Meta-analysis on the Diagnostic Performance of p16/Ki-67 Dual Immunostaining for Cervical Cancer Screening
Download
Abstract
Background: This article discusses cervical cancer and its higher incidence in LMIC compared to HIC due to limited resources and poor prevention strategies. It is preventable through early detection of persistent high-risk HPV infection, which is a critical promoter. Screening methods have evolved from pap smear cytology to HPV-DNA testing and currently to co-testing, but these methods still result in false positives and high colposcopy referrals. p16/Ki-67 dual immunostaining was proposed as a biomarker for cervical cancer triage due to its ability to detect HPV-mediated neoplastic transformation. The aim of the study was to evaluate the sensitivity and specificity of this dual staining method through a review and meta-analysis of published data.
Material and Methods: The search was based on PRISMA guidelines using specific MeSH terms and keywords. The search was limited to publications that compared p16/Ki-67 dual immunostaining to Pap cytology and/or high-risk HPV-DNA and included colposcopy biopsy results as the diagnostic standard. The study focused on diagnostic performance outcomes such as sensitivity, specificity, and diagnostic odds ratio. To be eligible for meta-analysis, a publication must report the diagnostic performance at predicting CIN2+.
Results: A total of 24 studies were included in the review and 21,450 samples were used for dual immunostaining. The results showed that dual immunostaining was significantly more sensitive than pap cytology (75.9% compared to 71.1%) and had a higher specificity (79.7% compared to 64.3% for pap cytology and 48.9% for HPV-DNA). A meta-analysis showed that dual immunostaining had a higher pooled diagnostic odds-ratio compared to pap cytology and HPV-DNA, indicating a better test power.
Conclusions: The study suggested that dual immunostaining is a better approach for detecting HPV-induced cervical cancer compared to traditional screening methods. The authors suggest that despite its reduced sensitivity, dual immunostaining should be considered as a promising alternative, especially in large-scale testing.
Introduction
The purpose of this review was to address the disproportionate incidence of cervical cancer in low- and middle-income countries (LMIC) compared to high-income countries (HIC). Cervical cancer is the 4th leading cancer worldwide and the leading gynecological cancer in low and middle income, countries (LMIC), with an incidence of 4-6 times higher compared to that of high-income countries (HIC) [1]. This distinction is due in part to the sub-optimal screening exercise borne from limited resources, poor prevention techniques and control strategies. Today’s cervical cancer prevention protocol includes detection of high-risk HPV (hrHPV) genotype and pathogen induced precancerous lesions with subsequent management protocol ranging from periodic monitoring with repeat screening, to colposcopy biopsy and possible definitive treatment [2]. Like most cancers, it begins as an accurately diagnosable and curable, pre-malignant lesion that is driven by a critical detectable promoter characterized as persistent high risk human papilloma virus (hrHPV) infection, thus making it preventable [2]. The greater understanding of pathogen induced cervical carcinogenesis has led in part to an evolving screening strategy which began from pap smear cytology in the 50s to HPV DNA genotyping which has a high pathogen sensitivity. However, its low specificity results in a high number of false positives compared to pap cytology which has a higher specificity thus, an effort to combine both strengths led to the adoption of co-testing screening for cervical cancer [1, 3, 4]. Today’s prevention protocol of co-testing, and primary vaccination has resulted in an 80% decrease in HIC incidence, and with point of care (POC) colposcopy biopsy plus definitive treatment, mortality has also reduced [5, 6]. In contrast, WHO for affordability reasons, advised visual inspection with acetic acid (VIA) with cryotherapy in LMIC, as both a screening protocol and a point of care (POC), for the so called “screen and treat” approach [7] and the incidence and mortality parameters in LMIC continue to be on the increase [8].
Despite advances made, today’s cervical cancer screening, reports indicate that co-testing with HPV-DNA genotyping, results in a higher detection of false positives, with 60 – 90% of colposcopy referrals still undergoing unbeneficial invasive biopsy [4, 9]. Consequently, there is need for a better assay that can detect persistent rather than transient infection to promote an effective colposcopy triage. p16/Ki-67 dual immunostaining cytology was proposed as a biomarker for colposcopy triage due to its ability to detect HPV mediated neoplastic transformation that correlates to cervical intraepithelial neoplasm II/III (CIN2/3) lesions [10].
The evidence for using p16/Ki-67 immunostaining for cervical cancer screening is that it serves as biomarkers for abnormal uterine cell growth which can indicate progression towards cervical cancer. p16 is a known surrogate protein of transforming HPV infection that regulates the cell cycle and triggers its arrest. It is overexpressed in the nucleus and cytoplasm of dysplastic uterine epithelium through the viral E7 oncoprotein mediated event indicating increased cell proliferation, and potential carcinogenesis [11, 12]. Due to a non-HPV related imperfection in p16’s immunostaining assay [11], another cellular proliferation marker in Ki-67 was cojoined as a dual assay to mitigate against this. Ki-67 is an established proliferation marker that is upregulated during mitotic activity with both proteins rarely expressed together [13]. Overexpression of both would suggest a cell cycle deregulation by HPV infection and both are used today to distinguish between intermediate to high grade (CIN2/3), from low grade dysplasia (CIN1) [13, 14]. It is suggested by the authors that using p16/Ki-67 dual immunostaining in some role for cervical cancer screening compared to the current VIA, would improve in early detection of abnormal uterine cells. Overall, the hypothesis is that p16/Ki-67 immunostaining can serve as valuable tools in improving the accuracy of cervical cancer screening and ultimately lead to improved patient outcomes.
The aim of this study is to review the effectiveness of p16/Ki-67 immunostaining by evaluating the sensitivity and specificity of p16/Ki-67 dual staining during its use for cervical cancer triage Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [15] of current published data that was used quantitative cervical screening tests.
Materials and Methods
Search Strategy
Our search methodology was modelled from the checklist of PRISMA guidelines (Figure 1) [15].
Figure 1. Flow Diagram.
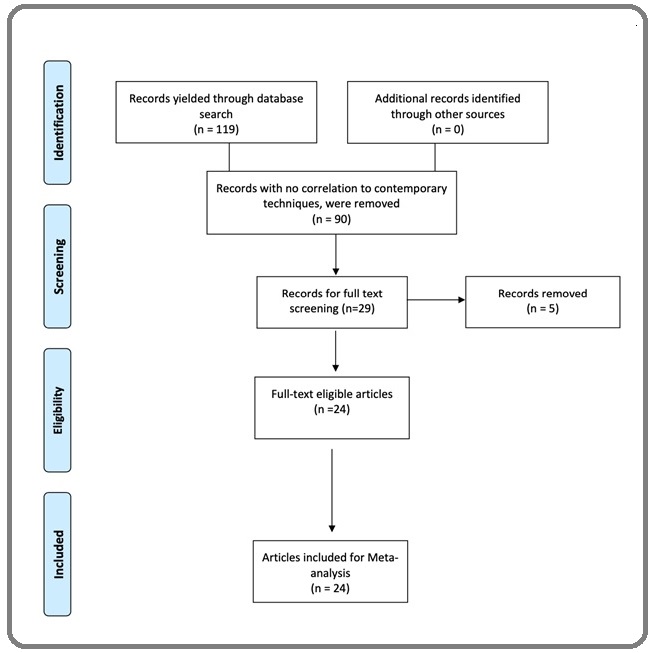
Cochrane style MeSH terms and keywords comprising; cervical cancer, cervical intraepithelial neoplasia, p16, / Ki-67, and Immunohistochemistry or Dual-Immunostaining, were used for the initial search by both authors through the search tools; Pubmed, Ovid Medline and Web of science, and results after June 2015 were considered inadmissible. Because the cervical cancer screening literature is extensive, search limits were instituted to narrow the search without compromising the inclusion of quality publications. The limits per publication inclusion were; (i) Comparing p16/Ki-67 dual immunostaining, with the two main comparators i.e., Pap cytology and (or) high risk HPV (hrHPV) DNA; (ii) Inclusion of colposcopy biopsy result of CIN2+ as the only diagnostic standard (iii) Inclusion of diagnostic performance or accuracy outcomes i.e., sensitivity and specificity, or positive predictive value (PPV), and negative predictive value (NPV), or diagnostic odds ratio (DOR) otherwise, calculated using the formula below:
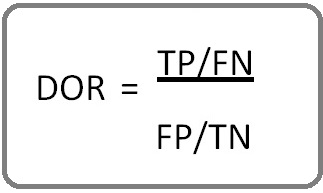
or
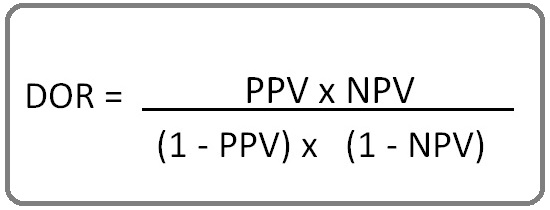
[16].
(TP, true positive; TN, true negative; FP, False positive; FN, false negative).
Eligibility criteria
English only publications were reviewed, and studies considered were - cross sectional studies, case control studies and controlled clinical trials. Publication titles and abstracts were reviewed to identify articles suitable for this review and selected manuscripts were proofread with results extracted. All studies must use proprietary named kits for dual immunostaining of cervical cytology slides according to manufacturer’s protocol. A sample was considered positive, only if one or more cervical epithelial cells stained positive for both brown cytoplasmic stain for p16 and red nuclear stain for Ki-67 with any other variation being negative [17]. Pap cytology abnormalities must be categorized as atypical squamous cells of unspecified significance (ASCUS), low grade squamous intraepithelial lesion (LSIL) and high grade squamous intraepithelial lesion (HSIL) while HPV DNA testing was either hrHPV positive or negative. Purely clinical or pathological papers, conference papers and abstracts only articles were excluded.
Sub-grouping
To be eligible for meta-analysis, a publication must include reports on diagnostic performance at predicting CIN2+ i.e., sensitivity, specificity, DOR based on Cochrane Handbook for systematic reviews of Diagnostic Test Accuracy [18]. Because of varying comparators and to remove these covariates (population characteristics), studies and assay performances were further sub-divided into 2 groups for meta-analysis:
1. Groups screened with pap cytology i.e., ASCUS or LSIL population
2. Groups screened with HPV DNA i.e., hrHPV +ve population
Quality appraisal
To qualitatively assess the quality of diagnostic accuracy in terms of risk of bias and assay applicability of the selected studies, a modified Quality Assessment of Diagnostic Accuracy Studies - 2 (modified QUADAS-2) was used covering three of the four domains i.e.; patient selection, and experimental design and assay accuracy for both dual immunostaining (index) and comparators (reference) [19, 20]. Risk of bias was assessed based on information used to support risk of bias judgement, while assay applicability was assessed based on study design and population characteristics, and both were judged as either “low”, “high” or “unclear” with the latter used only when insufficient data does not allow to report a judgement [20]. As part of the appraisal for applicability, proprietary named kits for dual immunostaining were used and colposcopy biopsy results were the only diagnostic standard. A table for index and reference qualitative assessment results was also generated using MS excel (Microsoft Excel for Mac version 16.59).
Data Extraction and Analysis
All results were extracted onto a data form for tabulation. Based on the assumption that no assay or screening technique is superior to the other, technique specificity and sensitivity, were preferred to PPV, NPV [21]. These test performances i.e., sensitivity and specificity, would be combined to a single standardized metric and jointly evaluated using the pooled effect model, and an estimate of the diagnostic accuracy of dual staining across different diagnostic tests across different studies would be predicted through logistic regression [22]. SPSS 27 software package (IBM Company, Armonk, NY, USA) was used for statistical analysis. Sample size, age range, mean or median of individuals with paired specimens, were captured for descriptive statistical information.
Results
The electronic search yielded 119 entries; of which 90 publications were removed for having no correlation to the set search objectives, and study duplicity, resulting in 29 publications being eligible for full text review. Of these, 24 studies [23-46] met the eligibility criteria and were included in the study, while 5 publications were removed for being review papers.
The studies cumulatively used 21,450 samples for dual immunostaining spread across these 24 studies.
16/24 studies utilized 12,436 samples with Pap cytology as comparator while 19/24 studies used 9,014 samples with HPV DNA as comparator (Tables 1 and 2).
| Sub-categories | Sample size (age range /mean/median) | Sensitivity (CI) | Specificity (CI) | DOR |
| Category I ASCUS or LSIL population | ||||
| · Zhang et al*. 2019 [23] | 537 | 88.1% (83.0-91.8) | 85.0% (80.7-88.5) | 41.81 |
| 80.0% (74.1-84.8) | 84.7% (80.4-88.2) | 22.14 | ||
| · Wentzensen et al 2015 [25] | 1509 | 83.4% (77.1-88.6) | 58.9% (56.2-61.6) | 7.2 |
| 76.6% (69.6-82.6) | 49.6% (46.9-52.3) | 3.2 | ||
| · Wright et al. 2017 [26] | 367(≥ 25) | 70.30% | 75.60% | 7.36 |
| 51.80% | 75.00% | 3.4 | ||
| · Liu et al*. 2020 [29] | 483 | 91.00% | 95.50% | NS |
| 42.80% | 95.20% | NS | ||
| · Ovestad et al. 2017 [30] | 266 (25-69) | 88.0% (79.0-94.0) | 31.0% (23.0-40.0) | 178.5 |
| 79.0% (68.0-87.0) | 35.0% (27.0-45.0) | 15.24 | ||
| · Luttmer et al*. 2016 [31] | 446 (18-66) | 85.5% (80.2-90.9) | 60.0% (54.3-65.7) | 3.27 |
| 86.7% (81.6-91.9) | 54.3% (48.5-60.1) | 1.05 | ||
| · Celewicz et al 2018 [27] | 93 (16-64) | 90% | 63% | 0.18 |
| 90% | 44% | 0.16 | ||
| · Wentzensen et al. 2019 [24] | 3225 (Mean 37.9) | 82.8% (79.4-86.2) | 55.7% (53.9-57.6) | 16.68 |
| 81.1% (77.6-84.7) | 44.6% (42.8-46.5) | 7.37 | ||
| · Rossi et al*. 2021 [32] | 2471 (25-64) | 75.2% (68.1-81.6) | 80.6% (70.9-88.3) | 6.1 |
| 61.0% (53.6-68.0) | 76.6% (74.5-78.5) | 3.45 | ||
| · Hu et al. 2020 [28] | 846 (≥ 21) | 63.5% (54.4-71.9) | 85.3% (82.5-87.8) | 10.44 |
| 61.9% (52.8-70.4) | 80.0% (76.9-82.9) | 5.99 | ||
| · Yu et al. 2016 [38] | 231 (≥ 30) | 90.9% (86.5–94.0) | 79.5% (77.0-81.8) | 10.02 |
| 93.5% (89.6-96.0) | 76.2% (73.6-78.7) | 6.5 | ||
| · Ebisch et al*. 2017 [36] | 462 (33-63) | 92.0% (84.0-97.0) | 61.0% (54.0-69.0) | 39.39 |
| 93.0% (85.0-98.0) | 49.0% (41.0-56.0) | 46.85 | ||
| · Torres-Ibarra et al. 2021 [37] | 475 (Median 37) | 55.2% (42.6-67.4) | 80.6% (76.5-84.4) | 20.58 |
| 23.9% (14.3-35.9) | 87.5% (83.9-90.5) | 12.82 | ||
| · Yu et al*. 2022 [33] | 335 (19-81) | 84.8% (80.4-88.4) | 81.7% (77.9-85.1) | 5.11 |
| 97.0% (94.4-98.5) | 41.8% (37.3-46.4) | 2.2 | ||
| · El-Zein et al*. 2021 [34] | 492 (19-73) | 80.7% (75.0-85.6) | 64.0% (57.9-69.8) | 24.98 |
| 91.7% (40.3-53.6) | 42.1% (36.0-48.3) | 23.57 | ||
| · Liao et al*. 2018 [35] | 198 (Mean 49.3) | 94.1% (73.0-99.0) | 80.1% (73.7-85.3) | 63.14 |
| 47.1% (26.2-69.0) | 91.7% (86.8-94.9) | 9.93 |
*, Tested for both comparators; CI, Confidence interval; DOR, Diagnostic odd ratio; Bold data, Pap cytology data; NS, Not stated
| Sub-categories | Sample size (age range /mean/median) | Sensitivity (CI) | Specificity (CI) | DOR |
| Category 2: hrHPV +ve population | ||||
| · Zhang et al*. 2019 [23] | 537 | 88.1% (83.0-91.8) | 85.0% (80.7-88.5) | 41.81 |
| 95.7% (92.1-97.7) | 71.2% (66.1-75.9) | 55.56 | ||
| · Prigenzi et al. 2017 [39] | 151 | 61.5% (31.6-86.1) | 91.1% (80.4-97.0) | 16.35 |
| 100% (75.3-100) | 23.2% (13.0-36.4) | ND | ||
| · Liu et al*. 2020 [29] | 483 | 91.00% | 95.50% | 178.5 |
| 100% | 38.70% | ND | ||
| · Luttmer et al*. 2016 [31] | 446 (18-66) | 85.5% (80.2-90.9) | 60.0% (54.3-65.7) | 0.18 |
| 60.8% (53.4-68.3) | 57.1% (51.3-62.9) | 0.34 | ||
| · Rossi et al. 2021 [32] | 2471 (25-64) | 75.2% (68.1-81.6) | 80.6% (70.9-88.3) | 10.44 |
| 94.4% (89.1-97.3) | 34.4% (31.9-37.0) | 12.67 | ||
| · Hu et al*. 2020 [28] | 846 (≥ 21) | 63.5% (54.4-71.9) | 85.2% (82.5-87.8) | 10.02 |
| 61.9% (52.8-70.4) | 72.4% (68.9-75.6) | 4.25 | ||
| · Yu et al. 2016 [38] | 231 (≥ 30) | 90.9% (86.5-94.0) | 79.5% (77.0-81.8) | 39.39 |
| 94.4% (89.6-96.0) | 76.9% (74.2-79.3) | 54.76 | ||
| · Tay et al. 2017 [40] | 97 (Mean 45.9) | 93.70% | 76.50% | 48.26 |
| 85.70% | 14.70% | 1.04 | ||
| · Packet et al. 2018 [41] | 111 (Mean 41) | 94% (84.3-98.2) | 58% (43.2-72.9) | NS |
| 92% (82.2-97.3) | 21% (11.2-33.3) | NS | ||
| · Pirtea et al. 2019 [42] | 161 (< - > 30) | 66% | 93% | NS |
| 79% | 72% | NS | ||
| · Magkana et al. 2021 [43] | 196 (mean 37.5) | 90.4% (68.0-98.0) | 97.2% (89.9-99.0) | 326.89 |
| 52.3% (30.0-74.0) | 76.4% (65.0-85.0) | 3.54 | ||
| · Ebisch et al*. 2017 [36] | 462 (33-63) | 92.0% (84.0-97.0) | 61.0% (54.0-69.0) | 20.58 |
| 75.0% (64.0-84.0) | 75.0% (68.0-82.0) | 9.24 | ||
| · Torres-Ibarra et al *2021 [37] | 475 (Median 37) | 55.2% (42.6-67.4) | 80.6% (76.5-84.4) | 5.11 |
| 31.3% (20.6-43.8) | 83.6% (79.6-87.0) | 2.32 | ||
| · Yu et al*. 2022 [33] | 335 (19-81) | 84.8% (80.4-88.4) | 81.7% (77.9-85.1) | 24.98 |
| 93.1% (89.7-95.5) | 26.3% (22.5-30.6) | 4.86 | ||
| · El-Zein et al*. 2021 [34] | 492 (19-73) | 80.7% (75.0-85.6) | 64.0% (57.9-69.8) | NS |
| 46.9% (40.3-53.6) | 87.9% (83.3-91.6) | NS | ||
| · Han et al. 2020 [44] | 468 (25-65) | 91.50% | 77.00% | 28.03 |
| 68.4% for hpv16 | 75.0% for hpv16 | -- | ||
| · Liao et al. 2018 [35] | 198 (Mean 49.3) | 94.1% (73.0-99.0) | 80.1% (73.7-85.3) | 63.14 |
| 29.4% (13.3-53.1) | 95.6% (91.7-97.8) | 9 | ||
| · Bergeron et al. 2015 [45] | 427 (Mean 35.9) | 94.4% (72.7-99.9) | 78.7% (74.4-82.6) | 64.72 |
| 100% (81.5-100) | 60.4% (55.5-65.2) | ND | ||
| · White et al. 2016 [46] | 427 (Median 41) | 75.4% (72.3-78.8) | 88.3% (87.2-89.4) | 11.72 |
| 92.8% (91.6-93.9) | 48.9% (46.3-51.6) | 14.99 |
*, Tested for both comparators; CI, Confidence interval; DOR, Diagnostic odd ratio; Bold data, Pap cytology data; ND, Not deducible; NS, Not stated
8/24 studies used both pap cytology and HPV DNA as comparators and overlapped both groups. All studies undertook colposcopy biopsy as diagnostic standard and a study search pre-condition. All studies also used several variations of result outcomes with sensitivity, specificity, PPV and NPV being the predominant. This review showed that in both categories of screening population, dual immunostaining was significantly more sensitive than pap cytology with a pooled sensitivity of 75.9% compared to pap cytology (71.1%) (p=0.01). Dual immunostaining pooled specificity of 79.7% was also significantly higher compared to that of pap cytology (64.3%) and HPV DNA (48.9%) (p=0.01) (Table 3).
| Category 1 | Category 2 | p value | |||
| P16/Ki-67 | Pap cytology | P16/Ki-67 | HPV DNA | ||
| Pooled sensitivity (%) | 76.5 | 72.3 | 75.4 | 92.8 | 0.011 |
| Pooled specificity (%) | 71.1 | 64.3 | 88.3 | 48.9 | |
| Total pooled sensitivity (%) | 75.9 | ||||
| Total pooled specificity (%) | 79.7 | ||||
| Pooled DOR | 28.98 | 10.92 | 55.63 | 14.38 | 0.37 |
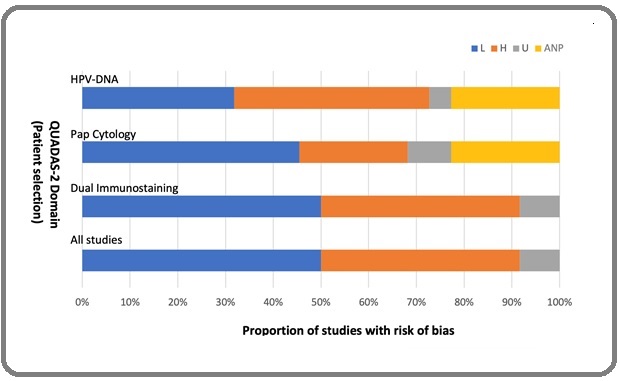
A total 14 of the 24 selected studies (24-28, 31, 32, 35-37, 39, 45) had low patient selection bias with 2/24 being unclear (38, 40) while all assay applicabilities had low biases ( Figure 2).
Figure 2. Graphical Illustration of QUADAS-2 Result. Key, L, low; H, High; U, Unclear; ANP, Assay Not Performed.
Meta-analysis
Diagnostic performance was evaluated using reported or calculated DOR, as earlier described.
Meta-analysis for sub-groups 1 and 3 were undertaken from 16 Pap cytology and 19 HPV DNA studies respectively and the study recorded a higher pooled DOR average for dual immunostaining (42.3) compared to that of pap cytology (10.9) and HPV DNA (14.4) that was adjudged non-significant (p=0.37) (see table 3), and which translates to dual immunostaining having a better test power.
Discussion
Cervical cancer remains a major cause of cancer related mortality, especially in LMIC and the upscaling of prevention measures through effective screening and HPV vaccination, can help reduce this mortality. The recent advances in cervical cancer screening towards HPV genotyping, illustrates the clinical relevance attached to HPV detection. While HPV status is clinically relevant, the lifetime risk of an individual contracting HPV infection is 90%, and over 90% of this infection resolves over time however, the persistent as opposed to transient HPV infection, remains the pathogenic driver of cervical carcinogenesis. Furthermore, despite the sensitivity HPV DNA test being > 90%, it has a low specificity resulting in false positive results and in addition, it is unable to distinguish between persistent and transient infection especially as, most HPV infection resolve within 2 years. Therefore, triaging co-tested HPV and pap cytology AS-CUS/LSIL positive women for colposcopy biopsy leads to increased unbeneficial referrals and this invasive biopsy is reported to discourage screening enrollment, which can further increase incidence, and mortality [9]. Our meta-analysis suggested that p16/ Ki-67 dual immunostaining has a better diagnostic, infection type distinction and triage tool compared to combined pap cytology and HPV-DNA. This is due to its higher sensitivity when compared to pap cytology, and importantly, a higher specificity and DOR when compared to both comparators, thus suggesting it to be a better predictor of transforming cervical cancer precursors. While the reduced specificity and cost of HPV DNA limits its use in most settings, the converse is true of p16/Ki-67 as it is cheap (anecdotally, cost $55), reproducible, easily interpreted, and can be easily upscaled to widespread use, bringing about a reduction in unwarranted colposcopy, which is a cost saving measure [47]. Moreso, reports suggest that dual immunostaining predictive positivity of histological diagnosis increases with increasing diagnostic severity, and no cytologically negative case displayed a positive p16/Ki-67 dual immunostaining [48]. There are reports of discrepancy in the evaluation of p16 immunostaining interpretation as exemplified in a review cum meta-analysis of p16 immunostaining, assisted cervical smear (cytology) [49], were there was difficulty in replication due to lack of consensus on evaluating p16 nuclear and cytoplasmic stains, but this has longed been resolved with more consistent evaluation methods [50].
In relation to socio-economic regions, while HPV DNA with quantitative PCR (qPCR), is the gold standard for HPV testing [50], its high testing cost combined with its relatively long assay time, voids the single visit “screen and treat” approach offered by VIA and cryotherapy. The average costs of the various cervical screening tests are estimated as: VIA = $15, cryotherapy = $56, pap smear = $25, p16/Ki-67 Immunostaining = $55 (anecdotal), HPV DNA PCR = $400 and colposcopy biopsy = $890 [51, 52]. VIA and pap smear remains in use in LMICs despite their highly suboptimal sensitivity concerns. Cryotherapy despite challenges with transportation of cryotherapy gas tank, absence of histopathology report, lack of quality assurance and privacy concerns, still subsists, due to it being offered during single VIA visit and it is relatively affordable compared to colposcopy biopsy and treatment [53, 54]. This mostly accounts for the high cervical cancer incidence recorded in LMIC. Therefore, it is imperative to develop an alternate screening and triage protocol that is suitable and limits the current mitigating factors impeding the current screening approaches in LMIC. Of particular emphasis, the cost of any screening technique would need to be low considering the minimum wage in Africa being approximately $9 - 388 (USD) / month with majority of the countries falling under the 75% percentile [55].
In conclusion, It is the authors suggestion that p16/Ki- 67 dual immunostaining being accurate at detecting HPV induced cervical cancer promoter [56], should be at the heart of this alternate cervical cancer screening approach in LMIC. That the limitation of a potentially reduced sensitivity may be tolerated, especially for the advantages to be derived from its low-cost use for large scale testing in regions of lower socio-economic or underdeveloped healthcare system.
Acknowledgements
Author contribution
Okoturo E: Contributed to conception, design, data acquisition, draft and review of manuscript.
Rabiu K: Contributed to design, data acquisition, and review of manuscript All authors gave their final approval and agreed to be accountable for all aspects of the work.
Declaration
Competing interest: None: Source of funding: None: Ethical approval: Adjudged none required.
References
- American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer Saslow D, Solomon D, Lawson HW , Killackey M, Kulasingam SL , Cain J, Garcia FAR , et al . CA: a cancer journal for clinicians.2012;62(3). CrossRef
- Management of cervical premalignant lesions Basu P, Taghavi K, Hu , Mogri S, Joshi S. Current Problems in Cancer.2018;42(2). CrossRef
- Circulating HPV cDNA in the blood as a reliable biomarker for cervical cancer: A meta-analysis Gu Y, Wan C, Qiu J, Cui Y, Jiang T, Zhuang Z. PloS One.2020;15(2). CrossRef
- HPV testing in primary cervical screening: a systematic review and meta-analysis Murphy J, Kennedy EB , Dunn S, McLachlin CM , Kee Fung MF , Gzik D, Shier M, Paszat L. Journal of obstetrics and gynaecology Canada: JOGC = Journal d'obstetrique et gynecologie du Canada: JOGC.2012;34(5). CrossRef
- Human papillomavirus DNA detection in plasma and cervical samples of women with a recent history of low grade or precancerous cervical dysplasia Cocuzza CE , Martinelli M M, Sina F, Piana A, Sotgiu G, Dell'Anna T, Musumeci R. PloS One.2017;12(11). CrossRef
- World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015 McGuire S. Advances in Nutrition (Bethesda, Md.).2016;7(2). CrossRef
- Chapter 8: Screening for cervical cancer in developing countries Denny L, Quinn M, Sankaranarayanan R. Vaccine.2006;24 Suppl 3. CrossRef
- Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society Fontham ETH , Wolf AMD , Church TR , Etzioni R, Flowers CR , Herzig A, Guerra CE , et al . CA: a cancer journal for clinicians.2020;70(5). CrossRef
- Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance Huh WK , Ault KA , Chelmow D, Davey DD , Goulart RA , Garcia FAR , Kinney WK . Gynecologic Oncology.2015;136(2). CrossRef
- Novel concepts in cervical cancer screening: a comparison of VIA, HPV DNA test and p16INK4a/Ki-67 dual stain cytology in Western Kenya Orang'o EO , Were E, Rode O, Muthoka K, Byczkowski M, Sartor H, Vanden Broeck D, et al . Infectious Agents and Cancer.2020;15. CrossRef
- Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience Singhi AD , Westra WH . Cancer.2010;116(9). CrossRef
- p16INK4A overexpression in precancerous and cancerous lesions of the uterine cervix in Tunisian women Missaoui N, Trabelsi A, Hmissa S, Fontanière B, Yacoubi MT , Mokni M, Korbi S, Frappart L. Pathology, Research and Practice.2010;206(8). CrossRef
- Immunocytochemical analysis of the cervical Pap smear Morgan TK , Berlin M. Methods in Molecular Biology (Clifton, N.J.).2015;1249. CrossRef
- Diagnostic performance of dual-staining cytology for cervical cancer screening: A systematic literature review Tjalma WAA . European Journal of Obstetrics, Gynecology, and Reproductive Biology.2017;210. CrossRef
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews Page MJ , McKenzie JE , Bossuyt PM , Boutron I, Hoffmann TC , Mulrow CD , Shamseer L, et al . BMJ (Clinical research ed.).2021;372. CrossRef
- The diagnostic odds ratio: a single indicator of test performance Glas AS , Lijmer JG , Prins MH , Bonsel GJ , Bossuyt PMM . Journal of Clinical Epidemiology.2003;56(11). CrossRef
- Meta-analysis on the performance of p16/Ki-67 dual immunostaining in detecting high-grade cervical intraepithelial neoplasm Sun M, Shen Y, Ren ML , Dong YM . Journal of Cancer Research and Therapeutics.2018;14(Supplement). CrossRef
- Systematic reviews of diagnostic test accuracy Leeflang MMG , Deeks JJ , Gatsonis C, Bossuyt PMM . Annals of Internal Medicine.2008;149(12). CrossRef
- QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies Whiting PF , Rutjes AWS , Westwood ME , Mallett S, Deeks JJ , Reitsma JB , Leeflang MMG , Sterne JAC , Bossuyt PMM . Annals of Internal Medicine.2011;155(8). CrossRef
- Systematic reviews and meta-analyses of diagnostic test accuracy Leeflang MM . Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases.2014;20(2). CrossRef
- Enabling Precision Medicine With Digital Case Classification at the Point-of-Care Obermeier P, Muehlhans S, Hoppe C, Karsch K, Tief F, Seeber L, Chen X, et al . EBioMedicine.2016;4. CrossRef
- Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews Reitsma JB , Glas AS , Rutjes AWS , Scholten RJPM , Bossuyt PM , Zwinderman AH . Journal of Clinical Epidemiology.2005;58(10). CrossRef
- Evaluation of p16/Ki-67 dual staining in the detection of cervical precancer and cancer in China Zhang SK , Jia MM , Zhao DM , Wu ZN , Guo Z, Liu YL , Guo PP , et al . Cancer Epidemiology.2019;59. CrossRef
- Clinical Evaluation of Human Papillomavirus Screening With p16/Ki-67 Dual Stain Triage in a Large Organized Cervical Cancer Screening Program Wentzensen N, Clarke MA , Bremer R, Poitras N, Tokugawa D, Goldhoff PE , Castle PE , et al . JAMA internal medicine.2019;179(7). CrossRef
- p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women Wentzensen N, Fetterman B, Castle PE , Schiffman M, Wood SN , Stiemerling E, Tokugawa D, et al . Journal of the National Cancer Institute.2015;107(12). CrossRef
- Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial Wright TC , Behrens CM , Ranger-Moore J, Rehm S, Sharma A, Stoler MH , Ridder R. Gynecologic Oncology.2017;144(1). CrossRef
- Clinical efficacy of p16/Ki-67 dual-stained cervical cytology in secondary prevention of cervical cancer Celewicz A, Celewicz M, Wężowska M, Chudecka-Głaz A, Menkiszak J, Urasińska E. Polish Journal of Pathology: Official Journal of the Polish Society of Pathologists.2018;69(1). CrossRef
- Evaluation of p16/Ki-67 Dual-Stained Cytology in Triaging HPV-Positive Women during Cervical Cancer Screening Hu Y, Hong Z, Gu L, Xie L, Yang B, Dai H, Chen H, et al . Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2020;29(6). CrossRef
- Good performance of p16/Ki-67 dual-stain cytology for detection and post-treatment surveillance of high-grade CIN/VAIN in a prospective, cross-sectional study Liu W, Gong J, Xu H, Zhang D, Xia N, Li H, Song K, et al . Diagnostic Cytopathology.2020;48(7). CrossRef
- Clinical value of fully automated p16/Ki-67 dual staining in the triage of HPV-positive women in the Norwegian Cervical Cancer Screening Program Ovestad IT , Dalen I, Hansen E, Loge JLD , Dybdahl BM , Dirdal MB , Moltu P, Berland JM . Cancer Cytopathology.2017;125(4). CrossRef
- p16/Ki-67 dual-stained cytology for detecting cervical (pre)cancer in a HPV-positive gynecologic outpatient population Luttmer R, Dijkstra MG , Snijders PJF , Berkhof J, Kemenade FJ , Rozendaal L, Helmerhorst TJM , et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2016;29(8). CrossRef
- p16/ki67 and E6/E7 mRNA Accuracy and Prognostic Value in Triaging HPV DNA-Positive Women Giorgi Rossi P, Carozzi F, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, De Marco L, et al . Journal of the National Cancer Institute.2021;113(3). CrossRef
- Significance of Triple Detection of p16/ki-67 Dual-Staining, Liquid-Based Cytology and HR HPV Testing in Screening of Cervical Cancer: A Retrospective Study Yu L, Chen X, Liu X, Fei L, Ma H, Tian T, Wang L, Chen S. Frontiers in Oncology.2022;12. CrossRef
- Dual staining for p16/Ki-67 to detect high-grade cervical lesions: Results from the Screening Triage Ascertaining Intraepithelial Neoplasia by Immunostain Testing study El-Zein M, Gotlieb W, Gilbert L, Hemmings R, Behr MA , Franco EL . International Journal of Cancer.2021;148(2). CrossRef
- The effect of p16/Ki-67 and p16/mcm2 on the detection of cervical intraepithelial neoplasia: a prospective study from China Liao GD , Kang LN , Li J, Zeng X, Chen W, Xi MR . International Journal of Clinical and Experimental Pathology.2018;11(8).
- Evaluation of p16/Ki-67 dual-stained cytology as triage test for high-risk human papillomavirus-positive women Ebisch RM , Horst J, Hermsen M, Rijstenberg LL , Vedder JE , Bulten J, Bosgraaf RP , et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2017;30(7). CrossRef
- Adjunctive testing by cytology, p16/Ki-67 dual-stained cytology or HPV16/18 E6 oncoprotein for the management of HPV16/18 screen-positive women Torres-Ibarra L, Lorincz AT , Wheeler CM , Cuzick J, Hernández-López R, Spiegelman D, León-Maldonado L, Rivera-Paredez B, et al . International Journal of Cancer.2021;148(9). CrossRef
- Evaluation of p16/Ki-67 dual staining in detection of cervical precancer and cancers: a multicenter study in China Yu L, Chen W, Lei X, Qin Y, Wu Z, Pan Q, Zhang X, et al . Oncotarget.2016;7(16). CrossRef
- Dual p16 and Ki-67 Expression in Liquid-Based Cervical Cytological Samples Compared to Pap Cytology Findings, Biopsies, and HPV Testing in Cervical Cancer Screening: A Diagnostic Accuracy Study Prigenzi KCK , Heinke T, Salim RC , Focchi GRDA . Acta Cytologica.2018;62(2). CrossRef
- Comparison of the sensitivity and specificity of p16/Ki-67 dual staining and HPV DNA testing of abnormal cervical cytology in the detection of histology proven cervical intraepithelial neoplasia grade 2 and above (CIN 2+) Tay TKY , Lim KL , Hilmy MH , Thike AA , GohST , SongLH , Hwang JSG , Mantoo S. The Malaysian Journal of Pathology.2017;39(3).
- The use of p16/Ki-67 dual staining technology on cervical cytology of patients undergoing a LLETZ procedure Packet B, Poppe W, Weynand B, Vanherck M. European Journal of Obstetrics, Gynecology, and Reproductive Biology.2018;228. CrossRef
- p16/Ki-67 dual staining has a better accuracy than human papillomavirus (HPV) testing in women with abnormal cytology under 30 years old Pirtea L, Secosan C, Margan M, Moleriu L, Balint O, Grigoras D, Sas I, Horhat F, Jianu A, Ilina R. Bosnian Journal of Basic Medical Sciences.2019;19(4). CrossRef
- The p16/ki-67 assay is a safe, effective and rapid approach to triage women with mild cervical lesions Magkana M, Mentzelopoulou P, Magkana E, Pampanos A, Daskalakis G, Domali E, Rodolakis A, Pappa K. PloS One.2021;16(6). CrossRef
- p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening Han Q, Guo H, Geng L, Wang Y. Chinese Journal of Cancer Research = Chung-Kuo Yen Cheng Yen Chiu.2020;32(2). CrossRef
- Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results Bergeron C, Ikenberg H, Sideri M, Denton K, Bogers J, Schmidt D, Alameda F, Keller T, Rehm S, Ridder R. Cancer Cytopathology.2015;123(6). CrossRef
- Triage of LSIL/ASC-US with p16/Ki-67 dual staining and human papillomavirus testing: a 2-year prospective study White C., Bakhiet S., Bates M., Keegan H., Pilkington L., Ruttle C., Sharp L., et al . Cytopathology: Official Journal of the British Society for Clinical Cytology.2016;27(4). CrossRef
- Interpretation of p16(INK4a) /Ki-67 dual immunostaining for the triage of human papillomavirus-positive women by experts and nonexperts in cervical cytology Allia E, Ronco G, Coccia A, Luparia P, Macrì L, Fiorito C, Maletta F, et al . Cancer Cytopathology.2015;123(4). CrossRef
- Clinical role of p16INK4a expression in liquid-based cervical cytology: correlation with HPV testing and histologic diagnosis Benevolo M, Vocaturo A, Mottolese M, Mariani L, Vocaturo G, Marandino F, Sperduti I, Rollo F, Antoniani B, Donnorso RP . American Journal of Clinical Pathology.2008;129(4). CrossRef
- p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis Tsoumpou I., Arbyn M., Kyrgiou M., Wentzensen N., Koliopoulos G., Martin-Hirsch P., Malamou-Mitsi V., Paraskevaidis E.. Cancer Treatment Reviews.2009;35(3). CrossRef
- Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination Schache AG , Liloglou T, Risk JM , Filia A, Jones TN , Sheard J, Woolgar JA , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2011;17(19). CrossRef
- Costs of cervical cancer screening and treatment using visual inspection with acetic acid (VIA) and cryotherapy in Ghana: the importance of scale Quentin W, Adu-Sarkodie Y, Terris-Prestholt F, Legood R, Opoku BK , Mayaud P. Tropical medicine & international health: TM & IH.2011;16(3). CrossRef
- p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma Lewis JS . Head and Neck Pathology.2012;6 Suppl 1(Suppl 1). CrossRef
- Primary HPV screening for cervical cancer Bhatla N, Singhal S. Best Practice & Research. Clinical Obstetrics & Gynaecology.2020;65. CrossRef
- Population-level scale-up of cervical cancer prevention services in a low-resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia Parham GP , Mwanahamuntu MH , Kapambwe S, Muwonge R, Bateman AC , Blevins M, Chibwesha CJ , et al . PloS One.2015;10(4). CrossRef
- World Population Review 2023. World Population Review 2023.
- Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, Snyman L, et al . International Journal of Cancer.2014;134(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details