Melanotic Neuroectodermal Tumor of Infancy: A Case Series and Comprehensive Review of this Rare Pediatric Neoplasm
Download
Abstract
Melanotic neuroectodermal tumor of infancy also known as progonoma is an exceedingly rare neoplasm occurring within first two years of life with around 550 cases reported so far. It is predominantly a benign neoplasm but clinical suspicion of malignancy is always high owing to its rapid progression. Thus, it is a cause of concern to the parents and clinicians alike. It mostly involves the craniofacial bones and mean age of occurrence is 5 months. On radiology, well-defined lytic lesions are encountered. Histopathology is the gold standard for diagnosis wherein a biphasic tumor is seen. It comprises larger polygonal cells with melanin pigment and smaller round blue cells. Surgical excision is the preferred modality of treatment. However, the recurrence rates vary from 15 to 27%. Very few cases with metastasis have been reported. We present two interesting cases of this rare neoplasm along with a comprehensive clinical and pathological review of this rare entity.
Introduction
Melanotic neuroectodermal tumor of infancy (MNTI) is a very rare benign neoplasm, originating from the neural crest, which is rapidly growing and has a potential for local recurrence [1]. It was first described by Krompecher in 1918 in his article on the histogenesis and morphology of adamantinoma and other swollen jaws [2]. It was earlier known as retinal anlage tumor, melanotic progonoma, melanotic hamartoma or congenital melanocarcinoma [3]. It occurs in the infants and predominantly involves the craniofacial sites [4]. Due to swift growth, it is a cause of concern for the parents and clinicians alike. We report two cases of this exceedingly rare entity with detailed discussion towards the diagnosis and management.
Case details
Case 1
An eight months old female infant was brought with complaints of a swelling on her left face for a period of 15 days. There was a history of minor trauma to the same side of the face around one month back. There was no other significant past or family history. On examination, a 2.5x2 cm, non-tender and non-inflamed soft tissue swelling was noted. Computed tomography (CT) scan revealed an expansile soft tissue mass measuring 4.3x2.3x3.3 cm, epicentered at the left upper alveolus, showing maxillary sinus extension and expansion of ethmoid infundibulum. The mass was causing pressure erosion of the left maxilla. Clinical as well as radiological diagnosis was rhabdomyosarcoma was made.
Biopsy of the lesion showed a tumor with nests of dual population of atypical cells in myxoid stroma. One population of cells displayed moderately pleomorphic hyperchromatic nuclei with cytoplasm showing pigment granules. The surrounding primitive smaller cells exhibited hyperchromatic nuclei and scant cytoplasm. On immunohistochemistry (IHC), the pigmented cells were positive for HMB-45, AE1/AE3, CD99, Melan-A and synaptophysinwhile they were negative for S-100, desmin, and EMA. Ki-67 labelling index was 20 to 25% in the hotspots (Figure 1).
Figure 1. A) Case 1, Contrast Enhanced CT Scan Shows an Expansile Soft Tissue Mass at Left Upper Alveolus, with Maxillary Sinus Extension. B) Microsection shows dual population of atypical cells in nests, pigmented cells are seen on the left side. C) Tumor cells are positive for HMB45 D) Melan A is positive E) CD99 shows diffuse membranous positivity. F) PanCK is diffusely positive. G) Tumor cells are positive for Synaptophysin. H) Ki-67 is nearly 20% in the highest proliferation zone. I ) Case 2, Two distinct population of tumor cells are seen.
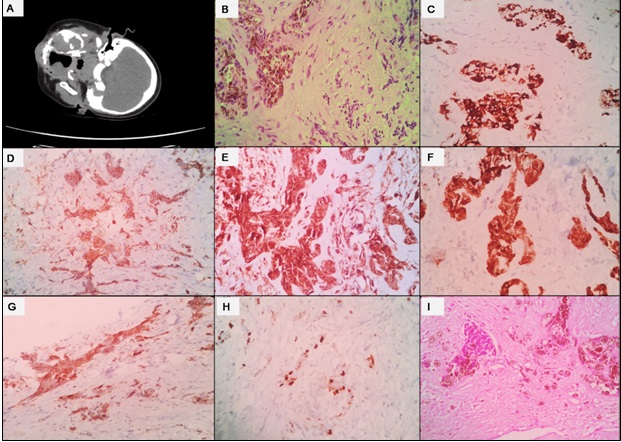
This concluded the diagnosis of MNTI.
Case 2
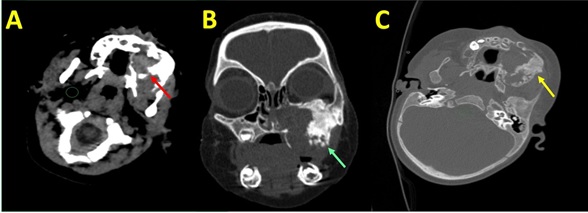
A nine months old female child was brought with swelling over the left upper jaw which was progressively increasing in size over the last four months. There was no other significant past or family history. In the previous health setting, it was clinically diagnosed as hemangioma. On examination, a hard swelling was noted over the upper jaw and left anterior maxilla. CT scan showed an expansile lytic lesion measuring 5 X 4 X 3 cm involving the alveolar arch in the right para-midline region crossing midline to involve the left half and extending into the overlying thinned eroded cortex (Figure 2).
Figure 2. CT Scan Showed an Expansile Lytic Lesion Measuring 5 X 4 X 3 cm Involving the Alveolar Arch (Case 2).
Biopsy of the lesion showed a tumor composed of biphasic population of cells embedded in a fibrotic stroma. The predominant cell type comprised oval to round cells with abundant cytoplasmic melanin pigment. The second population of small round blue cells was arranged in center of clusters or nests. On IHC, the large polygonal cells were positive for HMB-45 and AE1/AE3 and the background cells were positive for synaptophysin, while they were negative for CD99, S-100, Desmin and CD45. This rendered the diagnosis of MNTI. Surgical excision of the mass was done and the child is under regular follow up.
Discussion
MNTI is a rare neoplasm of infants with around 550 cases reported to date. Our patients were females and aged 8 and 9 months, respectively. In a large systematic review of 472 reported cases, Rachidi et al found that 59% of them were males and the median age of occurrence is 5 months [4]. They found the most common site to be maxilla (60.3%), followed by skull (18.1%), mandible (10.3%) and other sites [4]. Our patients also had the involvement of maxilla. However, occurrence at other sites like soft tissue, forearm and reproductive systems have been reported.1 Largely, it develops postnatally and only five cases of congenital MNTI are described in literature [5].
It presents as a painless rapidly enlarging mass usually beneath the intact epithelium and may show blue or black discoloration [1]. On imaging, these neoplasms are well-demarcated lytic lesions with local destruction of the bone in few cases secondary to pressure erosion [4]. The pressure erosion was noted in both of our cases. These locally destructive features, with history of rapid progression, point towards a malignant etiology. CT-scan is the modality of choice to decide the surgical approach although MRI scan helps to know the actual extent of tumor [1,4,5].
Histopathology is the gold standard for diagnosis. It typically comprises of a biphasic population of tumor cells, arranged in islands/nests, embedded in fibro-collagenous stroma [1]. The presence of large epithelioid cells with cytoplasmic melanin pigment and smaller primitive neurogenic cells is the hallmark and usually the larger cells are arranged at the periphery of nests of the smaller cells [6]. The differential diagnosis includes rhabdomyosarcoma, Ewing sarcoma, neuroblastoma, malignant melanoma and clear cell sarcoma of soft tissue [1,5,6].
On IHC, both the cell populations are positive for vimentin and neuron specific enolase (NSE), the larger cells are positive for cytokeratins and melanocytic markers namely HMB-45 and dopamine β-hydroxylase. Occasionally, they are positive for synaptophysin and epithelial membrane antigen. The smaller cells are positive for synaptophysin and occasionally positive for glial fibrillary acid protein (GFAP) [1,4,6]. They are mostly negative for Myogenin and MyoD-1 ruling out rhabdomyosarcoma, CD99 and FLI-1 ruling out Ewing sarcoma and S-100 ruling out clear cell sarcoma and melanoma [1]. In very rare cases, the positivity for these markers can lead to misdiagnosis, thus necessitating correlation with histomorphology and cytogenetics (for alternate diagnosis), if needed. In our cases also, the clinico-radiological diagnosis was rhabdomyosarcoma and hemangioma, respectively whereas histopathology with IHC rendered the final diagnosis.
Complete surgical resection is the treatment modality of choice. Chemotherapy and/or radiation has been used in locally advanced cases (up to 9.6%). The recurrence rates are high varying from 15% to 27% and generally encountered in multicentric tumor or incomplete surgical resection [1,4,7]. Age is reported as an important predictor of recurrence - infants 4.5 months and older had minimal risk of recurrence whereas those younger than 2 months had shorter disease free survival [4]. Metastasis is reported in 3% of cases to regional lymph nodes and central nervous system [1,7]. Few studies found predominance of primitive cells, mitotic rate of ≥2/10 hpf, Ki-67 labeling index >25% and CD99 positivity as predictors of aggressive behaviour.1 However, there are no radiological or histopathological criteria to differentiate benign and malignant neoplasms.
To conclude, MNTI is predominantly a benign neoplasm of infants occurring in the head and neck region. Since it grows rapidly, early diagnosis and complete surgical resection are essential to prevent local disfigurement and optimal patient care.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Melanotic Neuroectodermal Tumor of Infancy Soles BS , Wilson A, Lucas DR , Heider A. Archives of Pathology & Laboratory Medicine.2018;142(11). CrossRef
- Extensive Melanotic Neuroectodermal Tumor of Infancy Albuquerque AFM , Cunha JF , Avelar RL , Juca E, Costa FWG , Macedo MS . Head and Neck Pathology.2016;10(3). CrossRef
- Melanotic neuroectodermal tumor of infancy presenting in the subcutaneous soft tissue of the thigh Lacy SR , Kuhar M. The American Journal of Dermatopathology.2010;32(3). CrossRef
- Melanotic Neuroectodermal Tumor of Infancy: A Systematic Review Rachidi S, Sood AJ , Patel KG , Nguyen SA , Hamilton H, Neville BW , Day TA . Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons.2015;73(10). CrossRef
- Melanotic neuroectodermal tumor of infancy (MNTI) of the head and neck: A French multicenter study Moreau A, Galmiche L, Minard-Colin V, Rachwalski M, Belhous K, Orbach D, Joly A, Picard A, Kadlub N. Journal of Cranio-Maxillo-Facial Surgery: Official Publication of the European Association for Cranio-Maxillo-Facial Surgery.2018;46(2). CrossRef
- Neurogenic tumors of soft tissue Cates JMM , Coffin CM . Pediatric and Developmental Pathology: The Official Journal of the Society for Pediatric Pathology and the Paediatric Pathology Society.2012;15(1 Suppl). CrossRef
- Management of melanotic neuroectodermal tumor of infancy Gaiger de Oliveira M, Thompson LDR , Chaves ACM , Rados PV , Silva Lauxen I, Filho MS . Annals of Diagnostic Pathology.2004;8(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details