An Observational Study of Stromal CD10 Expression in Breast Carcinoma and Its Association with Clinicopathological Parameters at SMS Medical College and Hospital
Download
Abstract
Introduction: Female breast cancer is now more prevalent than lung cancer worldwide, with an estimated 2.3 million new cases worldwide, or 11.7% of all cancer cases. Breast cancer is an epithelial malignancy, but tumor microenvironment i.e. stroma plays remarkable role in invasion and metastasis. The cross talk between tumor cells and stromal cells is critical step in tumor progression as a result there is increased interest in developing novel therapies targeting the microenvironment.
Aim and Objectives: To study the frequency and intensity of stromal CD10 expression in invasive breast cancer and to correlate it with various clinicopathological parameters.
Materials and Methods: The study was conducted in Department of Pathology at S.M.S. Medical College, Jaipur. CD 10 expression in 70 patients diagnosed with carcinoma breast during the period of 2021-2022, assessed by immunohistochemistry and scored as negative (<10% stromal positivity), weak (10-30% stromal positivity) and strong (>30% stromal positivity) and statistically analyzed with other parameters.
Results and Discussion: Out of the 70 cases, 51 (72.86%) showed CD10 positivity in the stroma, with 28 (40%) showing strongly positive and 23 (32.86%) with weakly positive results. With respect to histopathological grade (P value = 0.0234), molecular subtype (P value = 0.0006), and PR negativity (P value = 0.0480), CD10 expression in the stroma showed a positive correlation.
Conclusion: The correlation between stromal expression of CD10 and histopathological grade, molecular subtype, and PR negativity was statistically significant. To confirm CD10 expression in invasive breast cancer as a predictor of overall survival rates and disease-free survival, more research is required..
Introduction
With an estimated 2.3 million new cases globally, equivalent for 11.7% of all cancer cases, female breast cancer has surpassed lung cancer as the most common cancer. With 685,000 deaths, it ranks 5th among the world’s leading causes of cancer mortality (2020). Every 4th cancer-related death in women is caused by breast cancer [1].
Breast cancer is the most prevalent cancer in women in India, according to NCRP (National Cancer Registry Programme), India. Reducing the incidence and mortality of cancer in Indian women requires a multi-disciplinary approach. The systemic spread of cancer cells is the main cause of these patients’ deaths. The process of a tumor spreading involves growth factors, oncoproteins, and tumor suppressor genes.
Each tumor is unique in terms of its capacity for metastasis and invasion as well as its rate of growth. This heterogeneity results from the interaction of tumor cells with the cells in their microenvironment.
The prognostic and therapeutic outcomes have been studied using a variety of markers, including ER, PR, and HER2neu, which are used to determine hormonal therapy and predictive markers that can be changed by targeting therapy against over expressed oncogenes [2,3]. The intrinsic metastatic potential of cancer cells varies in each individual patient, making it difficult to accurately predict the clinical course for all patients based on generally accepted prognostic factors like tumor size, grade, and axillary lymph node status.
The purpose of this study is to examine the stromal expression of CD 10 in patients with breast cancer and its associations with tumor size, histological grade, pathological stage, lymph node status, hormonal receptors, and HER2neu status. So, the role of stromal expression of CD10 as a prognostic marker in breast carcinoma will be assessed.
Materials and Methods
This cross-sectional study was carried out in the Department of Pathology, S.M.S Medical College, Jaipur (Rajasthan). 70 cases of breast carcinoma operated on and mastectomy specimens sent for histopathological examination to department of pathology were confirmed with the diagnosis of invasive ductal carcinoma by histopathological examination and included in the study after applying inclusion and exclusion criteria. The institutional ethical committee approval was obtained. Representative sections were taken for proper tissue processing, were paraffin embedded and then stained with hematoxylin and eosin stain. Histopathological grading was done according to Elston-Ellis modification of Scarff-Bloom-Richardson grading system and Nottingham’s Prognostic index was calculated. Staging was done according to pTNM status.
For immunostaining most representative section were de-waxed by three changes in xylene of 10 minutes followed by three changes of absolute alcohol of 5 minutes each and Antigen retrieval was done using a pressure cooker as a heating source. Primary antibodies used were: for ER– Rabbit monoclonal, Clone SP1, Biocare, for PR- Rabbit monoclonal, Clone SP2, Biocare, for Her2neu- Rabbit monoclonal, Clone EP3, Biocare, for Ki67- Rabbit monoclonal, Clone SP6, Biocare and for CD10- Mouse monoclonal, Clone 56C6, Biocare.
Scoring for ER, PR and Her2neu is done according to CAP (College of American Pathologists) protocol. Ki67 index of 14% or more than 14% was labeled as positive. CD10 in the stroma is scored as strong positive when more than 30% of the stromal cells showed positivity, as weak positive when 10-30% of stromal cells showed positivity and as negative when less than 10% of the stromal cells showed positivity for CD10.
The Chi-square test was used to determine the interrelationships between the analyzed stromal expression of CD10 and clinicopathological variables in Graph Pad software (Prism 6 version). The mean was used to present quantitative data. Frequency and percentage tables were used to present qualitative data. When the P value was less than 0.05 were considered statistically significant.
Results
In this study, 70 cases of breast cancer were included. Of these, 51 (72.86%) cases revealed CD10 positivity, while 23 (32.86%) cases did so weakly and 28 (40%) cases were strongly positive.
The majority of cases (32; 45.71%) belonged to the 25–45 age group. 33 cases (47.14%) had premenopausal status, while 37 (52.86%) cases were postmenopausal.
Table 1 illustrates the stromal expression of CD10 in breast carcinoma cases and how it relates to different clinicopathological characteristics. Increased tumor grade and stromal expression of CD10 were correlated significantly (χ2= 11.30; P=0.0234). Molecular subtype (P=0.0006) and PR negativity (χ2=6.072; P=0.048) were significantly correlated with stromal CD10 positivity.
| Variables (n=70)) | Stromal expression of CD10 | χ2 and p value | ||
| Number of cases showing negativity 19 (27%) | Number of cases showing weak positivity 23 (33%) | Number of cases showing strong positivity 28 (40%) | ||
| Age | ||||
| 25 to 45 years (32) | 9 (12.86) | 11 (15.71) | 12 (17.14) | 0.6063, 0.9624 |
| 46 to 65 years (29) | 7 (10) | 9 (12.8) | 13 (18.57) | |
| 66 to 90 years (9) | 3 (4.29) | 3 (4.29) | 3 (4.29) | |
| Menopausal status | ||||
| Premenopausal (33) | 10 (14.29) | 11 (15.7) | 12 (17.14) | 0.4404, 0.8024 |
| Postmenopausal (37) | 9 (12.86) | 12 (17.14) | 16 (22.86) | |
| Histologic tumor grade | ||||
| Grade I (11) | 6 (8.57) | 3 (4.29) | 2 (2.86) | 11.30, 0.0234 |
| Grade II (36) | 12 (17.14) | 10 (14.29) | 14 (20) | |
| Grade III (23) | 1 (1.43) | 10 (14.29) | 12 (17.14) | |
| Tumor Size | ||||
| ≤ 2 cm (9) | 4 (5.71) | 4 (5.71) | 1 (1.43) | 3.780, 0.4366 |
| 2.1 to 5.0 cm (42) | 10 (14.29) | 13 (18.57) | 19 (27.14) | |
| >5.0 cm (19) | 5 (7.14) | 6 (8.5) | 8 (11.43) | |
| ER | ||||
| Negative (32) | 5 (7.14) | 10 (14.29) | 17 (24.29) | 5.466, 0.065 |
| Positive (38) | 14 (20) | 13 (18.57) | 11 (15.71) | |
| PR | ||||
| Negative (38) | 6 (8.57) | 13 (18.57) | 19 (27.14) | 6.072, 0.048 |
| Positive (32) | 13 (18.57) | 10 (14.29) | 9 (12.86) | |
| Her 2 | ||||
| Negative (38) | 12 (17.14) | 14 (20) | 12 (17.14) | 4.392, 0.1112 |
| Positive (20) | 4 (5.71) | 4 (5.71) | 12 (17.14) | |
| Lymph Node Status | ||||
| Negative (29) | 8 (11.43) | 8 (11.43) | 13 (18.57) | 0.7107, 0.7009 |
| Positive (41) | 11 (15.71) | 15 (21.43) | 15 (21.43) |
Although it was statistically insignificant, CD10 stromal expression was primarily observed in ER negative breast carcinoma cases (χ2=5.466; P=0.065) and Her-2 positive breast carcinoma cases (χ2=4.392; P=0.1112). Age, menopausal status, lymph node status and tumor size did not correlate with CD10 stromal expression (Figure 1 and 2).
Figure 1. Microphotograph Showing Tumor with Strong CD10 Expression in Stromal Cells - >30% Cells are Positive for CD10 [IHC 100x].
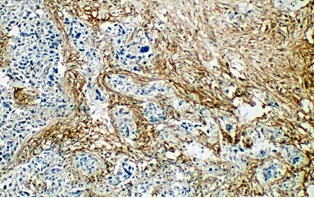
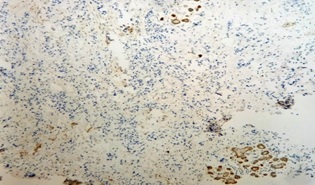
Figure 2. Microphotograph Showing Stromal Cells Negative for CD10 with Positive Myoepithelial Cells in Normal Breast Tissue.
The histological grade and CD10 expression are correlated in Graph 1.
Graph 1. Correlation of CD10 Expression with Histopathological Grade.
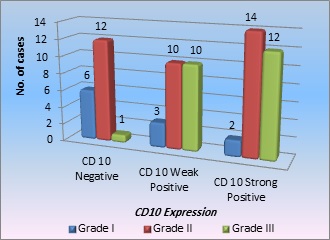
Out of 70 cases, 11 patients were of histological grade 1, of which 6 cases were negative for stromal CD10 expression, 3 cases were weakly positive, and 2 were strongly positive. Maximum cases were of grade 2 (36), 12 of which were negative, 10 cases were weakly positive, and 14 cases were strongly positive for stromal CD10 expression. There were 23 cases of grade 3, of which 1 was negative, 10 cases showed weak positivity for CD10, and 12 cases showed strong positivity,
In the Graph 2 above, CD 10 and molecular subtypes are correlated.
Graph 2. Correlation of CD10 Expression with Molecular Subtypes.
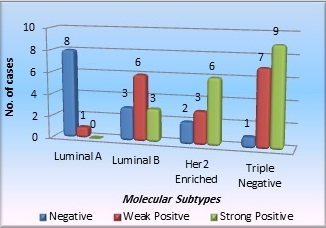
The cases were divided in 4 molecular subtypes on the basis of IHC and we found 17 cases were of Triple negative subtype, 12 cases of Luminal B subtype, 11 cases were of HER2neu enriched subtype and 9 cases of Luminal A subtype. 12 cases of HER2 neu equivocal category and 9 cases positive for ER, PR, and HER2 neu with Ki67 index less than 14% could not be categorized. Thus total 21 cases were unclassified. 9 cases out of a total of 49 cases are of the luminal A subtype, of which 8 had weak CD10 expression and 1 was negative. 3 of the 12 patients of luminal B subtype had negative CD10 results, 6 had weak positive results, and 3 had strong positive results. Among 11 HER 2 enriched patients, 2 were negative, 3 had mild CD10 positivity, and 6 had robust CD10 positivity. Only 1 case out of the 17 triple-negative cases was negative, 7 had weak positive CD-10 expression and 9 had strong positive CD-10 expression. These differences were statistically significant (P value 0.0006). Consequently, we discovered that the molecular subtype is closely related with CD10 expression and concluded that CD10 expression was more frequent and of more intensity in Her2neu positive and triple negative cases compared to luminal A and luminal B subtype.
Discussion
In terms of histopathological characteristics, metastatic patterns, molecular characteristics, outcome, and therapeutic response, breast cancer exhibits heterogeneity. The interaction between tumor cells and the cells of their surrounding microenvironment is what causes this heterogeneity. In breast carcinomas, interaction between stromal elements and tumor cells is a crucial stage in tumor development. Numerous studies have been conducted in this direction using different markers like vimentin, E-cadherin, ß-catenin, cytokeratins, and MMPs that alter the microenvironment predisposing cancer cells for greater progression.
It was discovered through a review of the literature that many researchers looked at the relationship between CD 10 expression in the stroma and breast cancer and suggested that CD10 expression in the stroma is linked to more aggressive tumors.
70 breast cancer patients were included in the current study. The goal was to investigate the relationships between various clinicopathological factors and stromal CD10 expression in breast cancer. Patients with ages ranging from less than 30 to more than 70 were included in this study; their mean age was 49.2 years, with the bulk of cases falling between the age of 25 and 45 and there was no correlation found between the age of the patients and stromal CD10 expression The study is comparable to the study done by Puri V. et al [3] which included patients from 30 to 80 years of age with a mean age of 48.5 years, and Sayantan H. Jana et al [4], which divided patients into age groups of less than 40, 40 to 60, and more than 60 years and found the majority of cases in the 40-60 years age group.
In the present study any statistical association between tumor size and CD10 expression was not found, the result of present study are concordant with the previous studies done by Keiichi Iwaya et al [5] in which 16/86 cases having tumor size <5cm, showed CD10 expression, Sayantan H. Jana et al [4] in which 15/30 cases having tumor size between 2-5 cm, showed CD10 expression while the results of present study are not concordant with the studies done by Ali Taghizadeh-Kermani et al [6] in which 53/81 cases with tumor size >2cm , showed CD10 expression and B.V. Anuradha Devi et al [7] in which 16/25 patients with tumor size 2-5 cm showed CD10 expression and had a statistically significant association between tumor size and CD10 expression.
Correlation between molecular subtype and CD10 expression was studied and it was found in our study, that there was a substantial correlation between molecular subtype and CD10 expression, and we discovered that triple-negative and HER2-neu positive cases had higher rates of stromal CD10 positivity than luminal A and B tumors (P value-0.0006). The findings are similar to those of a research conducted by Tahani Louhichi et al [8] however the association could not achieve statistical significance in their study, which had similar findings (P value-0.09).
In this study, CD10 was expressed in 24/36 cases of grade 2 tumors. Among grade 3 tumors 22 of the 23 cases were positive for CD10, with only one case being negative (10 showed weak positivity and 12 showed strong). This demonstrates that the expression of CD 10 rises with increasing grade, suggesting that it may serve as a marker for the aggressiveness of cancer. The correlation between the histological grade and the expression of CD 10 was statistically significant. (P=0.0234). The results from this study are consistent with those of Nikita A. Makretsov et al [9] who found that 100 out of 139 cases of grade 2 cases had positive CD10 expression (P value=0.002), and Fereshteh Mohammadizadeh et al [10] who discovered that 20 out of 25 patients with grade 2 cases had CD10 positive expression (P value=0.004). Similar findings were made by Sayantan H. Jana et al [4] who discovered that 16 out of 28 patients with histopathological grade 2 showed CD10 positive, and there was a significant correlation between CD10 expression and tumor grade (P value0.04). Although the link between stromal CD10 expression and ER positivity was shown to be negative in the current study, it was not statistically significant. There was discovered to be a statistically significant inverse relationship between stromal CD10 expression and PR positivity. In the current investigation, there was no correlation found between CD10 expression and HER2 neu status.
In conclusion, a statistically significant association was found between the stromal expression of CD10 and the histopathological grade, molecular subtype, and PR negativity. No statistically significant correlations could be found with menopausal status, tumor size, pathological stage, ER and HER2 neu status. More researches are needed to validate CD10 expression as a predictor of overall survival rates and disease-free survival in invasive breast cancer.
Limitations of the Study
Small sample size was an issue in our study. Only representative blocks of the tumor were subjected to IHC; as a result, there may be some variation in CD10 expression because of the heterogeneity of the tumor. All the cases could not be classified into molecular categories on the basis of immunochemistry.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Interrelationships Between Ki67, HER2/neu, p53, ER, and PR Status and Their Associations With Tumor Grade and Lymph Node Involvement in Breast Carcinoma Subtypes: Retrospective-Observational Analytical Study Shokouh TZ , Ezatollah A, Barand P. Medicine.2015;94(32). CrossRef
- Stromal Expression of CD10 in Invasive Breast Carcinoma and Its Correlation with ER, PR, HER2-neu, and Ki67 Puri V, Jain M, Thomas S. International Journal of Breast Cancer.2011;2011. CrossRef
- CD10-a new prognostic stromal marker in breast carcinoma, its utility, limitations and role in breast cancer pathogenesis Jana SH , Jha BM , Patel C, Jana D, Agarwal A. Indian Journal of Pathology & Microbiology.2014;57(4). CrossRef
- Stromal expression of CD10 in invasive breast carcinoma: a new predictor of clinical outcome Iwaya K, Ogawa H, Izumi M, Kuroda M, Mukai K. Virchows Archiv: An International Journal of Pathology.2002;440(6). CrossRef
- The Stromal Overexpression of CD10 in Invasive Breast Cancer and its Association with Clincophathologic Factors Taghizadeh-Kermani A, Jafarian AH , Ashabyamin R, Seilanian-Toosi M, Pourali L, Asadi M, Mashhadi L. Iranian Journal of Cancer Prevention.2014;7(1).
- A study on stromal CD10 expression in invasive breast carcinoma BV Anuradha Devi S , Chandra Sekhar C , Saritha S , Sanhya Anil H , Sandhya Rani . IAIM.2016;3(6):142-147.
- Stromal CD10 expression in breast cancer correlates with tumor invasion and cancer stem cell phenotype Louhichi T, Saad H, Dhiab MB , Ziadi S, Trimeche M. BMC cancer.2018;18(1). CrossRef
- Stromal CD10 expression in invasive breast carcinoma correlates with poor prognosis, estrogen receptor negativity, and high grade Makretsov NA , Hayes M, Carter BA , Dabiri S, Gilks CB , Huntsman DG . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2007;20(1). CrossRef
- CD10 expression in stromal component of invasive breast carcinoma: A potential prognostic determinant Mohammadizadeh F, Salavati M, Moghaddam NA . Journal of Research in Medical Sciences.2012;17(0).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details