Study of PD-L1 Expression in Tumours Based on Site and Histology of Tumour – The Experience of a Tertiary Referral Laboratory
Download
Abstract
Background: PD-L1 IHC test is used as a predictive biomarker using FDA-approved assays to select patients likely to benefit from immunotherapy in several advanced-stage tumors. We aim to present our data regarding the prevalence and expression pattern of PD-L1 across various tumors based on site and histology and compare them with those of the reported literature.
Material and Methods: A retrospective study of 301 cases of various tumors at different sites was done for PD-L1 IHC using the 22С3 pharmDx assay on the recommended platform.
Results: Out of 237 non small cell lung carcinoma cases, 14.7% were squamous and 85.2% were of nonsquamous histotype, with adenocarcinomas comprising the majority (82.2%). Fifty-seven percent of non small cell lung carcinoma was PD-L1 positive, 28.6% showed high expression. Sixty percent of the squamous and 56.4% of the non-squamous histotypes showed positive immunoexpression. Amongst non-squamous types, 56.4% of adenocarcinomas and 66.6% of sarcomatoid carcinomas were positive. At metastatic sites, 54.3% of on small cell lung carcinoma were positive. In head and neck squamous cell carcinoma, the majority (10/11) of cases were from the oral cavity; 81.8% of total cases were positive, 27.2% were strong expressors. For other sites, the number of cases showing PD-L1 immunopositivity is as follows: oesophageal squamous cell carcinoma (2/6), gastric adenocarcinoma (2/6), triple negative breast carcinoma (0/3), urothelial carcinoma (2/5), gall bladder (2/5), pancreatico-biliary (2/11), and colorectal (2/17) adenocarcinomas. In these tumors, PD-L1 immunoexpression did not differ significantly by age or gender.
Conclusion: Our study showed PD-L1 immunopositivity in 57% of non small cell lung carcinoma and 81.8% of head and neck squamous cell carcinoma, which is comparable to international studies. Further studies with larger sample sizes are needed to see their expression pattern in tumors at other sites and with different histologies.
Introduction
The programmed cell death protein–1 (PD-1) – programmed death-ligand 1 (PD-L1) axis is involved in immune regulation. The interaction between PD-1 on activated T-lymphocytes and its ligand PD-L1 on tumor cells or antigen-presenting cells sends immunosuppressive signals, leading to the evasion of tumor cells from the host anti-tumor immune response. Inhibiting this interaction with antibodies against PD-1 or PD-L1 is evolving as a valuable therapeutic strategy [1].
The development of immune checkpoint inhibitors (ICIs) aims to restore antitumor immunity by targeting PD-L1 and has led to an alternative, revolutionary therapeutic approach for different tumors, especially advanced non small cell lung carcinoma (NSCLC) [2-4]. The PD-L1 IHC test is used as a predictive biomarker to predict response to immunotherapy [5]. Several studies showed PD-L1 immunopositive tumors to have a better outcome with immunotherapy. However, the prognostic impact of this biomarker is yet to be established [6,7].
Immunotherapies targeting the PD-L1 have been assessed in clinical trials by evaluating PD-L1 protein expression in tumor cells and tumor-infiltrating immune cells (ICs) via immunohistochemistry using different assays. Hence, various diagnostic assays have been developed, and different antibodies, clones, platforms, scoring systems, and cutoff values have been introduced and validated for a specific inhibitor as first- or second-line treatment. Some of these assays have been approved and used as Companion Diagnostics for specific anti–PD-L1 agents. These are mandatory tests that must be performed before treatment can be initiated with a particular drug [3][8].
All FDA-approved commercial PD-L1 IHC assays (22C3, 28-8, SP263, and SP142) require assay-specified autostainer platforms [3]. Currently, the Food and Drug Association (FDA) has approved the clone 22C3 (DAKO; PD-L1 IHC 22C3 PharmDx) as a companion diagnostic test for the treatment of advanced NSCLC with pembrolizumab (KEYTRUDA) [9]. The test should be performed using EnVision FLEX visualization system on the DAKO Autostainer Link 48 [10]. The VENTANA PD-L1 (SP263) assay has received CE-IVD designation in Europe for durvalumab, pembrolizumab and nivolumab therapies. This detection kit should be used on a VENTANA BenchMark instrument [3].
Even though evaluation of PD-L1 has great therapeutic potential against various unresectable and/or advanced- stage tumors with poor prognoses, there is not much data available on the prevalence and pattern of its expression in various tumors from an Indian perspective.
Therefore, the aim of our study is to present our observations and experiences regarding the frequency and expression pattern of PD-L1 across various tumors based on site and histology using the 22С3 pharmDx assay via immunohistochemical technique and compare them with those in the already reported literature.
Materials and Methods
This was a retrospective study of 301 cases analyzed over a two-year period from November 2019 to October 2021. The PDL-1 testing was done when requested by the referring doctor on tissue paraffin blocks submitted for review. The exclusion criteria were- i) an inadequate number of tumor cells (a minimum of 100 viable tumor cells is required); ii) unsatisfactory fixation of tissue or preservation of paraffin blocks. Fifteen cases could not be evaluated because of lack of adequate tumor cells or inadequate tissue fixation. These cases were rejected and not included in this study. The patient’s demographic records and clinical details were retrieved from our digital archive. Hematoxylin and eosin (H&E)-stained slides were reviewed for confirming the microscopic diagnosis, and the IHC results were interpreted by the consultant pathologists.
PDL-1 IHC 22C3 pharmDx and interpretatio
IHC analysis was conducted using the FDA-approved IVD 22C3 pharmDx (mouse monoclonal primary anti- PD-L1 antibody) assay on the DAKO Autostainer Link 48 (Agilent) with the EnVision FLEX visualization system. Here, the detection and quantification of immunoreactivity were done according to the manufacturer’s instructions [10]. Tissue sections 3-4 microns thick were made from the formalin-fixed, paraffin-embedded tissue specimen blocks. Quality controls were run with each batch or test and included: an H&E-stained patient tissue specimen, a Dako-supplied Control Cell Line slide containing cell lines NCI-H226 (a positive control) and MCF-7 (a negative control), and lab-supplied positive and negative control tissues (known PD-L1 positive tumor tissue, where negative controls were applied by omitting the primary antibody).
PD-L1 immunostaining was evaluated by scanning the whole tumor section by the pathologist. A minimum of 100 viable tumor cells were assessed. Areas with extensive necrosis and hemorrhage, folded tissue, suboptimal preservation, and technical artifacts were avoided.
Criteria for Pd-L1 Expression in [10]
Tumor Cells—Any perceptible complete or partial membrane staining of any intensity in the viable cells distinct from any cytoplasmic staining was considered a positive expression. Normal cells and immune cells were not included.
Immune Cells (lymphocytes, monocytes and macrophages)- Any convincing membrane and/or cytoplasmic staining of any intensity of mononuclear inflammatory cells within tumor nests and/or adjacent supporting stroma was considered a positive expression. Only stroma that is contiguous to individual tumor nests was included in the tumour-area definition. Neutrophils, eosinophils, plasma cells, and immune cells associated with in situ components, benign structures, or ulcers were excluded.
PD-L1 protein expression in NSCLC was determined by using the tumor proportion score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining of any intensity.
PD-L1 protein expression in HNSCC, gastric or gastro-esophageal junction (GEJ) adenocarcinoma, oesophageal squamous cell carcinoma (SCC), breast carcinoma and urothelial carcinoma was determined using the combined positive score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. Although the result of the calculation can exceed the absolute value of 100, the maximum score was defined as CPS 100.
PD-L1 was considered expressed when TPS ≥1%; high PD-L1 expression was considered when TPS ≥50% [10] FDA-approved combined positive score (CPS) cut-offs for PD-L1 expression by the 22C3 clone in various tumours are as follows:- [10]
1. Gastric or gastro-esophageal junction adenocarcinoma CPS ≥ 1
2. Esophageal squamous cell carcinoma CPS ≥ 10
3. Head and Neck squamous cell carcinoma CPS ≥ 1 with CPS 20 being an additional positive finding.
4. Urothelial carcinoma CPS ≥ 10
5. Breast CPS ≥ 10 [11]
Results
A total of 301 cases of different histologic tumors were evaluated for PD-L 1 expression. The details of tumor at different sites and their demographic data are listed in Table 1.
| Primary Site of tumor | Diagnosis | No of cases | Age range | Male | Female | |
| 1 | Lung | Non small cell lung carcinoma | 237 | 29-87 | 155 | 82 |
| 2 | Head and Neck | Squamous cell carcinoma | 11 | 40-81 | 9 | 2 |
| 3 | Oesophagus | Squamous cell carcinoma | 6 | 55-80 | 4 | 2 |
| 4 | GE junction/ Gastric | Adenocarcinoma | 6 | 23-78 | 3 | 3 |
| 5 | Gall bladder | Adenocarcinoma | 5 | 40-75 | 2 | 3 |
| 6 | Pancreatico-biliary tree | Adenocarcinoma | 11 | 39-82 | 6 | 5 |
| 7 | Colorectal | Adenocarcinoma | 17 | 36-75 | 10 | 7 |
| 8 | Breast | Triple negative breast carcinoma | 3 | 53-68 | - | 3 |
| 9 | Urinary system | Urothelial carcinoma | 5 | 64-81 | 3 | 2 |
| Total | 301 |
Lung tumors comprised the majority of our study and included only NSCLC. About 15% (35/237) and 85% (202/237) were of squamous and nonsquamous histologies respectively. Majority of the nonsquamous type comprised of adenocarcinomas (82.2%, 195/237). PDL-1 expression pattern according to patient demographic profile, tumor characteristics and histologic type are illustrated in Tables 2 and 3.
| Lung Tumors (n=237) | PDL-1 Expression | ||||
| Characteristics | Negative (%) | Low expression (%) | High expression (%) | Total positives (%) | |
| Age (years) | ≤60 (n=86) | 33 (38.4) | 23 (26.8) | 30 (34.8) | 53 (61.6) |
| >60 (n=151) | 69 (45.7) | 44 (29.1) | 38 (25.2) | 82 (54.3) | |
| Sex | Male (n=155) | 67 (43.3) | 41 (26.4) | 47 (30.3) | 88 (56.7) |
| Female (n=82) | 35 (42.7) | 27 (33.0) | 20 (24.3) | 47 (57.3) | |
| Specimen source | Primary (n=202) | 86 (42.6) | 58 (28.7) | 58 (28.7) | 116 (57.4) |
| Metastases (n=35) | 16 (45.8) | 9 (25.7) | 10 (28.5) | 19 (54.2) | |
| Histology | Nonsquamous (n=202) | 88 (43.6) | 53 (26.3) | 61 (30.1) | 114 (56.4) |
| Squamous (n=35) | 14 (40) | 14 (40) | 7 (20) | 21 (60) |
| Total cases | Negative | Low expression | High expression | Total positives | ||
| Lung Tumors | ||||||
| Adenocarcinoma lung | Primary site | 163 | 71 | 43 | 49 | 92 |
| Metastatic site | 32 | 14 | 9 | 9 | 18 | |
| Squamous cell carcinoma lung | Primary site | 34 | 13 | 14 | 7 | 21 |
| Metastatic site | 1 | 1 | 0 | 0 | 0 | |
| Sarcomatoid carcinoma | Primary site | 5 | 2 | 1 | 2 | 3 |
| Metastatic site | 1 | 0 | 0 | 1 | 1 | |
| Adenosquamous carcinoma | Metastatic site | 1 | 1 | 0 | 0 | 0 |
| Total NSCLC | 237 | 102 | 67 | 68 | 135 |
Approx 57% (135/237) of NSCLC showed PD-L1 expression with 28.6% (68/237) showing high expression. About 56.4% (110/195) of adenocarcinomas, 60% (21/35) of SCCs and 66.6% (4/6) of sarcomatoid carcinomas were immunopositive, with 29.7% (58/195), 20% (7/35) and 50% (3/6) showing high expression, respectively. A single case of adenosquamous and subtypes of adenocarcinoma with neuroendocrine differentiation (2), lepidic pattern (1), mucinous type (2) were immunonegative. Out of 35 cases (32 adenocarcinoma and 3 others) of primary NSCLC at different metastatic sites (lymph node, pleural deposit/pleural fluid, hepatic, bone etc), 19 (54.3%) expressed PD-L1, with lymph node (6/8, 75%) and pleural (7/11, 63.6%) metastases showing higher expression.
All 11 cases in the head and neck region were squamous cell carcinoma. All cases (4/4) of <60 yrs and 71.4% (5/7) of ≥ 60 yrs showed immunopositivity. Almost 89% (8/9) of the males and 50% (1/2) of the females showed positive expression. Of the total cases, 81.8% (9/11) were positive (CPS≥1), and 27.2% (3/11) were strong expressors (CPS≥20). Ten cases (8 primary, 2 metastatic sites) were of the oral cavity, of which 9 cases (7 primary, 2 metastatic sites) were positive. A single case of poorly differentiated carcinoma of the larynx (epiglottis) was negative.
In the gastro-intestinal system, male predominance was seen in all the carcinomas except gall bladder adenocarcinomas, which showed female predominance. Two out of six cases (33.3%) of oesophageal squamous cell carcinoma showed PD-L1 positivity (CPS ≥ 10). Two out of six cases (33.3%) of GE junction/gastric adenocarcinoma, both of which were poorly differentiated (one with signet cell morphology), were PD-L1 positive (CPS ≥ 1). Forty percent (2/5) of gall bladder, 18.1% (2/11) of pancreatico-biliary and 11.7% (2/17) of colorectal adenocarcinomas (including both primary and metastatic sites) were immunopositive.
Out of 5 cases of primary urothelial carcinoma, 2 (40%) were positive (CPS≥10). All 3 cases of triple negative breast carcinoma were negative (CPS<10) (Figure 1,2 and 3).
Figure 1. Photomicrograph of PD-L1 Expression in Lung Adenocarcinoma A) TPS 90% (high expression) B) TPS 35% (low expression) C) TPS <1% (Negative).
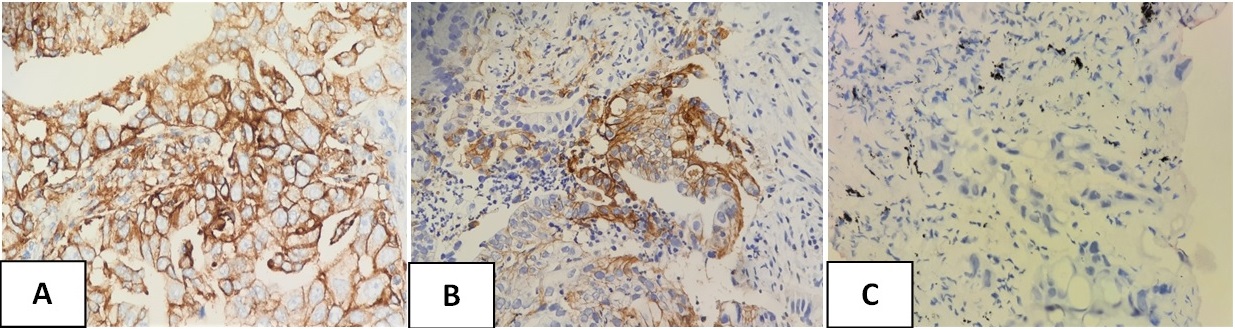
Figure 2. Photomicrograph of PD-L1 Expression in Lung A) Squamous Cell Carcinoma 1) TPS 95% (high expression) 2) TPS 3% (low expression) and B) Sarcomatoid Carcinoma TPS 35%.
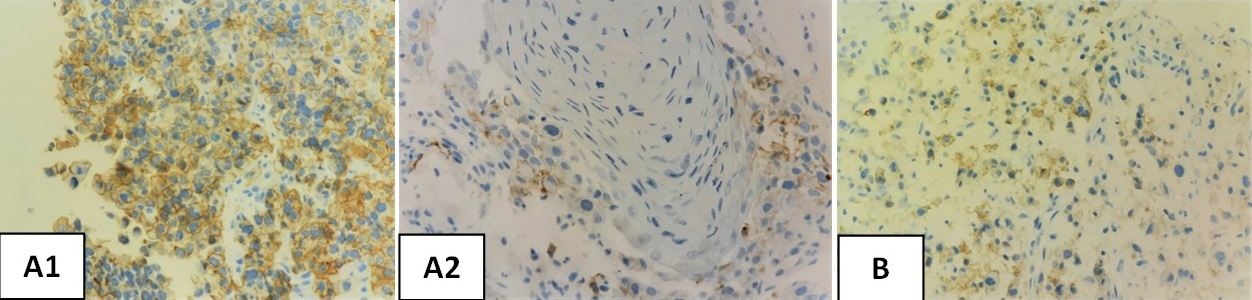
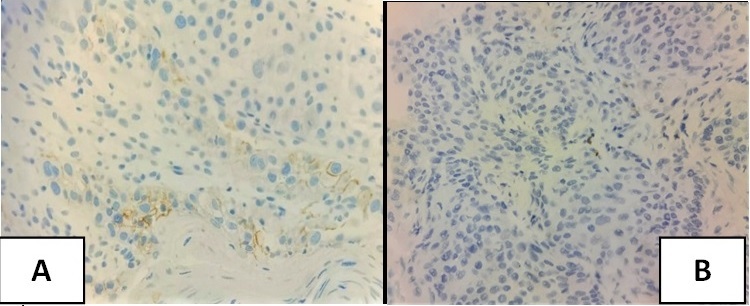
Figure 3. Photomicrograph of PD-L1 Expression in Head and Neck Squamous Cell Carcinoma A) CPS ≥1 (positive) B) CPS <1 (negative).
Discussion
PD-L1 immunopositivity in NSCLC shows wide variation in the literature. Aggarwal et al analysed the prevalence of PD-L1 expression in advanced NSCLC across 3 global clinical trials conducted using the 22C3 clone and observed that 68% were immunopositive (TPS ≥1%) and 28% had high expression (TPS ≥50%) [12]. They concluded that the prevalence was similar across demographic (age, sex) and tumor characteristics, viz. specimen source (primary or metastatic sites, 65% each), treatment status (treatment-naive or previously treated), and histology (squamous 81%, nonsquamous 74%). Zhu et al using clone 22C3 reported 72.8% immunopositivity (49.3% low expression, 23.5% high expression) [13]. Domadia et al demonstrated positive expression in 47% cases (23.1% low expression, 23.9% high expression) with similar prevalence between histotypes (squamous 47.7%, non-squamous 50.5%) [14]. The prevalence of PD-L1 positivity (57% with 28.6% high expression) with no significant difference in its expression between histotypes (squamous 60%, non-squamous 56.4%) and specimen sources (primary 57.4%, metastases 54.2%) seen in our study is in concordance with the above studies. In contrast, Archana et al reported a lower positivity (27%), with squamous cell carcinoma as the predominant type; however, the positivity frequency in SCC was similar (50%) but lower (33%) in adenocarcinomas as compared to our study [7]. Sarcomatoid carcinomas in our study showed relatively higher immunopositivity (66.6%), similar to observations by Archana (100%) and Kim et al. (90%) [15]. Our study did not show much variation in PD-L1 expression with respect to age (61% in ≤60, 54% in >60 yrs) and gender (males 56%, females 57%). Several of the above studies reported similar age range with male predominance and stating that age and sex did not correlate with PD-L1 expression [7][13][14]. Domadia demonstrated approximately 50% positivity in tumors at metastatic sites similar to our study (54.3%). PD-L1 expression was higher in metastases to lymph nodes and pleura/pleural fluid compared to other sites (bone, liver), which was similar to the study by Zhu [13]. This could be related to decalcification in bone sites and absence of lymphoid/immune cells in liver metastases. Although, Archana and Domadia used different clones (SP142 and SP263), several harmonization studies by Ratcliffe [16], Tsao [17] and Marchetti [18] et al demonstrated high agreement between 22C3 and SP263 assays, stating they can be used interchangeably. However, Tsao demonstrated less sensitivity with the SP142 assay.
Squamous cell carcinoma accounts for more than 90% of head and neck cancers and includes cancers of the nasal sinus, nasopharynx, oropharynx, hypopharynx, larynx, and oral cavity [19,20]. In accord with our study, Mishra et al. reported a similar age range with male predominance and the oral cavity as the most common site of tumour [21]. Immunopositivity was seen in 81.8% of our cases, which was in concordance with studies by Cerebelli [22], Crosta [23] and Keynote 048 [24] (88.4%, 80% and 85%, respectively) using clone 22C3. However, these studies reported a higher proportion of strong expressors (39.5%, 47% and 43%, respectively) than our study (27.2%). Mishra reported a slightly lower positivity (63.4%) using the same clone and stated there was no statistical correlation between PD-L1 positivity and patient demographics or tumor site. Ninty percent (9/10) of the tumors from the oral cavity showed positivity, similar to Chen et al (87%) [20].
We have just presented our data at other sites since the numbers were very small. Though there are no standard criterias for evaluation of PD-L1 immunoexpression in adenocarcinomas of the gall bladder, pancreatico-biliary tree and colorectum, they were evaluated using TPS with positive score ≥ 1% and negative score of <1%. A disclaimer was put at the end of the report, stating that the diagnostic assay used was applicable for lung tumours only and that their interpretation in other tumors has not been clinically validated. Though our numbers were small, they reflect the findings of other studies, e.g., Keynote-181 study using clone 22C3 showed 34% immunopositivity in oesophageal squamous cell carcinoma [25]. Liu reported 59.3% positivity in gastric carcinoma using the same clone [26].
Appropriate implementation and interpretation of the PD-L1 IHC test are critical as a predictive biomarker and are challenging owing to factors like multiple diagnostic assays available, different methods of interpretation and cut-off values, pre-analytic factors (cold ischemia time, volume and type of fixative, time for fixation), size of the sample (surgical resection versus biopsy), and site that influence the immunoexpression [5]. Prolonged delay in fixation, use of alcohol-containing fixatives or decalcification of bone tissue result in loss of PD-L1 specific staining. Sampling of tissue is important given PD-L1 heterogeneity, and therefore, small biopsy specimens can pose a challenge. However, fresh samples or archival tissue blocks and the site of tumor (primary or metastatic) do not much affect the immunoexpression. [3][27].
Although expression of PD-L1 has become the most widely used biomarker for selecting patients for ICI therapy, another emerging potential biomarker to predict response to immunotherapy is the tumor mutational burden (TMB). TMB is defined as the total number of mutations per coding area of a tumor genome. Each tumor mutation within a cancer cell has the potential to give rise to tumor-specific neo-antigens, which will be recognized by the immune system. High mutation load correlates with an immunogenic tumor microenvironment with increased expression of neo-antigens that can be targeted by activated immune cells . Thus ICI therapy has proven to be most effective in tumors with a high TMB. A high TMB would induce a high density of neoantigen-specific tumor- infiltrating lymphocytes, leading to secretion of IFN-γ and upregulation of PD-L1 on tumor cells. Hence, PD-L1 and TMB may be independent predictive biomarkers that can individually contribute to the identification of patients for immune checkpoint therapy [28,29].
There are certain limitations to our study. The cases have not been systematically and consecutively studied to ascertain the proportion of PD-L1 positive cases in a given tumor type. Nothing is known about the stage, prior treatment, if any, or what treatment (chemotherapy or targeted) was received. Except for lung tumors, the sample sizes of other tumors were quite small. Comparing the results of our study with those of previous studies posed a challenge due to the different platforms and/or interpretation methods used to evaluate PD-L1 expression.
In conclusion, in India, the literature related to PD-L1 testing is sparse in comparison to global studies. Data from our study shows PD-L1 immunopositivity in 57% of NSCLC and 81.8% of HNSCC, which is comparable to international studies. Further studies with larger sample sizes are needed to see their expression pattern in tumors at other sites and of different histologies.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- PD-1 and PD-L1 Expression in Indian Women with Breast Cancer Bharadwa KR , Dasgupta K, Narayana SM , Ramachandra C, Babu SMC , Rangarajan A, Kumar RV . European Journal of Breast Health.2022;18(1). CrossRef
- From Hope to Reality: Durable Overall Survival With Immune Checkpoint Inhibitors for Advanced Lung Cancer Rangachari D, Costa DB . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2019;37(28). CrossRef
- PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee Lantuejoul S, Sound-Tsao M, Cooper WA , Girard N, Hirsch FR , Roden AC , Lopez-Rios F, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2020;15(4). CrossRef
- Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC Socinski MA , Jotte RM , Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, et al . The New England Journal of Medicine.2018;378(24). CrossRef
- Agreement between PDL1 immunohistochemistry assays and polymerase chain reaction in non-small cell lung cancer: CLOVER comparison study Tsimafeyeu I, Imyanitov E, Zavalishina L, Raskin G, Povilaitite P, Savelov N, Kharitonova E, et al . Scientific Reports.2020;10(1). CrossRef
- PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data Aguiar PN , De Mello RA , Hall P, Tadokoro H, Lima Lopes G. Immunotherapy.2017;9(6). CrossRef
- Clinicopathologic correlation of programmed death ligand-1 expression in non-small cell lung carcinomas: A report from India Vallonthaiel AG , Malik PS , Singh V, Kumar V, Kumar S, Sharma MC , Mathur S, Arava S, Guleria R, Jain D. Annals of Diagnostic Pathology.2017;31. CrossRef
- Immunotherapy of non-small cell lung cancer: report from an international experts panel meeting of the Italian association of thoracic oncology Gridelli C, Ascierto PA , Barberis MCP , Felip E, Garon EB , O'brien M, Senan S, Casaluce F, Sgambato A, Papadimitrakopoulou V, De Marinis F. Expert Opinion on Biological Therapy.2016;16(12). CrossRef
- U.S. Food and Drug Administration. PD-L1 IHC 22C3 pharmDx https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013S014C.pdf .
- Dako. PD-L1 IHC 22C3 pharmDx. https://www.agilent.com/ cs/library/packageinsert/public/P03951%20rev%2004.pdf .
- https://www.agilent.com/cs/library/usermanuals/public/29389_22c3_pharmdx_tnbc_interpretation_manual_kn355.pdf .
- Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024 Aggarwal C., Abreu DR , Felip E., Carcereny E., Gottfried M., Wehler T., Ahn MJ , et al . Annals of Oncology.2016;27. CrossRef
- Study on PD-L1 Expression in NSCLC Patients and Related Influencing Factors in the Real World Zhu Y, Lin S, Wang Y, Shen B, Lin L, Wang L, He S. Computational and Mathematical Methods in Medicine.2021;2021. CrossRef
- Retrospective evaluation of PD-L1 expression in tumor tissue of patients with lung carcinoma and correlation with clinical and demographical data from a tertiary care institute of northern India Domadia KR , Batra U., Jain P., Sharma M., Gupta S., Bothra SJ , Pasricha S., Chaudhari K., Vishwakarma G.. Annals of Oncology.2018;29. CrossRef
- Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas Kim S, Kim MY , Koh J, Go H, Lee DS , Jeon YK , Chung DH . European Journal of Cancer (Oxford, England: 1990).2015;51(17). CrossRef
- Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer Ratcliffe MJ , Sharpe A, Midha A, Barker C, Scott M, Scorer P, Al-Masri H, Rebelatto MC , Walker J. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2017;23(14). CrossRef
- PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project Tsao MS , Kerr KM , Kockx M, Beasley MB , Borczuk AC , Botling J, Bubendorf L, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2018;13(9). CrossRef
- Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors Marchetti A, Barberis M, Franco R, De Luca G, Pace mv , Staibano S, Volante M, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2017;12(11). CrossRef
- Cancer statistics, 2018 Siegel RL , Miller KD , Jemal A. CA: a cancer journal for clinicians.2018;68(1). CrossRef
- Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance Chen SW , Li SH , Shi DB , Jiang WM , Song M, Yang AK , Li YD , Bei JX , Chen WK , Zhang Q. The International Journal of Biological Markers.2019;34(4). CrossRef
- Determining PD-L1 expression in head and neck squamous cell carcinoma using immunohistochemistry Mishra PS , Sidhu A, Dwivedi G, Mulajker DS , Awasthi S. Indian Journal of Cancer.2022;59(4). CrossRef
- Evaluating programmed death-ligand 1 (PD-L1) in head and neck squamous cell carcinoma: concordance between the 22C3 PharmDx assay and the SP263 assay on whole sections from a multicentre study Cerbelli B, Girolami I, Eccher A, Costarelli L, Taccogna S, Scialpi R, Benevolo M, et al . Histopathology.2022;80(2). CrossRef
- PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols Crosta S, Boldorini R, Bono F, Brambilla V, Dainese E, Fusco N, Gianatti A, et al . Cancers.2021;13(2). CrossRef
- Translating KEYNOTE-048 into practice recommendations for head and neck cancer Szturz P, Vermorken JB . Annals of Translational Medicine.2020;8(15). CrossRef
- Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer Kojima T, Shah MA , Muro K, Francois E, Adenis A, Hsu CH , Doi T, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2020;38(35). CrossRef
- High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features Liu X, Choi MG , Kim K, Kim KM , Kim ST , Park SH , Cristescu R, Peter S, Lee J. Pathology, Research and Practice.2020;216(4). CrossRef
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future Kim H, Chung JH . Journal of Pathology and Translational Medicine.2019;53(4). CrossRef
- Tumor mutation burden in lung cancer: a new predictive biomarker for immunotherapy or too soon to tell? Alexander M, Galeas J, Cheng H. Journal of Thoracic Disease.2018;10(Suppl 33). CrossRef
- PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers Yarchoan M, Albacker LA , Hopkins AC , Montesion M, Murugesan K, Vithayathil TT , Zaidi N, et al . JCI insight.2019;4(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details