythm. Furthermore, healthcare professionals can reduce additional disturbances to the body’s circadian rhythm by strategically scheduling chemotherapy, radiation, and other treatments, which may result in improved outcomes for cancer treatment [59]. Recent advancements in these fields are highlighted by the unique perspective that circadian medicine has brought to more potent anticancer treatments [61].
According to the results, disrupted circadian rhythms may affect a person’s vulnerability to environmental influences and reaction to therapy for skin cancer. In the medical field, this work emphasizes the possibility of customized circadian-based treatments [62]. The difficulties with chronotherapy are summed up by Kuo and Ladurner in 2019 as follows: (a) conducting clinical trials that will provide a better understanding of how biological clocks affect that how anticancer medications work (b) investigating how specific factors affect how well a patient responds to chronotherapy (c) pressuring pharmaceutical companies to assess the toxicity and efficacy of their products in accordance with chronotherapy criteria. Furthermore, it is imperative to create and optimize more efficient delivery methods, including nanoparticle systems, to ensure that pharmaceuticals are administered according to circadian rhythm [63, 64]. Control theory, which takes into account the potential impact of certain environmental conditions on the response to therapy, has gained significant attention in recent times. In particular, there is increasing interest in using control theory to pharmacokinetic and pharmacodynamic models for anticancer treatments. With more tumor data becoming available, mathematical models that forecast the best therapy delivery parameters to regulate treatment response and progression time can be developed. But before it can be used effectively, a lot more study in this area is still required [65].
Acknowledgments
None
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Both authors played an important role in research and document writing. Each author thoroughly reviewed and gave their approval to the submitted manuscript and affirm that there are no competing interests with respect to this article’s publication.
References
- Timely Questions Emerging in Chronobiology: The Circadian Clock Keeps on Ticking Chawla S, O’Neill J, Knight MI , He Y, Wang L, Maronde E, Rodríguez SG , et al . Journal of Circadian Rhythms.;22. CrossRef
- A period without PER: understanding 24-hour rhythms without classic transcription and translation feedback loops Millius A, Ode KL , Ueda HR . F1000Research.2019;8. CrossRef
- Molecular mechanisms and physiological importance of circadian rhythms Patke A, Young MW , Axelrod S. Nature Reviews. Molecular Cell Biology.2020;21(2). CrossRef
- A molecular mechanism for circadian clock negative feedback Duong HA , Robles MS , Knutti D, Weitz CJ . Science (New York, N.Y.).2011;332(6036). CrossRef
- Oscillatory dynamics of the mammalian circadian clock induced by the core delayed negative feedback loop Wang C, Liu H, hang Y. Nonlinear Dynamics.2024;112(9):7527-40.
- Role of circadian transcription factor REV-ERB in cardiovascular diseases: a review Wang C, Yang J, Yuan J, Wang X, Li Q, Ren C, Zhi X, et al . Frontiers in Cardiovascular Medicine.2025;12. CrossRef
- Melanopsin, a Canonical Light Receptor, Mediates Thermal Activation of Clock Genes Moraes MN , Assis LVM , Magalhães-Marques KK , Poletini MO , Lima LHRG , Castrucci AMDL . Scientific Reports.2017;7(1). CrossRef
- Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons Ikeda M, Sugiyama T, Wallace CS , Gompf HS , Yoshioka T, Miyawaki A, Allen CN . Neuron.2003;38(2). CrossRef
- Distinct roles for REV-ERBα and REV-ERBβ in oxidative capacity and mitochondrial biogenesis in skeletal muscle Amador A, Campbell S, Kazantzis M, Lan G, Burris TP , Solt LA . PloS One.2018;13(5). CrossRef
- Cancer and the Circadian Clock Shafi AA , Knudsen KE . Cancer Research.2019;79(15). CrossRef
- Clocks, cancer, and chronochemotherapy Sancar A, Van Gelder RN . Science (New York, N.Y.).2021;371(6524). CrossRef
- Metabolic rivalry: circadian homeostasis and tumorigenesis Kinouchi K, Sassone-Corsi P. Nature Reviews. Cancer.2020;20(11). CrossRef
- Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML Puram RV , Kowalczyk MS , Boer CG , Schneider RK , Miller PG , McConkey M, Tothova Z, et al . Cell.2016;165(2). CrossRef
- Upregulation of circadian gene 'hClock' contribution to metastasis of colorectal cancer Wang Y, Sun N, Lu C, Bei Y, Qian R, Hua L. International Journal of Oncology.2017;50(6). CrossRef
- Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment Sulli G, Lam MTY , Panda S. Trends in Cancer.2019;5(8). CrossRef
- Hallmarks of cancer: the next generation Hanahan D, Weinberg RA . Cell.2011;144(5). CrossRef
- Cellular senescence and metabolic reprogramming: Unraveling the intricate crosstalk in the immunosuppressive tumor microenvironment Zhang F, Guo J, Yu S, Zheng Y, Duan M, Zhao L, Wang Y, Yang Z, Jiang X. Cancer Communications (London, England).2024;44(9). CrossRef
- Nicotinamide: Oversight of Metabolic Dysfunction Through SIRT1, mTOR, and Clock Genes Maiese K. Current Neurovascular Research.2020;17(5). CrossRef
- Histone methylation: at the crossroad between circadian rhythms in transcription and metabolism González-Suárez M, Aguilar-Arnal L. Frontiers in Genetics.2024;15. CrossRef
- Epigenetic gene silencing in cancer initiation and progression Nephew KP , Huang TH . Cancer Letters.2003;190(2). CrossRef
- Molecular Links between the Circadian Clock and the Cell Cycle Farshadi E, Horst GTJ , Chaves I. Journal of Molecular Biology.2020;432(12). CrossRef
- Cross-talk between the circadian clock and the cell cycle in cancer Soták M, Sumová A, Pácha J. Annals of Medicine.2014;46(4). CrossRef
- Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle Qu M, Zhang G, Qu H, Vu A, Wu R, Tsukamoto H, Jia Z, et al . Proceedings of the National Academy of Sciences of the United States of America.2023;120(2). CrossRef
- The role of circadian clock genes in tumors Li H. OncoTargets and Therapy.2019;12. CrossRef
- p53 regulates Period2 expression and the circadian clock Miki T, Matsumoto T, Zhao Z, Lee CC . Nature Communications.2013;4. CrossRef
- Association of the circadian factor Period 2 to p53 influences p53's function in DNA-damage signaling Gotoh T, Vila-Caballer M, Liu J, Schiffhauer S, Finkielstein CV . Molecular Biology of the Cell.2015;26(2). CrossRef
- Circadian regulation of c-MYC in mice Liu Z, Selby CP , Yang Y, Lindsey-Boltz LA , Cao X, Eynullazada K, Sancar A. Proceedings of the National Academy of Sciences of the United States of America.2020;117(35). CrossRef
- Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1 Kang T, Leem S. Nucleic Acids Research.2014;42(7). CrossRef
- Circadian angiogenesis Jensen LD , Gyllenhaal C, Block K. Biomolecular Concepts.2014;5(3). CrossRef
- The Circadian System Is Essential for the Crosstalk of VEGF-Notch-mediated Endothelial Angiogenesis in Ischemic Stroke Zhang Y, Zhao X, Guo C, Zhang Y, Zeng F, Yin Q, Li Z, Shao L, Zhou D, Liu L. Neuroscience Bulletin.2023;39(9). CrossRef
- The Relationship between Circadian Rhythm and Cancer Disease Munteanu C, Turti S, Achim L, Muresan R, Souca M, Prifti E, Mârza SM , Papuc I. International Journal of Molecular Sciences.2024;25(11). CrossRef
- Significance of Melatonin in the Regulation of Circadian Rhythms and Disease Management Megha K. B., Arathi A., Shikha S, Alka P, Ramya P, Mohanan P. V.. Molecular Neurobiology.2024;61(8). CrossRef
- Expression of the Circadian Clock Gene BMAL1 Positively Correlates With Antitumor Immunity and Patient Survival in Metastatic Melanoma Assis LVM , Kinker GS , Moraes MN , Markus RP , Fernandes PA , Castrucci AMDL . Frontiers in Oncology.2018;8. CrossRef
- Associations among Metabolism, Circadian Rhythm and Age-Associated Diseases Cao Y, Wang R. Aging and Disease.2017;8(3). CrossRef
- Metabolism and cancer: the circadian clock connection Sahar S, Sassone-Corsi P. Nature Reviews. Cancer.2009;9(12). CrossRef
- MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation Shostak A, Ruppert B, Ha N, Bruns P, Toprak UH , Eils R, Schlesner M, Diernfellner A, Brunner M. Nature Communications.2016;7. CrossRef
- Glycolysis under Circadian Control Zlacká J, Zeman M. International Journal of Molecular Sciences.2021;22(24). CrossRef
- Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH , Alexander RK , Unluturk U, et al . Cell Metabolism.2015;22(4). CrossRef
- Circadian adaptation to cell injury stresses: a crucial interplay of BMAL1 and HSF1 Tamaru T, Ikeda M. The journal of physiological sciences: JPS.2016;66(4). CrossRef
- Epigenetic Basis of Circadian Rhythm Disruption in Cancer Reszka E, Zienolddiny S. Methods in Molecular Biology (Clifton, N.J.).2018;1856. CrossRef
- The rhythms of histones in regeneration: The epigenetic modifications determined by clock genes Silveira EJD , Barros CCDS , Bottino MC , Castilho RM , Squarize C. Experimental Dermatology.2024;33(1). CrossRef
- Circadian clock as a possible control point in colorectal cancer progression (Review) Rao X, Lin L. International Journal of Oncology.2022;61(6). CrossRef
- Functional inversion of circadian regulator REV-ERBα leads to tumorigenic gene reprogramming Yang Y, Zhang X, Cai D, Zheng X, Zhao X, Zou JX, Zhang J, et al . Proceedings of the National Academy of Sciences of the United States of America.2024;121(42). CrossRef
- Chromatin remodeling and nuclear receptor signaling Buranapramest M, Chakravarti D. Progress in Molecular Biology and Translational Science.2009;87. CrossRef
- Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets Wang Y, Guo H, He F. Cancer Metastasis Reviews.2023;42(1). CrossRef
- Roles of circadian clocks in cancer pathogenesis and treatment Lee Y. Experimental & Molecular Medicine.2021;53(10). CrossRef
- Circadian chronotherapy for human cancers Lévi F.. The Lancet. Oncology.2001;2(5). CrossRef
- Medicine in the Fourth Dimension Cederroth CR , Albrecht U, Bass J, Brown SA , Dyhrfjeld-Johnsen J, Gachon F, Green CB , et al . Cell Metabolism.2019;30(2). CrossRef
- Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, Smaaland R, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2006;24(22). CrossRef
- Circadian chemotherapy for gynecological and genitourinary cancers Kobayashi M, Wood PA , Hrushesky WJM . Chronobiology International.2002;19(1). CrossRef
- Radiation chronotherapy-clinical impact of treatment time-of-day: a systematic review Shuboni-Mulligan DD , Breton G, Smart D, Gilbert M, Armstrong TS . Journal of Neuro-Oncology.2019;145(3). CrossRef
- Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment? Rahn DA , Ray DK , Schlesinger DJ , Steiner L, Sheehan JP , O'Quigley JM , Rich T. Cancer.2011;117(2). CrossRef
- Does chronomodulated radiotherapy improve pathological response in locally advanced rectal cancer? Squire T, Buchanan G, Rangiah D, Davis I, Yip D, Chua YJ, Rich T, Elsaleh H. Chronobiology International.2017;34(4). CrossRef
- Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases Chan S, Zhang L, Rowbottom L, McDonald R, Bjarnason GA , Tsao M, Barnes E, et al . Annals of Palliative Medicine.2017;6(1). CrossRef
- A protective effect of morning radiotherapy on acute skin toxicity in patients with breast cancer: A prospective cohort study Fuzissaki MA , Paiva CE , Oliveira MA , Maia MA , Canto PPL , Maia YCP . Medicine.2021;100(42). CrossRef
- Metastasis of Breast Cancer Promoted by Circadian Rhythm Disruption due to Light/Dark Shift and its Prevention by Dietary Quercetin in Mice Numata M, Hirano A, Yamamoto Y, Yasuda M, Miura N, Sayama K, Shibata M, et al . Journal of Circadian Rhythms.2021;19. CrossRef
- Circadian nutritional behaviours and cancer risk: New insights from the NutriNet-santé prospective cohort study: Disclaimers Srour B, Plancoulaine S, Andreeva VA , Fassier P, Julia C, Galan P, Hercberg S, et al . International Journal of Cancer.2018;143(10). CrossRef
- Clocking cancer: the circadian clock as a target in cancer therapy Battaglin F, Chan P, Pan Y, Soni S, Qu M, Spiller ER , Castanon S, et al . Oncogene.2021;40(18). CrossRef
- Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation Haus EL , Smolensky MH . Sleep Medicine Reviews.2013;17(4). CrossRef
- Carcinogenicity of night shift work Ward EM , Germolec D, Kogevinas M, McCormick D, Vermeulen R, Anisimov VN , et al . The Lancet. Oncology.2019;20(8). CrossRef
- A new time dimension in the fight against metastasis Diamantopoulou Z, Gvozdenovic A, Aceto N. Trends in Cell Biology.2023;33(9). CrossRef
- Population-level rhythms in human skin with implications for circadian medicine Wu G, Ruben MD , Schmidt RE , Francey LJ , Smith DF , Anafi RC , Hughey JJ , et al . Proceedings of the National Academy of Sciences of the United States of America.2018;115(48). CrossRef
- Exploiting the Circadian Clock for Improved Cancer Therapy: Perspective From a Cell Biologist Kuo TT , Ladurner AG . Frontiers in Genetics.2019;10. CrossRef
- Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle Lee Y, Fong SY , Shon J, Zhang SL , Brooks R, Lahens NF , Chen D, Dang CN , Field JM , Sehgal A. Science Advances.2021;7(7). CrossRef
- Control Theory and Cancer Chemotherapy: How They Interact Lecca P. Frontiers in Bioengineering and Biotechnology.2020;8. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Nursing , 2025
Author Details
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times