Adults Myelodysplastic Syndromes in Algeria: A Study by the Algerian MDS Group
Download
Abstract
Introduction: Myelodysplastic syndromes (MDS) are a group of haematological disorders, whose diagnosis is based mainly on cytological studies of blood and marrow cells and cytogenetic analysis. Moreover, national epidemiological data on MDS are very scarce, especially in Maghreb countries where the population is on average younger than in Europe or the USA. The objective of the present study was to describe demographic and clinical features and the overall survival of patients with MDS in Algeria.
Materials and Methods: This study is retrospective and national multicenter (n=19 centres), performed between 2014 to 2019. The evaluation was performed using EPI-INFO and SPSS version 21 software. Survival data were calculated using the Kaplan-Meier method and comparison of survival curves using the Log Rank test. Univariate and multivariate analysis of survival was performed using the Cox regression method. The study has been approved by the Ethical and Scientific Council of the participating hospitals. The closing date of the study is 31/12/2019.
Results: A total of 670 patients with newly diagnosed MDS have been identified. The average number of new cases was 112/year, with an annual progression rate of 19%. Demographics show a slight female predominance (M/F of 317/353=0, 89; sex ratio F/M=1.11). The median follow-up was 29,3 months (range, 1 to 77 months). The overall median age was 69 years (range 16-96). The crude mean annual incidence rate was 0, 38 per 100,000 inhabitants aged ≥15 years old and it was 0, 17/100,000 in men and 0, 21/100,000 in women. Overall survival was 39 months. According to the IPSS score, the high-risk forms are low and their overall survival was 15 months. The rate of transformation into acute myeloid leukaemia (AML) is 32%.
Conclusion: This national epidemiological survey shows an annual progression rate of 19% and an increase in incidence from 0.007/100.000 in 2005 to 0.45/100.000 in 2019.
Introduction
Myelodysplastic syndromes (MDS) are bone marrow stem cell disorders characterized by ineffective hematopoiesis with morphologic abnormalities. The diagnosis is mainly based upon morphologic evaluation of peripheral blood and bone marrow smears and the exclusion of other causes of cytopenia and dysplasia [1]. MDS are regarded as preleukemic states, which may, in one third of the patients, progress to acute myeloid leukemia [2]. The main risk factors for MDS are old age, male gender, previous chemotherapy or radiotherapy, family history of hematopoietic neoplasms, and exposure to chemical carcinogens such as benzene and formaldehyde [3]. The prognosis of the disease depends on the proportion of blasts in bone marrow, severity of cytopenia and somatic gene or karyotype abnormalities [4]. The World Health Organization (WHO) developed the subsequent classifications published in 2001, 2008 and 2016 [5]. Research on occurrence of MDS is complicated by changes over time in diagnostic activities and criteria as well as classification practices at cancer registries [6]. This has also led to paucity of studies on MDS incidence. Previous studies of the incidence of MDS, in Algeria, have reported overall age-standardized incidence rate (ASIR) ranging 0.07–0.27 per 100,000 person-years [7].
A large study based on different cancer registries estimated that the ASIR of MDS in Europe years 2000– 2002 was 1.24 per 100,000 person-years [8]. According to an Oceanian study, the ASIR of MDS was 3.2 in Australia and 3.7 in New Zealand [9].
Several studies have found higher incidence of MDS among men than women and major increases with age [10]. In general, the incidence of MDS increased exponentially after 60 years of age [11]. In a US study, the incidence rate of MDS was triple among 70–74-year-olds compared to the age group of 60–64 years, and almost sevenfold at age 80–84 years [12]. In the Netherlands, the incidence rate in the age group of 70–79 years was about double and after age 80 years about fourfold compared to ages 60-69 years [13]. Their treatment is currently based on prognostic scores, which are regularly updated [14] and vary from therapeutic abstention to allogeneic stem cell transplantation [15]. Cytogenetic and molecular analysis of MDS has allowed the development of hypomethylating treatments such as Azacytidine [16] or Decitabine [17] or immunomodulating treatments such as Lenalidomide [18] and more recently of Luspatercept [19]. National multicenter epidemiological surveys allow an evaluation of the incidence, and an analysis of the patients’ recruitment profile, allowing a posteriori an evaluation of the performance of the country’s health coverage but also planning of the diagnostic and therapeutic means, necessary for the evaluation of the public health budgets. In general, epidemiological studies on MDS remain scarce, and national epidemiological data on MDS are very scarce, especially in Maghreb countries where the population is on average younger than in Europe or the USA. The objective of the present study was to describe demographic and clinical features and for the first time, therapeutic results of patients with MDS in Algeria.
Materials and Methods
This study is retrospective, descriptive, and national multicenter, performed between 2014 to 2019. The survey data were collected through an Excel spreadsheet including epidemiological, clinical, and biological items at diagnosis as well as the evaluation of therapeutic modalities of MDS and sent to all haematology departments of the country. Demographic data, as well as disease information (French American British classification, FAB [20] and WHO classification, cytogenetic, laboratory findings at diagnosis, International Prognostic System Scoring (IPSS) [21], transfusion dependency, revised IPSS (R-IPSS) [22], and leukemic evolution), were collected and analyzed for all patients (aged ≥15 years) diagnosed during the study period. The crude or specific incidence rates according to age and sex were calculated according to estimates of the Algerian population aged over 15 years from January 1, 2014, to January 2019 (Sources: World Health Organization (WHO) classification, Office National des Statistiques (ONS) and World Bank) [23]. The evaluation was performed on all the above items using EPI-INFO and Statistical Package for the Social Sciences (SPSS version 21 software). Survival data were calculated using the Kaplan-Meier method and comparison of survival curves using the Log Rank test. Univariate and multivariate analysis of survival was performed using the Cox regression method. The study has been approved by the Ethical and Scientific Council of the participating hospitals. The closing date of the study is 31/12/2019.
Results
Demographic data
As of 12/31/2019, 670 patients were collated over 6 years, at 19 haematology departments in the country (Figure 1(a)).
Figure 1a: Department of Haematology in Algeria. Footnotes, Centre Pierre et Marie Curie, Algiers (n=143 patients), Centre de Lutte contre le Cancer Blida (n=74 patients), Centre Hospitalier Universitaire de Tlemcen (n=53 patients), Centre Hospitalier Universitaire de Sidi Belabbès (n=52 patients), Centre Hospitalier Universitaire de Annaba (n=49 patients), Centre Hospitalier Universitaire de Tizi-Ouzou (n=48 patients), Hôpital civil des Armées Med Seghier Nekkache (n=41 patients), Centre Hospitalier Universitaire de Sétif (n=33 patients), Centre Hospitalier Universitaire de Beni Messous Algiers (n=29 patients), Etablissement Hospitalier Universitaire d’Oran (n=22 patients), Centre Hospitalier Universitaire de Bejaia (n=21 patients), Centre de Lutte contre le Cancer Batna (n=20 patients), Centre Hospitalier Universitaire de Blida (n=15 patients), Centre Hospitalier Universitaire d’Oran (n=15 patients), Etablissement Public Hospitalier de Mascara (n=14 patients), Centre Transfusion Sanguine de Beni Messous Algiers (n=13 patients), Centre Hospitalier Universitaire de Constantine (n=12 patients), Centre Hospitalier Universitaire de Batna (n=10 patients), Hôpital Militaire Régional Universitaire d’Oran (n=6 patients).
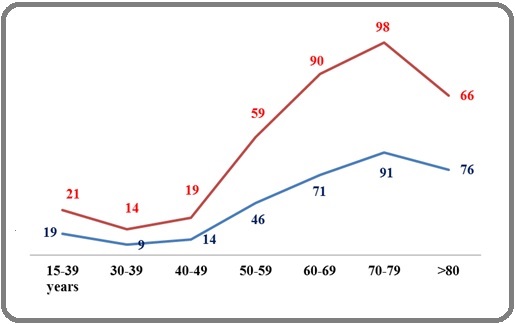
The distribution by years is as follows: 2014 (n=77), 2015(n=124), 2016(n=99), 2017(n=108), 2018 (n=127) and 2019 (n=135). The number of new MDS cases gradually increased from 77 during the year 2014, to 135 during the year 2019. The average number of new cases is 112/year, with an annual progression rate of 19%. The regional distribution shows Central (n=363pts; 54%), Western (n=162 patients; 24%) and Eastern (n=145 patients; 22%). Demographics show a slight female predominance (M/F=317/353=0,89; sex ratio F/M=1.11). The mean age at diagnosis was 67 years (16-96 years; median= 69 years). The mean age of males was 68 years (19-96; the median was 70 years) and the mean age of females was 66, 2 years (16-94; the median was 68 years). The age distribution shows a low rate =11% between 15-50 years and a peak frequency of 52% (n=350) between 60-79 years. Patients aged 60-79 years show a female predominance of 54% (n=189 patients) and a sex ratio of 1,17 (Figure 1(b)).
Figure 1b: Age and Sex Distribution of MDS in Algeria. Footnotes; MDS, Myelodysplastic syndromes, Reed, Female; Blue, Male.
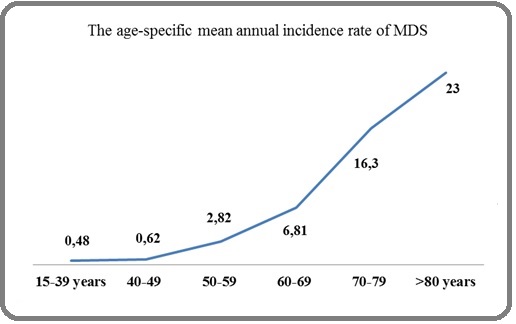
The average annual crude incidence for the period 2014-2019 was 0,38/100.000 (Figure 1(c)). The mean annual gender-specific incidence was 0,17/100.000 inhabitants for males and 0,21/100.000 for females.
Figure 1c: Age-specific Mean Annual Incidence Rate of MDS in Algeria. Footnotes, MDS, Myelodysplastic syndromes.
Clinical results
Baseline haematological parameters are summarized in Table 1.
| Parameter | Number | % | Normal ranges |
| Total patients | 670 | 100 | - |
| Median age (range, years) | 69 (16-96) | ||
| Median age (range, years), Male | 70 (19-96) | - | |
| Median age (range, years), Female | 68 (16-94) | ||
| Gender M: F ratio | 317/353; 0.89 | - | |
| Pallor | 590/652 | 90 | - |
| Fatigue | 578/652 | 89 | - |
| Fever/infection | 75/654 | 11 | - |
| Bleeding/Bruising | 114/654 | 17 | - |
| Mean Hemoglobin (g/dl) | 8 (3-14) | 16-Dec | |
| Mean White Blood Cells (G/L) | 4.62 (0.4-186) | 4-10 G/L | |
| Mean Absolute Neutrophil Count (ANC) (G/L) | 2.17 (0,99-2,8) | 2-7.5 | |
| Mean platelets (G/L) | 126 (33-683) | 150-450 G/L | |
| Mean Lacticodehydrogenase (IU/l) | 378 (213-429) | 135-214 | |
| Cytopenia | |||
| § Single cytopenia | 231/654 | 35 | |
| § Bicytopenia | 222/654 | 34 | - |
| § pancytopenia | 201/654 | 31 | |
| Marrow Blasts (median, range,%) | 4.5 (0-23) | <5% | |
| Ring sideroblasts>15% | 150/495 | 30,3 | 0% |
The mean Absolute Neutrophil Count (ANC) was 4, 62 G/L, and the mean platelets were 126 G/L. Moreover, 34 % of the patients had various cytopenias and 31% were pancytopenia. Clinical signs were dominated by anaemia symptoms in 90% (590/652), asthenia in 89% (578/652), infection in 11% (75/654) and haemorrhage in 17% (114/654). Perl’s staining was performed in 74% of patients (n=495/670) of which 173/495 (35%) were Perls positive: Perls<15%=23(13%) patients, Perls≥15%=150 (87%) patients.
FAB and WHO classifications
According to FAB classification (n=641 patients; 96%), the distribution was as follows (Table 2(a)): refractory anaemia (RA) 330 (51,48%) patients, RA with ring sideroblasts (RARS) 79 (12,32% ), RA with an excess of blasts (RAEB) 195 (30,42% ), RAEB in transformation (RAEB-T) 11 (2%), chronic myelomonocytic leukaemia (CMML) 24 (4%), and unclassified MDS 2 (0,31%) patients.
| FAB classification (n=641) | number | % |
| RA | 330 | 51 |
| RARS | 79 | 12 |
| RAEB | 195 | 30.7 |
| RAEB-t | 11 | 2 |
| CMML | 24 | 4 |
| Unclassified | 2 | 0.3 |
Footnotes, FAB, French-American-British classification; RA, refractory anaemia; RARS, refractory anaemia with ring sideroblasts; RAEB, refractory anaemia with excess of blasts; RAEB-t, refractory anaemia with excess of blasts in transformation; CMML, chronic myelomonocytic leukaemia.
WHO classification According to the WHO 2016- classification (n=649 patients; 97%), the distribution was as follows (Table 2 (b)): 120 (18%) patients had MDS with single lineage dysplasia (MDS SLD), 171 (26%) patients had MDS with multilineage dysplasia (MDS MLD), 65 (10%) patients had MDS with ring sideroblasts with single lineage dysplasia (MDS RS (SLD)), 45 (7%) patients had MDS with ring sideroblasts with multilineage dysplasia (MDS RS (MLD)), 94 (14%) patients had MDS with excess blasts-1 (MDS EB1), 94 (14%) patients had MDS with excess blasts-2 (MDS EB2), 15 (2%) patients had AML, 24 (4%) patients had CMML and 21/196 (10,7%) patients had MDS with isolated del (5q).
| WHO classification (n=649) | Number patients | % |
| MDS SLD | 120 | 18 |
| MDS MLD | 171 | 26 |
| MDS RS (SLD) | 65 | 10 |
| MDS RS (MLD) | 45 | 7 |
| MDS EB1 | 94 | 14 |
| MDS EB2 | 94 | 14 |
| AML | 15 | 2 |
| CMML-1 | 16 | 2,4 |
| CMML-2 | 8 | 1 |
| 5q- syndrome | 21/196 | 10,7 |
Footnotes, WHO, World Health Organization; MDS, myelodysplastic syndromes; SLD, single lineage dysplasia; MLD, multilineage dysplasia; RS, ring sideroblasts; EB-1or 2, excess of blasts; AML, acute myeloblastic leukaemia; CMML, chronic myelomonocytic leukaemia-1 and 2.
Cytogenetic classification
Cytogenetic analysis was performed in 196 patients (29%). The karyotype was normal in 139 (71%) and abnormal in 57 (29%). The most commonly detected cytogenetic abnormalities were: trisomy 8 (4,08%), chromosome 7 abnormalities in 4,08%, and multiple (≥3) or complex chromosomal abnormalities in 6,12%. Del (5q) was found in 10,7% (n=21) of the patients and was more frequent in women (p=0.006).
Prognostic classifications
Prognostic classifications were shown in Table 2(c).
| Prognostic Classifications | number | % |
| IPSS (n=647 patients) | ||
| - Low risk | 210 | 32.45 |
| - Intermediate risk-I | 338 | 52.24 |
| - Intermediate risk-II | 79 | 12.21 |
| - High risk | 20 | 3.09 |
| R-IPSS (n=196 patients) | ||
| - Very low risk | 32 | 16 |
| - Low risk | 66 | 34 |
| - Intermediate risk | 57 | 29 |
| - High risk | 25 | 13 |
| - Very high risk | 16 | 8 |
| Cytogenetic classification (IPSS) (n=196 patients) | ||
| - Favourable | 157 | 80 |
| - Intermediate | 16 | 8 |
| - Unfavourable | 23 | 12 |
Footnotes, IPSS, International Prognostic System Scoring; R-IPSS, Revised International Prognostic System Scoring.
According to IPSS classification (n=647 patients) (96,5%) the distribution was as follows, low-risk in 210 (32,4%), intermediate-1 in 338 (52,24%), intermediate-2 in 79 (12,21%), and high-risk in 20 (3,09%) patients (Table 3).
| univariate analysis | multivariate analysis | |||||
| HR | CI:95% | p-value | HR | CI:95% | p-value | |
| Age>69 years | 0.84 | 0.7-0.95 | 0.004 | 0.8 | 0.7-0.95 | 0.005 |
| Male | 0.7 | 0.5-0.8 | 0.002 | 0.8 | 0.6-1.03 | 0.09 |
| Hb<6g/dl | 0.8 | 0.7-09 | 0.008 | 0.8 | 0.7-0.9 | 0.006 |
| ANC<0.5 G/l | 0.7 | 0.6-08 | <0.001 | 0.86 | 0.7-1.5 | 0.14 |
| Platelets<50 G/l | 1 | - | <0.001 | 1 | - | 0.6 |
| Cytopenia>1 | 1 | - | <0.001 | 1 | - | 0.003 |
| Serum Ferritin>500 | 1 | - | 0.001 | - | - | - |
| LDH>700 IU/L | 0.75 | 0.58-0.96 | 0.023 | - | - | - |
| Marrow Blasts>5% | 1 | 0.98-1.01 | <0.001 | 1 | - | 0.002 |
Footnotes, Hb, hemoglobin; ANC, absolute neutrophil count; LDH, lacticodehydrogenase; HR, hazard ratio; CI, confidence interval.
According to R-IPSS classification (n=196 patients) (29%), the distribution was as follows, very-low-risk group in 32 (16%) patients, low-risk in 66 (34%) patients, Intermediate-risk in 57 (29%) patients, the high-risk group in 25 (13%) and very-high-risk in 16 (8%) patients. In 474 (71%) patients cytogenetic results were unavailable, and therefore the R-IPSS could not be calculated.
According to cytogenetic classification (n=196 patients), the distribution was as follows: Favorable risk in 157 patients (80%), intermediate risk group in 16 patients (8%) and unfavourable risk group in 23 patients (12%) (Table 2(c)).
Therapeutic results
In terms of supportive care, Reed Blood Cells (RBC) transfusion was performed in 435 /654 (67%) patients with a mean number of 9, 53RBC concentrates/year. Iron chelation was performed in 19% (124/639) of patients. Platelet transfusions were performed in 108/652 (17%) patients. Erythropoietin (EPO) was prescribed in 282/654 (43%) patients, Hematopoietic growth factors (Granulocyte Colony Stimulating Factor, G-CSF) in 73/654 (11%) patients, androgens in 27/654 (4%) patients, Ciclosporin in 9/654 (1,3%) patients, Lenalidomide in 40/668 (6%) patients, low dose Cytarabine in 21/654 (3%) patients and Azacytidine in 117/650 (18%) patients. Finally, a hematopoietic stem cell transplant was performed in only 11 /647 (2%) patients with a mean age of 36 years (range 22-65 years).
Survival results
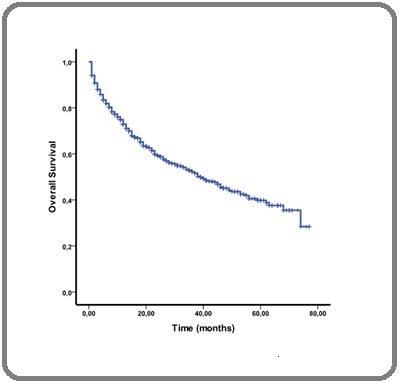
The median follow-up was 29,3 months (range, 1 to 77 months). Survival analysis showed a median overall survival (OS) for the entire cohort of 39 months (Figure 2(a)).
Figure 2a. Overall Survival for the Whole 2014-2019 Period.
The median OS for the three health regions, West, Central and East, were 38 months, 35 months and 50 months respectively (p=0,17; p=0,33). OS according to IPSS was shown in Figure 2(b).
Figure 2b. Overall Survival Stratified by MDS Type According to 2016 IPSS Classification. Footnotes, MDS, Myelodysplastic Syndromes; IPSS, International Prognostic System Scoring.
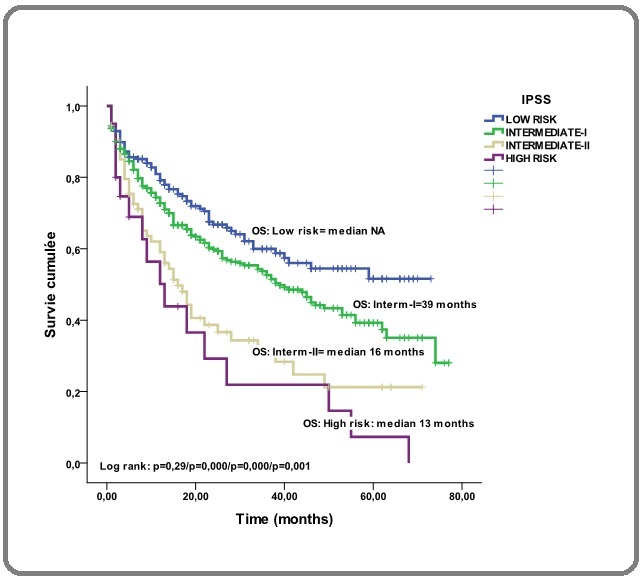
In the low-risk, the median was not reached, in intermediate –I-risk, the median was 39 months (p=0,29), in the intermediate-II-risk, the median was 16 months (p=0,0001) and in the high-risk group, the median was 13 months (p=0,001). OS according to R-IPSS was shown in Figure 2(c).
Figure 2c. Overall Survival Stratified by MDS Type According to 2016 R-IPSS classification. Footnotes, MDS, myelodysplastic syndromes; R-IPSS, Revised International Prognostic System Scoring.
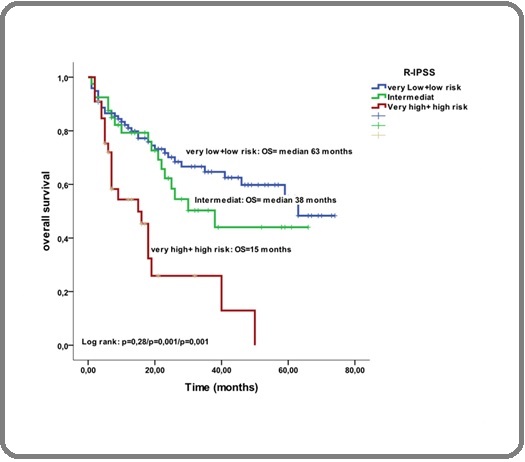
The median OS in the very-low and the low-risk group, was 63 months, while was 38 months in the intermediate-risk group (p=0,001) and 15 months in the high and very-high-risk group (p=0,001). Del-5q syndrome was more frequent in women and the OS was 54% at 5 years. The results of the search for predictive prognostic factors on survival in univariate and multivariate analysis are reported in Table 3. After a mean duration of the dysplastic phase of 23 months (range 1–77 months), the disease progressed to secondary AML in 216 patients (32%), whereas no progression was observed in 370 (55%). In 84 patients (13%), the progression status was unknown. The rate of death was 45% (n=300), among them 216 (72%) patients with AML.
Discussion
Demographic data
The incidence varies from 4 to 5 new cases/100.000 inhabitants/year and is constantly increasing with age, to reach 20 new cases/100.000 inhabitants/year in patients older than 70 years [24]. In Greece, the crude mean annual incidence rate was 6,0 per 100,000 inhabitants [25]. Our study shows an average annual number of 112 new cases per year and an annual progression rate of 12% with a lower gross annual incidence of 0,38/100.000 inhabitants, due to the younger age of the Algerian population, half of which is under 30 years old, but also, probably due to insufficient diagnosis. In addition, in Europe, the population aged over 65 years represents 17, 33% while in Algeria; it represents only 6,17% in 2020 i.e almost 3 fold more. The same results are found in the other Maghreb countries (Tunisia and Morocco), as they have the same demographic characteristics [26-27]. At the local level, more than half of the patients are diagnosed in the centre of the country (54%), 24% in the west (p<0.0001) and 22% in the east of the country (p<0.0001) and this is related to the distribution of the Algerian population and the medical density, more concentrated in the centre of the country. At diagnosis, the mean age was 67 years (16-96) with 30% of patients below 60 years and 52% of patients between 60 and 79 years. These results show a higher age than in a previous survey conducted between 1995 and 2005, where the mean age was 65 years, 60% of patients ≤ 60 years (p<0.0001) and 28% between 60 and 79 years (p<0.0001). This is therefore likely to represent an epidemiological transition as there was a 24% increase in MDS cases over 14 years, reflecting an ageing population, whose life expectancy has increased from 73 years in 2005 to 76.5 in 2017 [28]. Moreover, the age of our MDS patients is higher than in Tunisia (mean age=55 years) [26], Morocco (mean age=66 tears) [27], Egypt (mean age=55 years) [29] or Pakistan (mean age=58 years) [30], but still lower than in Greece (mean age=73,1 years) [25] or in Europe (mean age=71 years) [31-32]. In our study, a slight female predominance (ratio F/M=1.11) was found, particularly in the age group between 65 and 70 years (ratio F/M=1,17), as well as in Egypt (ratio=1,3), which differs from the slight male predominance generally reported in western countries. This difference can be explained by the ethnic and racial heterogeneity of MDS in the world and may be also attributable to differences in geographic areas [6,33].
Clinical data
The clinical characteristics are dominated by anaemia (90%) and asthenia (89%), and biologically a single lineage involvement in 35% of the cases and more than one lineage in 65% of the cases, like those found in Mahmoud et al [30].
FAB and WHO classifications
The FAB classification, shows a distribution similar to the literature with a predominance of RA in our series (51%) versus 42,7% in Greece [25], and 36,6% in El Husseiny Noha M et al [29] and 36% in Lee JH. Et al [34], RARS 12,32% versus 11,2% in Greece, RAEB 30,42% versus 39% in Greece. The WHO classification is dominated by MDS SLD (18%) and multi-cell lineage involvement (26%). The 5q- syndrome was found in 10,7% (n=21/196) in our study, with an average age of 66 years and a clear female predominance (F/M:2,5). Although some cases of MDS with 5q- syndrome may have been overlooked in our study due to the lack of cytogenetic analysis in most patients. This same rate was found in Chaubey et al with a rate of 10% [33], and 10,5% in Voso et al [35].
IPSS and R-IPSS Classifications
The IPSS classification of 647 patients shows that only one-third of the patients are at low risk (32,45%) and small patients had a high-risk 3%. These findings were similar in Greece.
According to the R-IPSS classification, 50% of our patients were in low and very-low-risk, 29% in Intermediate-risk and 21% in high and very-high-risk. These results were similar to those of Greenberg et al [21], who reported 57% in low and very-low-risk, 20% in Intermediate-risk and 23% in high and very-high-risk groups. Also, in an Italian study, Voso et al showed 38% in the very-low-risk group, 33% in the low-risk, and 18% in the Intermediate-risk group [35].
Therapeutic results
Regarding treatment, the national survey revealed a great heterogeneity, apart from a blood transfusion, which was performed in 67% of patients with an average rate of 10 RBC concentrates per year, and the absence of a consensual approach, particularly in the high-risk forms, with only 18% of patients treated with hypomethylating agents (Azacytidine). Allogeneic hematopoietic stem cell transplantation (HSCT) was performed in only 2% of patients, due to the advanced age of patients and the limited number of allogeneic transplantation centres in our country (only two centres).
Survival
The median follow-up in our study was 29,3 months (range, 1-77) and OS was 39 months for the entire cohort. Three hundred (45%) of the patients were dead and 370 (55%) were alive. These outcomes were similar to those published in the literature [12,36]. As expected, the probabilities of OS according to the IPSS score were better in the low and intermediate-risk groups than in the high- risk groups (Figure 2(b)) (p=0.02-p<0.0001-p<0.0001). These results are substantially the same as those published by Greenberg et al [21].
The search for factors predictive of OS revealed, in multivariate analysis, age over 69 years as well as Hb<6g/ dl, marrow blasts>5% and the number of cytopenias>1.
In the future, we are thinking of developing cytogenetics and molecular biology platforms in MDS in Algeria. We will also further treat patients with lenalidomide, azacytidine and allogeneic stem cell transplantation in high-risk forms.
In conclusion, this third national epidemiological survey shows an annual progression rate of 19% and an increase in incidence from 0.007/100.000 in 2005 to 0.45/100.000 in 2019. The incidence of MDS has increased due to the increase in life expectancy at birth in Algeria. This is an epidemiological transition, but also the increase in the number of haematology departments and the improvement of diagnosis of MDS. Today, the age at diagnosis and the peak frequency are identical to those described in the literature; however, a female predominance was noted in our study. The low rate of cytogenetic use (29%) at the national level remains the most important negative point in the diagnosis of MDS. The therapeutic management of MDS patients, with in particular 19% of iron chelation and only 6% of the use of IMiDs, 18% of 5-Azacytidine and 2% of hematopoietic stem cell transplant is still limited and must be improved. Our retrospective study was limited and our results should be verified in other studies.
Authors’ contribution
All authors worked on the conception of the article. MAB, PF wrote the first draft of the article. All authors reviewed and vouched for content.
Declaration of Competing Interest
The authors report no conflicts of interest.
Ethics approval
The study was approved by the Ethics Committee of Etablissement Hospitalier Universitaire Hospital Oran compliant with the Helsinki Declaration.
Acknowledgements
We thank the medical staff and patient participants.
References
- Myelodysplastic syndromes Tefferi A, Vardiman JW . The New England Journal of Medicine.2009;361(19). CrossRef
- Myelodysplastic syndromes: From pathogenesis and prognosis to treatment Fenaux P. Seminars in Hematology.2004;41(2 Suppl 4). CrossRef
- Validation of the revised international prognostic scoring system (IPSS-R) in patients with myelodysplastic syndrome: a multicenter study Neukirchen J, Lauseker M, Blum S, Giagounidis A, Lübbert M, Martino S, Siragusa S, et al . Leukemia Research.2014;38(1). CrossRef
- Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010 Sekeres MA . Journal of the National Comprehensive Cancer Network: JNCCN.2011;9(1). CrossRef
- Changes in the Updated 2016: WHO Classification of the Myelodysplastic Syndromes and Related Myeloid Neoplasms Bennett JM . Clinical Lymphoma, Myeloma & Leukemia.2016;16(11). CrossRef
- Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics Aul C., Giagounidis A., Germing U.. International Journal of Hematology.2001;73(4). CrossRef
- Epidemiology and Clinical Features of Adults Myelodysplastic Syndromes in Algeria: A Population-Based Study. Review of the Algerian Myelodysplastic Syndromes Study Group. Blood 2015 126:5253; published ahead of print December 4, 2015 Bekadja MA , Ahmed Nacer R, Hamladji RM , Boudjerra N, Belhani M, Ardjoun FZ , et al . .
- Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadié M, Simonetti A, Lutz JM , Berrino F. Blood.2010;116(19). CrossRef
- Myelodysplastic syndrome in New Zealand and Australia Rodger EJ , Morison IM . Internal Medicine Journal.2012;42(11). CrossRef
- Age-related incidence and other epidemiological aspects of myelodysplastic syndromes Aul C., Gattermann N., Schneider W.. British Journal of Haematology.1992;82(2). CrossRef
- Incidence of myelodysplastic syndromes in Finland 1997-2016 Kontro S, Raitanen J, Porkka K, Auvinen A. Leukemia Research.2022;116. CrossRef
- Myelodysplastic syndromes: incidence and survival in the United States Ma X, Does M, Raza A, Mayne ST . Cancer.2007;109(8). CrossRef
- Trends in incidence, initial treatment and survival of myelodysplastic syndromes: a population-based study of 5144 patients diagnosed in the Netherlands from 2001 to 2010 Dinmohamed AG , Visser O, Norden Y, Huijgens PC , Sonneveld P, Loosdrecht AA , Jongen-Lavrencic M. European Journal of Cancer (Oxford, England: 1990).2014;50(5). CrossRef
- How we manage adults with myelodysplastic syndrome Fenaux P, Platzbecker U, Ades L. British Journal of Haematology.2020;189(6). CrossRef
- Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS Deeg HJ , Scott BL , Fang M, Shulman HM , Gyurkocza B, Myerson D, Pagel JM , et al . Blood.2012;120(7). CrossRef
- Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study Fenaux P, Mufti GJ , Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, et al . The Lancet. Oncology.2009;10(3). CrossRef
- Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome Kantarjian HM , O'Brien S, Shan J, Aribi A, Garcia-Manero G, Jabbour E, Ravandi F, Cortes J, Davisson J, Issa JP . Cancer.2007;109(2). CrossRef
- Impact of somatic mutations on response to lenalidomide in lower-risk non-del(5q) myelodysplastic syndromes patients Santini V, Fenaux P, Giagounidis A, Platzbecker U, List AF , Haferlach T, Zhong J, et al . Leukemia.2021;35(3). CrossRef
- Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes Fenaux P, Platzbecker U, Mufti gj , Garcia-Manero G, Buckstein R, Santini V, Díez-Campelo M, et al . The New England Journal of Medicine.2020;382(2). CrossRef
- Proposals for the classification of the myelodysplastic syndromes Bennett JM , Catovsky D, Daniel MT , Flandrin G, Galton DA , Gralnick HR , Sultan C. British Journal of Haematology.1982;51(2).
- International scoring system for evaluating prognosis in myelodysplastic syndromes Greenberg P, Cox C, LeBeau MM , Fenaux P, Morel P, Sanz G, Sanz M, et al . Blood.1997;89(6).
- Revised international prognostic scoring system for myelodysplastic syndromes Greenberg PL , Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM , et al . Blood.2012;120(12). CrossRef
- ONS and World Bank. ONS.dz 15 .
- WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed Swerdlow , Campo E, Harris NL , Jaffe ES , Pileri SA , Stein H, Thiele J, Vardiman JW . Lyon: International Agency for Research on Cancer.2008.
- The incidence of myelodysplastic syndromes in Western Greece is increasing Avgerinou C, Alamanos Y, Zikos P, Lampropoulou P, Melachrinou M, Labropoulou V, Tavernarakis I, et al . Annals of Hematology.2013;92(7). CrossRef
- Evolution in the diagnosis of myelodysplastic syndromes: Experience of Farhat Hached hospital Braham-Jmili N, Becha M, Achour B, Mezrigui R, Ben youssef Y, Sendi-Sennana H, et al . Rev Tun Biol Clin.2019;27(02):5-13.
- The epidemiology of myelodysplastic syndromes. A retrospective study in the Hematology Department of the Military Hospital of Rabat. Yahyaoui A, Zahid H, Labrini F, Hadef R, Messaoudi N. International Journal of Medicine & Health Research.2016;:Edition 1.
- https://www.aps.dz (ons.dz) .
- Myelodysplastic Syndrome: An Egyptian Experience El Husseiny Noha M, Shereef AM , Mattar Mervat M. J Blood Disord Transfus.2012;3:3.
- Myelodysplastic Syndrome in Pakistan: Clinicohematological Characteristics, Cytogenetic Profile, and Risk Stratification Mahmood R, Altaf C, Ahmed P, Khan SA , Malik HS . Turkish Journal of Haematology: Official Journal of Turkish Society of Haematology.2018;35(2). CrossRef
- Trends of classification, incidence, mortality, and survival of MDS patients in Switzerland between 2001 and 2012 Bonadies N, Feller A, Rovo A, Ruefer A, Blum S, Gerber B, Stuessi G, et al . Cancer Epidemiology.2017;46. CrossRef
- Incidence and prevalence of myelodysplastic syndromes: data from the Düsseldorf MDS-registry Neukirchen J, Schoonen WM , Strupp C, Gattermann N, Aul C, Haas R, Germing U. Leukemia Research.2011;35(12). CrossRef
- Cytogenetic profile of Indian patients with de novo myelodysplastic syndromes Chaubey R, Sazawal S, Dada R, Mahapatra M, Saxena R. The Indian Journal of Medical Research.2011;134(4).
- Application of different prognostic scoring systems and comparison of the FAB and WHO classifications in Korean patients with myelodysplastic syndrome Lee JH , Shin YR , Lee JS , Kim WK , Chi HS , Park CJ , Seo EJ , Lee KH . Leukemia.2003;17(2). CrossRef
- Revised International Prognostic Scoring System (IPSS) predicts survival and leukemic evolution of myelodysplastic syndromes significantly better than IPSS and WHO Prognostic Scoring System: validation by the Gruppo Romano Mielodisplasie Italian Regional Database Voso MT , Fenu S, Latagliata R, Buccisano F, Piciocchi A, Aloe-Spiriti MA , Breccia M, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(21). CrossRef
- Incidence and survival estimates for patients with myelodysplastic syndrome in the early 21st century: no evidence of improvement over time Pulte D, Jansen L, Brenner H. Leukemia & Lymphoma.2022;63(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details