Value of Glypican-1 Expression in Pleural Epithelioid Mesothelioma, Adenocarcinoma and Squamous Cell Carcinoma of the Lung
Download
Abstract
Background: Distinguishing between pleural epithelioid mesothelioma, lung adenocarcinoma, and poorly differentiated squamous cell carcinoma (SCC) is a challenge in some cases. A panel of markers has been recommended by the International Mesothelioma Interest Group (IMIG) guideline to differentiate epithelioid mesothelioma from lung adenocarcinoma and SCC. However, the use of novel highly specific immunohistochemical (IHC) markers is still required. Objectives: This study aimed to evaluate glypican-1 (GPC1) expression in epithelioid mesothelioma, lung adenocarcinoma, and SCC, correlating its expression with some known clinicopathological parameters to clarify its diagnostic and prognostic value.
Methods: This study included seventy specimens designated as 20 cases of pleural epithelioid mesothelioma, 30 cases of lung adenocarcinoma, and 20 cases of lung SCC. GPC1 expression was evaluated using immunohistochemistry (IHC). The data was analyzed statistically by SPSS software 25. GPC1 expression was correlated to different clinicopathologic data using Chi-square test. The study was conducted according to local Ethical Committee regulations.
Results: This study detected positive GPC1 reactivity in all cases (100%) of pleural mesothelioma and lung SCC, in which increased GPC1 expression was correlated to large-sized, high grade, advanced stage tumors, and the presence of lymph node metastasis (LNM). In contrast, GPC1 expression was absent in about 93% of lung adenocarcinoma cases, and only 2 cases exhibited weak focal expression.
Conclusion: Our findings demonstrated that GPC1 expression is upregulated in advanced pleural mesothelioma and pulmonary SCC; tumors with high GPC1 expression exhibited more advanced biological behavior.
Introduction
Lung carcinoma is ranked the 2nd most frequent malignancy and a major factor in cancer-related mortality globally, representing approximately 18% of all cancer deaths [1]. Lung carcinoma is the 5th most common type of cancer in Egypt. It affects males more than females, and most patients are diagnosed at the age of 60 or older. Smoking is the main risk factor for lung carcinoma [2].
Non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) are the main histologic subtypes of lung carcinoma. More than 80% of lung carcinoma are NSCLC. Histologically, NSCLC is categorized into three subtypes: lung adenocarcinoma, SCC, and large cell carcinoma. Adenocarcinoma accounts for about 40% of cases and is the most frequent subtype [3]. Based on the histologic and molecular status of the primary tumors, specific therapeutic strategies can be recommended. Consequently, it has become increasingly important to accurately discriminate between solid predominant lung adenocarcinoma and poorly differentiated SCC, as gene- targeted treatment requires the proper differentiation of these two histological subtypes [4].
Malignant pleural mesothelioma is a rare, highly aggressive neoplasm that arises from mesothelial cells that line the pleural cavity. It represents the most common primary pleural malignant tumor among Egyptians, accounting for more than 50% of cases. Exposure to asbestos is the main risk factor [5]. Three histologic subtypes are recognized: epithelioid, sarcomatoid, and biphasic variants. The majority, about 70%, of all histologic subtypes are epithelioid variants [6]. Epithelioid mesothelioma and lung adenocarcinoma share many similarities, and some peripheral lung carcinomas exhibit a pleurotropic growth pattern like mesothelioma. An accurate diagnosis of mesothelioma is required because it has a different prognosis and treatment [7].
GPC1 is a cell surface heparan sulfate proteoglycan, belongs to the six glypicans family. It is encoded by the GPC1 gene located at 2q37 [8]. It is involved in the signaling of growth factors such as transforming growth factor beta (TGF-β), fibroblast growth factor-2 (FGF-2), and vascular endothelial growth factor (VEGF) that regulate cell division and growth. It has three heparan sulfate chains and contains 588 amino acids in total [9]. It has been investigated as a possible target for cancer treatment in different neoplasms. Numerous studies have demonstrated the role of GPC1 in cancer cell growth, metastasis, and angiogenesis [10]. Previous studies reported its overexpression in various tumors, including pancreatic cancer, breast cancer, and glioma [11]. Studies evaluating the expression of GPC1 in pleural mesothelioma and lung carcinoma are insufficient and controversial. Further studies are required to fully understand its prognostic role and its potential value in distinguishing between pleural epithelioid mesothelioma, lung adenocarcinoma, and SCC.
Materials and Methods
Clinical data and specimens’ collection
Seventy specimens, including 20 cases of pleural epithelioid mesothelioma, 30 cases of lung adenocarcinoma, and 20 cases of lung SCC, were included. After receiving approval from the Sohag University Ethical Committee (Registration number: Soh-Med-22-01-29), tissue blocks were obtained from the Pathology Laboratory of the Sohag University Hospitals and Sohag Oncology Center. Exclusion criteria included insufficient tissue for IHC, neoadjuvant chemotherapy or radiotherapy, and incomplete clinical data. Each block yielded two tissue sections stained with H and E and anti-human GPC1. The following parameters were re-assessed in the H and E stained sections: histologic type and tumor grade.
Immunohistochemical staining of GPC1
Serial sections from the tissue blocks were submitted for IHC. The staining process is based on the streptavidin- biotin immunoperoxidase complex procedure. 4 µm thick sections were placed in heated xylene to be deparaffinized. Descending levels of alcohol were used to rehydrate the tissue sections. Following a 10-minute incubation with 3% H2O2 to inhibit endogenous peroxidase activity, two rounds of washing in phosphate buffer solution (PBS) were performed. Using citrate buffer fluid at a concentration of 0.01 mmol/L for 20 minutes in the microwave at 650 W, antigen retrieval was accomplished. Sections were incubated with rabbit polyclonal anti-Glypican-1 antibody (1/100, ab217339, Abcam, UK) overnight at 4°C. After each step, tissue sections were washed in PBS. The slides were incubated for 10 minutes with goat serum secondary antibody, followed by streptavidin biotin. Diaminobenzidine (DAB) was used as a chromagen. Finally, tissue sections were counterstained using Harris’ hematoxylin, then dehydrated, cleared, and mounted. Bronchial epithelium was used as an internal positive control. The negative controls were stained parallel to the positive controls but without the addition of the primary antibody.
Evaluation of GPC1 immunostaining
The evaluation of GPC1 immunostainig was based on the intensity and percentage of positive tumor cells. Membranous and cytoplasmic reactivity were detected within the tumor cells. The intensity of staining was scored as follows: (0= no stain, 1= weak, 2= moderate, and 3= strong). The percentage of positive cells was scored as follows: 0<5%, 1 (6–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The immunoreactive score (IRS) was calculated by multiplying intensity and percentage of positivity. Low expression was defined as a total IRS <4, while high expression was defined as a total score ≥4 [12].
Statistical analysis
Data were collected, revised, coded, and entered to the Statistical Package for Social Science (IBM SPSS, version 25) to perform the statistical analysis. Mean ± standard deviation (SD), and range were used for quantitative data. Frequency and percentage were used for qualitative data. The means of the two groups were compared using a student t-test. The expression rate between the categories was compared using the Chi-square test. If P value less than 0.05, it was regarded as statistically significant.
Results
Patients’ characteristics
The current study included a total of 70 specimens: 20 cases of pleural Epithelioid mesothelioma, 30 cases of lung adenocarcinoma, and 20 cases of lung SCC. The clinical and histopathological characteristics of the studied cases were described in Table 1.
| Variable | Mesothelioma | Lung Adenocarcinoma | Lung SCC |
| (20 cases) | (30 cases) | (20 cases) | |
| N (%) | N (%) | N (%) | |
| Age (Year) | |||
| Range | 43-77 | 55-75 | 60-75 |
| Mean ± SD | 58±8.6 | 63.4±5.4 | 67±4.7 |
| Gender | |||
| Male | 14 (70) | 15 (50) | 13 (65) |
| Female | 6 (30) | 15 (50) | 7 (35) |
| Smoking | |||
| Smoker | 11 (55) | 17 (56.7) | 14 (70) |
| Non-smoker | 9 (45) | 13 (43.3) | 6 (30) |
| Tumor size (cm.) | |||
| T1 | 4 (13.3) | 3 (15) | |
| T2 | ---- | 15 (50) | 10 (50) |
| T3 | 11 (36.7) | 7 (35) | |
| Laterality | |||
| Right side | 13 (65) | 20 (66.7) | 15 (75) |
| Left side | 7 (35) | 10 (33.3) | 5 (25) |
| Grade | |||
| I | 4 (20) | 3 (10) | 1 (5) |
| II | 10 (50) | 15 (50) | 6 (30) |
| III | 6 (30) | 12 (40) | 13 (65) |
| Stage | |||
| I | 2 (10) | 3 (10) | 2 (10) |
| II | 10 (50) | 13 (43.3) | 10 (50) |
| III | 8 (40) | 11 (36.7) | 6 (30) |
| IV | 0 (0) | 3 (10) | 2 (10) |
| LNM | |||
| Yes | 9 (45) | 17 (56.7) | 13 (65) |
| No | 11 (55) | 13 (43.3) | 7 (35) |
Immunohistochemical detection of GPC1
Membranous and cytoplasmic expression were observed within the neoplastic cells, and both patterns were considered positive. All cases of epithelioid mesothelioma and lung SCC showed positive reactivity. Epithelioid mesothelioma and lung SCC cases exhibited high GPC1 expression (score ≥4) in 16/20 (80%) and 17/20 (85%), respectively, while 4/20 (20%) and 3/20 (15%) of cases expressed low expression levels (score<4). In contrast, only 2/30 (6.7%) of lung adenocarcinoma cases revealed focal weak expression, and the remainder of cases didn’t show any expression (Figure 1).
Figure 1. High GPC1 Expression in Pleural Epithelioid Mesothelioma (A and B), High GPC1 Expression in Lung SCC (C), Weak Focal GPC1 Expression in Lung Adenocarcinoma (D). X400.
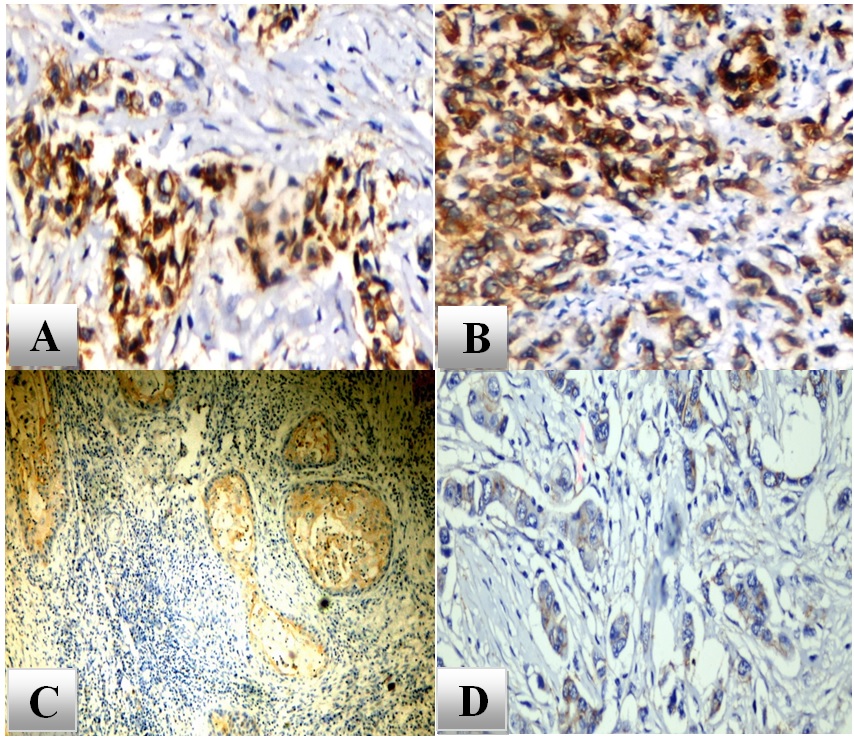
Our findings revealed that GPC1 expression levels were significantly higher in pleural mesothelioma and lung SCC compared to its level in lung adenocarcinoma (p<0.0001) (Tables 2 and 3).
| Variable | GPC1 IHC Expression | |||
| Negative | Positive | P value | ||
| Low N (%) | High N (%) | |||
| Pleural mesothelioma | 0 | 4 (20) | 16 (80) | <0.0001** |
| Lung Adenocarcinoma | 28 (93.3) | 2 (6.7) | 0 |
Chi-square test was used; ** highly significant.
| Variable | GPC1 IHC Expression | |||
| Negative | Positive | P value | ||
| Low N (%) | High N (%) | |||
| Lung SCC | 0 | 3 (15) | 17 (85) | <0.0001** |
| Lung adenocarcinoma | 28 (93.3) | 2 (6.7) | 0 |
Chi-square test was used; ** highly significant.
On the other hand, there was no statistically significant difference in GPC1 expression between pleural mesothelioma and lung SCC (p = 0.677), Table 4.
| Variable | GPC1 IHC Expression | P value | |
| Low N (%) | High N (%) | ||
| Pleural mesothelioma | 4 (20) | 16 (80) | 0.677 |
| Lung SCC | 3 (15) | 17 (85) | (NS) |
Chi-square test was used; NS, non-significant.
Comparing the statistical results of the GPC1 immunostaining to the available cliniopathological data in epithelioid mesothelioma cases revealed increased expression levels in advanced, less differentiated tumors. The intensity of GPC1 immunostaining was noticed to be increased in higher tumor grades and stages (p=0.008) and (p=0.007), respectively. Additionally, GPC1 expression and LNM were shown to be statistically significantly correlated (p=0.043). However, statistical analysis of GPC1 expression in relation to patients’ age, gender, smoking, and tumor laterality didn’t reveal any statistically significant difference (Table 5).
| Variable | No. of cases | GPC1 expression | ||
| -20 | High expression | Low expression | P value | |
| 16 (80%) | 4 (20%) | |||
| N (%) | N (%) | |||
| Age (Year) | ||||
| Mean ±SD | - | 59 ± 8.9 | 53.8 ± 6.6 | 0.284* |
| Gender | ||||
| Male | 14 | 11(78.6) | 3 (21.4) | 0.807 |
| Female | 6 | 5 (83.3) | 1 (16.7) | |
| Smoking | ||||
| Smoker | 11 | 8 (72.7) | 3 (27.3) | 0.369 |
| Non-smoker | 9 | 8 (88.9) | 1 (11.1) | |
| Laterality | ||||
| Right side | 13 | 9 (69.2) | 4 (30.8) | 0.101 |
| Left side | 7 | 7 (100) | 0 | |
| Grade | ||||
| I | 4 | 1 (25) | 3 (75) | |
| II | 10 | 9 (90) | 1 (10) | 0.008 |
| III | 6 | 6 (100) | 0 | |
| Stage | ||||
| I | 2 | 0 | 2 (100) | 0.007 |
| II | 10 | 8 (80) | 2 (20) | |
| III | 8 | 8 (100) | 0 | |
| LNM | ||||
| Yes | 9 | 9 (100) | 0 | 0.043 |
| No | 11 | 7 (63.6) | 4 (36.4) |
P value was calculated by Chi-Square test. *P value was calculated by Independent Sample T-Test.
Concerning lung SCC cases, GPC1 expression revealed a positive statistically significant correlation in relation to tumor size (p= 0.021), grade ( p= 0.045), stage (p= 0.005), and LNM (p= 0.01). In addition, GPC1 expression was statistically analyzed in relation to the other studied clinicopathological characteristics, but no statistically significant correlations were found (Table 6).
| Variable | No. of cases | GPC1 expression | ||
| High expression | Low expression | P value | ||
| 17 (85%) N (%) | 3 (15%) | |||
| N (%) | ||||
| Age (Year) | ||||
| Mean ±SD | - | 67.3 ±4.6 | 65.7 ± 6 | 0.596* |
| Gender | ||||
| Male | 13 | 11 (84.6) | 2 (15.4) | 0.948 |
| Female | 7 | 6 (85.7) | 1 (14.3) | |
| Smoking | ||||
| Smoker | 14 | 12 (85.7) | 2 (14.3) | 0.891 |
| Non-smoker | 6 | 5 (83.3) | 1 (16.7) | |
| Laterality | ||||
| Right side | 15 | 12 (80) | 3 (20) | 0.278 |
| Left side | 5 | 5 (100) | 0 | |
| Size | ||||
| T1 | 3 | 1 (33.3) | 2 (66.7) | 0.021 |
| T2 | 10 | 9 (90) | 1 (10) | |
| T3 | 7 | 7 (100) | 0 | |
| Grade | ||||
| I | 1 | 0 | 1 (100) | |
| II | 6 | 5 (83.3) | 1 (16.7) | 0.045 |
| III | 13 | 12 (92.3) | 1 (7.7) | |
| Stage | ||||
| I | 2 | 0 (0) | 2 (100) | |
| II | 10 | 9 (90) | 1 (10) | 0.005 |
| III | 6 | 6 (100) | 0 | |
| IV | 2 | 2 (100) | 0 | |
| LNM | ||||
| Yes | 13 | 13 (100) | 0 | 0.01 |
| No | 7 | 4 (57) | 3 (43) |
P value was calculated by Pearson Chi-Square test. *P value was calculated by Independent Sample T-Test.
Discussion
GPC1 regulates cell proliferation and differentiation. It has been reported to contribute to carcinogenesis, and its overexpression is associated with poor prognosis and chemoresistance in various types of human cancers. According to previous reports, GPC1 has been suggested as a potential therapeutic target in several neoplasms as it interacts with different growth factors such as TGF-β, FGF-2, and VEGF to regulate cell division and growth; furthermore, these growth factors are known to control tumor cell proliferation, angiogenesis, and metastasis [13, 14]. Several trials have developed antibody-based therapeutics that target GPC1 for the treatment of solid tumors such as oesophageal and pancreatic cancer [15, 16].
Few previous studies were conducted to evaluate GPC1 expression levels in pleural mesothelioma and lung carcinoma. In this study, we evaluated GPC1 expression and its applicability in distinguishing epithelioid mesothelioma from lung adenocarcinoma, and SCC correlated its expression with some known clinicopathological parameters.
GPC1 reactivity was observed in all cases of pleural epithelioid mesothelioma and lung SCC; high expression (score ≥4) was detected in 80% and 85% of cases, respectively. In contrast, only two cases of lung adenocarcinoma exhibited positive expression that was focal and weak. According to our findings, the GPC1 expression level was significantly higher in pleural epithelioid mesothelioma compared to its level in lung adenocarcinoma. These findings were in concordance with the findings of Amatya and his colleagues, who reported that GPC1 expression was detected in all epithelioid mesothelioma cases, and only 3 cases of pulmonary adenocarcinoma revealed focal weak expression. They described the value of GPC1 immunostaining to distinguish between epithelioid mesothelioma and lung adenocarcinoma, where GPC1 expression demonstrated 100% sensitivity, 97% specificity, and 98% accuracy. Therefore, they recommended using GPC1 as an additional positive marker for pleural epithelioid mesothelioma [17]. Meanwhile, our results differed significantly from those of Chiu and his colleagues, who reported positive GPC1 expression in all cases of pleural epithelioid mesothelioma as well as pulmonary adenocarcinoma and reported that GPC1 IHC can’t distinguish between pleural mesothelioma and lung adenocarcinoma [18]. Moreover, Kai and his colleagues investigated the differential GPC1 expression in poorly differentiated SCC versus solid predominant adenocarcinoma of the lung and reported strong reactivity in all SCC cases, while weak positivity was observed only in two cases of adenocarcinoma. They hypothesized that GPC1 can help in distinguishing between poorly differentiated lung SCC and solid predominant adenocarcinoma, which was in agreement with our findings [19]. This variation in GPC1 expression levels makes it a promising target for future therapeutic approaches. Several studies investigated GPC1 expression in various human cancers and correlated its expression with different clinical and histopathological features. To the best of our knowledge, no previous studies have discussed these correlations in either pleural mesothelioma or lung carcinoma. Analyzing the correlations between GPC1 expression and clinicopathological data in pleural mesothelioma and lung SCC cases, we found that GPC1 expression is significantly correlated with tumor grade and size. High GPC1 expression was more frequently observed in poorly differentiated tumors compared with well-differentiated tumors. Moreover, GPC1 expression was upregulated in larger tumors in lung SCC cases. These findings are in line with previous studies conducted on different neoplasms that reported higher levels of GPC1 expression in larger sizes and higher grades of pancreatic ductal adenocarcinoma [20] and breast carcinoma [21]. Additionally, there was an increase in GPC1 expression levels as the tumor became more advanced and invasive. There was a significantly higher frequency of GPC1 expression in advanced stages than in early stages. These findings are in agreement with a previous study by Chen and his colleagues, who observed higher levels of GPC1 immunoreactivity in advanced stages of hepatocellular carcinoma compared with early stages [12]. We observed that GPC1 expression was upregulated in association with the presence of nodal metastasis. This was in accordance with previous studies reporting that GPC1 plays a role in controlling cell migration [22]. In addition, GPC1 plays a key role in cancer progression and metastasis; GPC1 down-regulation has been noted to inhibit metastasis in pancreatic cancer cell lines [20]. On the other hand, no significant correlations were detected between GPC1 expression and other patients’ characteristics, including age, sex, and smoking, as reported by previous studies [12].
In conclusion, our findings revealed that high GPC1 expression may be associated with more advanced biological behavior in pleural mesothelioma and lung SCC, so it may be a potentially valuable biomarker to predict the prognosis. GPC1 expression level was significantly lower in lung adenocarcinoma, which may help differentiate it from pleural epithelioid mesothelioma and poorly differentiated lung SCC. However, these findings must be interpreted with caution, and further large-scale prospective studies are recommended.
Limitations of the study
The sample size was not large enough, which in turn weakened the statistical power of the results.
Recommendations
Further large-scale prospective studies on GPC1 expression in pleural epithelioid mesothelioma, adenocarcinoma, SCC, and non-malignant lesions of the lung are recommended to obtain more accurate results. In addition, follow-up of patients is important to emphasize the correlation between GPC1 expression and patient survival and disease outcome.
Acknowledgements
Not applicable.
Abbreviations
GPC1, Glypican-1; H and E, Hematoxylin and eosin; HCC, Hepatocellular carcinoma; IHC, Immunohistochemistry/Immunohistochemical; IMIG,The International Mesothelioma Interest Group; IRS, Immunoreactive score; LNM, lymph node metastasis; NSCLC, Non-small cell lung cancer; PBS, Phosphate buffer solution; SCC, Squamous cell carcinoma; SCLC, Small-cell lung cancer.
Declarations
- Ethical approval and consent to participate: The study protocol was reviewed and approved by Ethical Committee of Sohag Faculty of Medicine.
Consent for publication
Not applicable.
Availability of data and material
All data and materials were available.
Competing interests
The authors declare that they have no conflict of interest.
Funding
No fund was received for this work.
Authors Contribution
Noha ED Hassab El-Naby and Nagwa Abd EL-Sadek Ahmed carried out manuscript writing & design, figures analysis & manipulation and histopathological diagnosis with archival revision of paraffin blocks and slides. Mohsen Saber Mohammed Ahmed collected the clinical, demographic and radiological data.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Gender-based epidemiological study of lung cancer patients: single center study, Egypt Abdelwahab H, Farrag N, Akl F, Radi A. Egyptian Journal of Chest Diseases and Tuberculosis.2021;70. CrossRef
- Global Epidemiology of Lung Cancer Barta JA , Powell CA , Wisnivesky JP . Annals of Global Health.2019;85(1). CrossRef
- Novel biomarkers that assist in accurate discrimination of squamous cell carcinoma from adenocarcinoma of the lung Takamochi K, Ohmiya H, Itoh M, Mogushi K, Saito T, Hara K, Mitani K, et al . BMC cancer.2016;16(1). CrossRef
- Environmental And Occupational Risk Factors And Predictors Of Survival Among Malignant Pleural Mesothelioma Patients Abdel-Hamid MA , Ammar NE , Shoman A, Elhoussinie M, Elwakeel H. Egyptian Journal of Occupational Medicine.2019;43(2). CrossRef
- Malignant pleural mesothelioma subtypes Wadowski B, Hung YP , De Rienzo A. Atlas Genet Cytogenet Oncol Haematol.2020;24:373-378. CrossRef
- The pathological and molecular diagnosis of malignant pleural mesothelioma: a literature review Alì G, Bruno R, Fontanini G. Journal of Thoracic Disease.2018;10(Suppl 2). CrossRef
- Glypicans as Cancer Therapeutic Targets Li N, Gao W, Zhang Y, Ho M. Trends in cancer.2018;4(11). CrossRef
- The Expression, Regulation, and Biomarker Potential of Glypican-1 in Cancer Wang S, Qiu Y, Bai B. Frontiers in Oncology.2019;9. CrossRef
- Role of glypican-1 in regulating multiple cellular signaling pathways Pan J, Ho M. American Journal of Physiology. Cell Physiology.2021;321(5). CrossRef
- The Role of Glypicans in Cancer Progression and Therapy Li N, Spetz MR , Ho M. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society.2020;68(12). CrossRef
- High glypican-1 expression is a prognostic factor for predicting a poor clinical prognosis in patients with hepatocellular carcinoma Chen G, Wu H, Zhang L, Wei S. Oncology Letters.2020;20(5). CrossRef
- Glypican-1 Is a Novel Target for Stroma and Tumor Cell Dual-Targeting Antibody-Drug Conjugates in Pancreatic Cancer Tsujii S, Serada S, Fujimoto M, Uemura S, Namikawa T, Nomura T, Murakami I, Hanazaki K, Naka T. Molecular Cancer Therapeutics.2021;20(12). CrossRef
- Targeted beta therapy of prostate cancer with 177Lu-labelled Miltuximab® antibody against glypican-1 (GPC-1) Yeh MC , Tse BWC , Fletcher NL , Houston ZH , Lund M, Volpert M, Stewart C, et al . EJNMMI research.2020;10(1). CrossRef
- Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma Harada E, Serada S, Fujimoto M, Takahashi Y, Takahashi T, Hara H, Nakatsuka R, et a; . Oncotarget.2017;8(15). CrossRef
- The Proteoglycan Glypican-1 as a Possible Candidate for Innovative Targeted Therapeutic Strategies for Pancreatic Ductal Adenocarcinoma Busato D, Mossenta M, Dal Bo M, Macor P, Toffoli G. International Journal of Molecular Sciences.2022;23(18). CrossRef
- Glypican-1 immunohistochemistry is a novel marker to differentiate epithelioid mesothelioma from lung adenocarcinoma Amatya VJ , Kushitani K, Kai Y, Suzuki R, Miyata Y, Okada M, Takeshima Y. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2018;31(5). CrossRef
- Glypican-1 immunohistochemistry does not separate mesothelioma from pulmonary adenocarcinoma Chiu K, Lee L, Cheung S, Churg AM . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2018;31(9). CrossRef
- Glypican-1 is a novel immunohistochemical marker to differentiate poorly differentiated squamous cell carcinoma from solid predominant adenocarcinoma of the lung Kai Y, Amatya VJ , Kushitani K, Kambara T, Suzuki R, Fujii Y, Tsutani Y, Miyata Y, Okada M, Takeshima Y. Translational Lung Cancer Research.2021;10(2). CrossRef
- Elevated glypican-1 expression is associated with an unfavorable prognosis in pancreatic ductal adenocarcinoma Lu H, Niu F, Liu F, Gao J, Sun Y, Zhao X. Cancer Medicine.2017;6(6). CrossRef
- Glypican-1 Overexpression in Different Types of Breast Cancers Alshammari FOFO , Al-Saraireh YM , Youssef AMM , Al-Sarayra YM , Alrawashdeh HM . OncoTargets and Therapy.2021;14. CrossRef
- Glypican 1 promotes proliferation and migration in esophagogastric adenocarcinoma via activating AKT/GSK/β-catenin pathway Pratap A, Li A, Westbrook L, Gergen AK , Mitra S, Chauhan A, Cheng L, et al . Journal of Gastrointestinal Oncology.2022;13(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details