Multi-parametric MR Evaluation of Post Neo-adjuvant Chemo-radiation Cases of Rectal Cancer
Download
Abstract
Introduction: In India as per ICMR|NCDIR newly diagnosed rectal cancer cases in 20211 were 15,782. Among these new cases, more cases were registered in males (9506) than females (6276). MRI is the imaging modality of choice for staging and restaging after chemo radiotherapy. Multi-parametric MRI implies addition of multiple parameters including diffusion weighted imaging, dynamic contrast enhancement, conventional MRI and specific sequences tailored to rectal cancer. As such Dynamic contrast enhanced MRI and Diffusion weighted imaging remains an active area of ongoing research. Characterization of lymph nodes based on morphologic criteria helps in diagnosing metastatic lymph nodes.
Objectives: Primary objective is to establish the role of novel MRI pulse sequences and protocols in patients with rectal cancer post-treatment (neo-adjuvant chemo-radiation). Secondary objective is to evaluate post-treatment morphological components of tumour on MRI and its correlation with treatment response.
Methods: Present prospective study was conducted in the Department of Radio-diagnosis of a tertiary level care.
Results: Total 40 patients were enrolled in this study. Dynamic contrast enhanced and diffusion weighted sequences gave most significant results, where the exact localization of tumour was done, however in DCE MRI and DWI MRI there were 1 (97.5%) and 2 (95%) patients respectively, showed inconclusive results in T staging after post NACRT. As per the study, conventional sequences in post NACRT patients are less sensitive in T staging due to the post NACRT fibrosis and necrosis.
Conclusion: Among conventional sequences oblique axial T2 weighted imaging with minimal spacing and slice thickness along with post contrast T1 images allow the most accurate assessment of residual tumor. In novel sequences DWI and dynamic contrast imaging also shows accurate results and should be added to routine sequences if possible.
Introduction
Rectal cancer is usually diagnosed after the age of 50 years. It constitutes the main subset of colorectal cancer (CRC) requiring a dedicated multidisciplinary modality. Magnetic resonance imaging (MRI) gained wide acceptance in the pre-surgical assessment of rectal cancer, mainly for local staging. MRI has an overall good sensitivity in local staging of primary tumors. It can evaluate and predict resection margin, extramural vascular invasion with good accuracy. Certain studies have shown that MRI is a reproducible technique with specificity as high as 92% in predicting negative circumferential resection margin (CRM), depth of invasion, and relationship with CRM.
Recently, neoadjuvant therapy that consists of chemo- and radiotherapy (CRT) is need of the hour for local staging of rectal carcinomas. Treatment response can be assessed using MRI. The sensitivity of MRI for assessing the treatment response is less compared to sensitivity for local primary staging. The presence of inflammation, edema and fibrosis due to treatment may mask the identification of complete response to therapy, as their signal intensities on T2-weighted images resemble tumour. The sensitivity and specificities were mostly based on rectal classical MRI with conventional sequences depending on the morphological assessment. Due to these disadvantages, a functional response assessment approach with advanced sequences is required than just deepening on morphologic data. CRT can cause certain side effects like incontinence and bowel dysfunction. So, it is vital to predict non-responders during the time of initial diagnosis itself.
Conventional rectal MRI includes high-resolution TWI oriented as per the axis of tumour in the rectum. The word “multi-parametric” MRI of rectum includes adding diffusion- and perfusion-weighted sequences to conventional MRI. It is focused on tumour biology, which is relatively novel. There were 15,782 new cases of rectal cancer in India in 2021 as per ICMR|NCDIR [1]. Among these new cases more cases were registered in males (9506) than females (6276).
Aim and Objectives
Aim
To know the role of multi-parametric MRI in rectal cancer patients after neo-adjuvant chemo-radiation therapy.
Objectives
Primary objective is to establish the role of novel MRI pulse sequences and protocols in patients with rectal cancer post-treatment (neo-adjuvant chemo-radiation).
Secondary objective is to evaluate post-treatment morphological components of tumour on MRI and its correlation with treatment response.
Study Design And Sampling
The current study was conducted in the department of Radio-diagnosis,Dr Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Type of study
Prospective study
We included 40 patients in the current study.
Materials and Methods
Methodology
Histo-pathologically diagnosed rectal cancer patients were evaluated with pre-treatment multi-parametric MRI.
Neo-adjuvant Chemo-radiotherapy was given.
Post NACRT patients were then evaluated with multi-parametric MRI.
MRI of the rectum was taken with same protocols as in pre treatment with greater emphasis on novel sequences in post treatment.
The shown images are of rectal cancer patient, pre NACRT status, Figure 1a) axial T2 weighted image, Figure 1b) T2 sagittal image, Figure 1c) DWI, Figure 1d) CE MRI, showing circumferential altered signal intensity lesion showing restriction on DWI and enhancement on post contrast study. Arrow in the ‘d’ is pointed at the lesion (Figure 1).
Figure 1. a) Axial T2 Weighted Image, b) T2 Sagittal Image, c) DWI, d) CE MRI, Showing Circumferential Altered Signal Intensity Lesion Showing Restriction on DWI and Enhancement on Post Contrast Study.
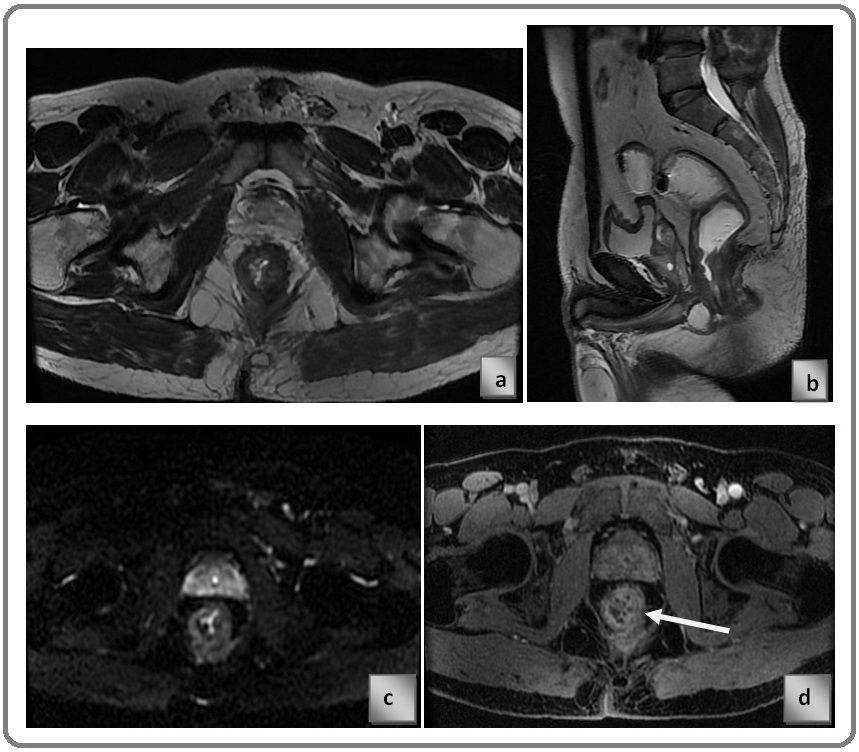
The same patient discussed before underwent neo-adjuvant chemo-radiotherapy Figure 2 a) axial T2 weighted image, Figure 2b) T2 sagittal image, Figure 2c) DWI, Figure 2d) CE MRI, circumferential altered signal intensity lesion which is inconclusive on T2 and DWI due to post NACRT fibrosis, CE-MRI image showing enhancing component in residual tumour and non-enhancing component is likely to be post NACRT fibrosis. Post NACRT Multi-parametric MRI scan suggests significant reduction in tumour volume. Arrow in the‘d’ is pointed at the post treatment fibrosis (Figure 2).
Figure 2. a) Axial T2 Weighted Image, b) T2 Sagittal Image, c) DWI, d) CE MRI.
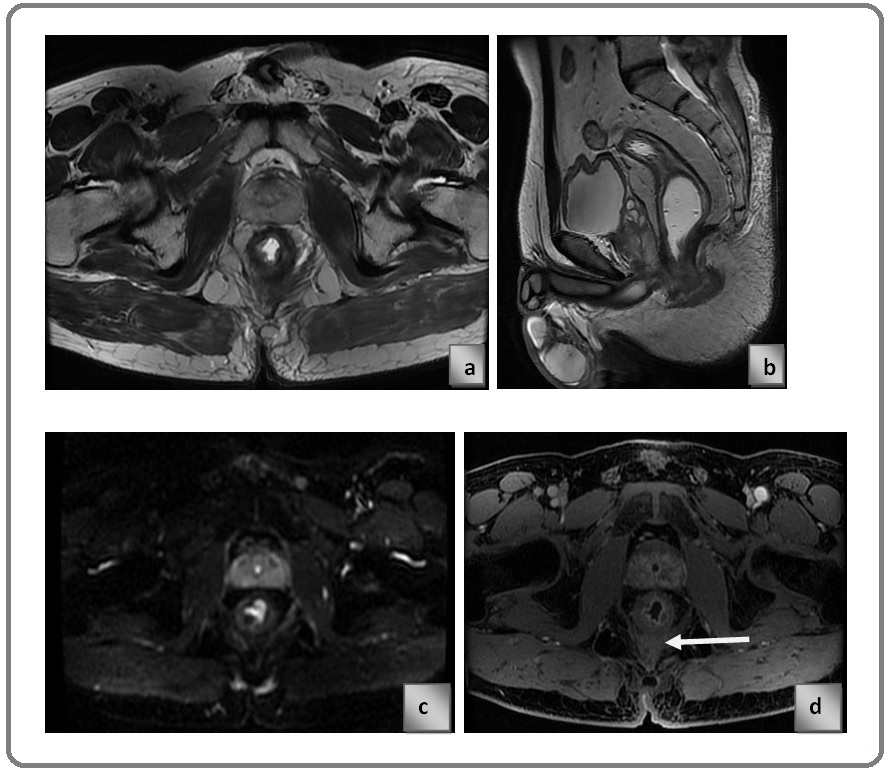
Contrast enhanced MRI in another patient with rectal cancer Figure 3 a) pre NACRT image shows circumferentially enhancing thickening in wall of rectum and extending into mesorectal fat Figure 3 b) post NACRT image shows the lesion is extending into the meseorectal fat and infiltrating the mesorectal fascia which is suggestive of progressive disease. Arrow showing the focal infiltration of lesion into mesorectal fascia (Figure 3).
Figure 3. a) Pre NACRT Image Shows Circumferentially Enhancing Thickening in Wall of Rectum and Extending into Mesorectal Fat. b) post NACRT image shows the lesion is extending into the meseorectal fat and infiltrating the mesorectal fascia which is suggestive of progressive disease. c) Post Contrast T1 LAVA Sequence Showing Stage T3b Lesion. d) Post Contrast T1 LAVA Sequence Showing Stage T3c Lesion.
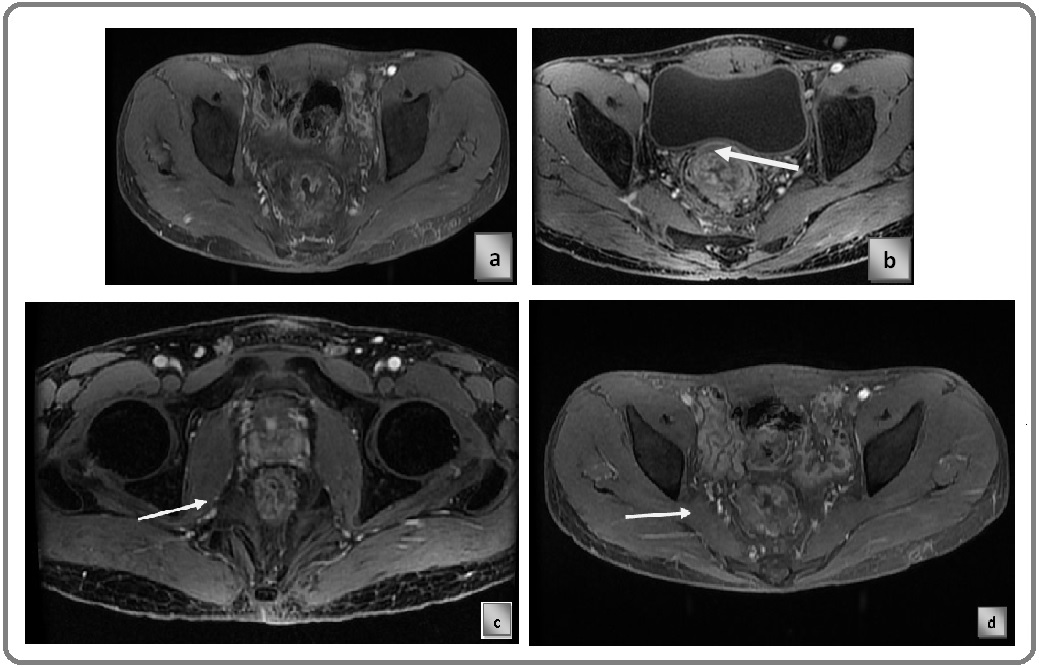
Known case of Carcinoma Rectum, post contrast T1 LAVA sequence showing stage T3b lesion. Arrow in the image is pointed at the lesion (Figure 3c).
Known case of Carcinoma Rectum, post contrast T1 LAVA sequence showing stage T3c lesion. Arrow in the image is pointed at the lesion (Figure 3d).
Known case of Carcinoma Rectum, post contrast T1 LAVA sequence Figure 4a) axial plane Figure 4b) sagittal plane, showing stage T3d lesion. Arrow in the image is pointed at the lesion (Figure 4c).
Figure 4. a) Axial Plane; b) Sagittal Plane, Showing Stage T3d lesion. Arrow in the image is pointed at the lesion. c) Post Contrast T1 LAVA Sequence Showing Stage T4a Lesion .
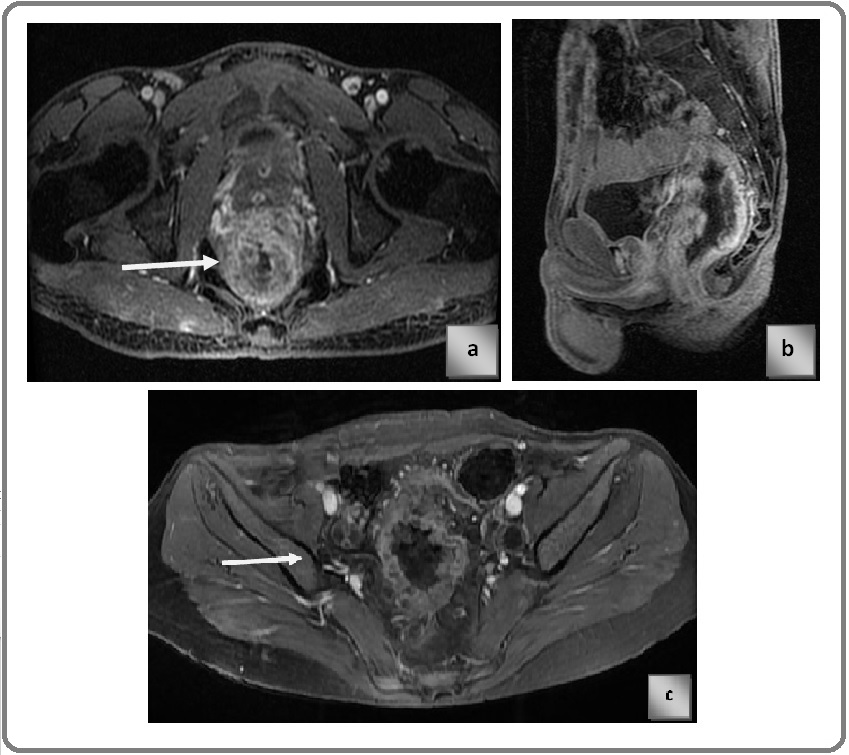
Known case of Carcinoma Rectum, post contrast T1 LAVA sequence showing stage T4a lesion. Arrow in the image is pointed at the lesion.
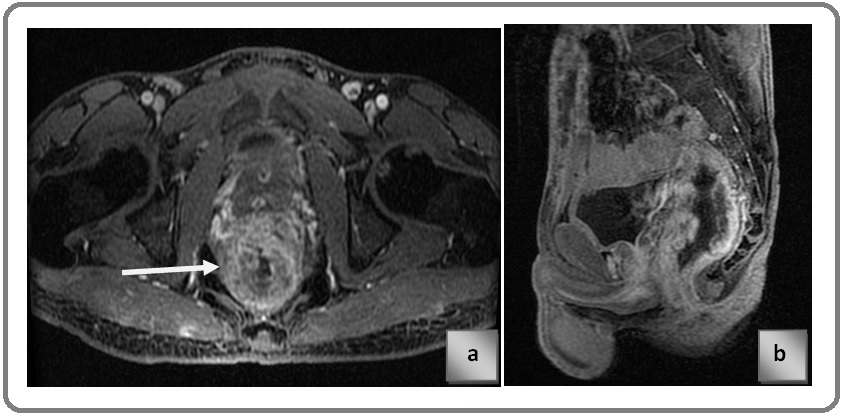
Known case of carcinoma Rectum, post contrast LAVA T1 sequence Figure 5 a) axial plane Figure 5 b) sagittal plane, showing T4b stage cancer anteriorly infiltrating the posterior wall of prostate. Arrow demonstrates the infiltration of lesion into prostate focally.
Figure 5. a) Axial plane, b) Sagittal plane.
Results
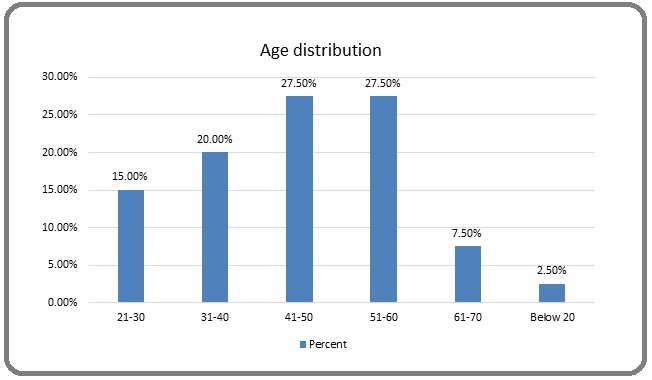
The above table show the distribution of patients according to age group. The age of the patients varied from 16 to below 70 years. The mean age was 44.17±13.4 years (Figure 6).
Figure 6. Age Group Distribution.
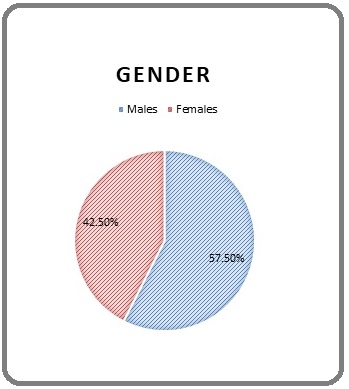
The graph shows the distribution of patients according to sex. Out of the 40 patients, 23 (57.50%) were male and 17 (42.50%) were female (Figure 7).
Figure 7. Sex distribution.
Diagnosis of rectal cancer was conclusive in 95% of patients. It was inconclusive in 5% of patients as per DWI before treatment (Table 1).
| Diagnosis DWI | Frequency | Percent (%) | Cum. Percent (%) |
| Conclusive | 38 | 95 | 95 |
| Inconclusive | 2 | 5 | 100 |
| Total | 40 | 100 | 100 |
Diagnosis of rectal cancer was conclusive in 97.5% of patients in contrast enhanced MRI (Table 2).
| CE-MRI | Frequency | Percent (%) | Cum. Percent (%) |
| Conclusive | 39 | 97.50 | 97.50 |
| Inconclusive | 1 | 2.50 | 100.00 |
| Total | 40 | 100.00 | 100.00 |
Diagnosis was inconclusive in 17.5% of patients in the current study after treatment in DWI (Table 3).
| T2-after treatment | Frequency | Percent (%) |
| Conclusive | 33 | 82.50 |
| Inconclusive | 7 | 17.50 |
| Total | 40 | 100.00 |
Diagnosis was inconclusive in 10% of patients in the current study after treatment in CE MRI (Table 4).
| T2-after treatment | Frequency | Percent (%) |
| Conclusive | 36 | 90 |
| Inconclusive | 4 | 10 |
| Total | 40 | 100.00 |
Downgrading of tumours (regression and stabilization)- improvement after treatment was seen in 77.5% of patients. Progression was seen in 22.5% of patients in the current study (Table 5).
| Downgrading | Frequency | Percent (%) | Cum. Percent (%) |
| No | 9 | 22.5 | 22.5 |
| Yes | 31 | 77.5 | 100 |
| Total | 40 | 100 | 100 |
Discussion
Age
The mean age of the patients in the current study was 44.17±13.4 years and the age varied from 16 to 70 years, with males commonly affected by rectal cancer more than females. The mean age of the patients with rectal cancer found in a study on 978 patients at a tertiary care center in India was 45.7 years, with a range of ages from 11 to 85 years. Also, 517 (65%) males were seen as affected predominantly with CRC over 283 (35%) females, out of 978 patients assessed in one year.33% of the participants in their study were under the age of 40, while 44% were in the 40 to 60 age range [2]. The median age at diagnosis in research by Hussain et al [22] 2013, in central India, on 233 patients over 8 years, was reported to be 43 years; 39% of the study participants were identified with CRC at 40 years or below [3]. In a study from eastern India, the mean age was reported to be 47.01 years, while in a retrospective examination of 220 patients, it was 58.4 years [4,5]. Similar results with CRC at younger ages have also been found in other trials [6,7]. In contrast, the data from western nations shows higher ages. 90% of newly diagnosed CRC cases in the USA are over 50 at the time of diagnosis, and 58% of newly diagnosed CRC cases are over 65 years of age [8].
Our findings are consistent with other researches done in India, which found that rectal cancer occurs more frequently in the younger age group and is most common in people in their fourth decade of life. Although the precise cause is unknown, the increasing prevalence may be related to changes in lifestyle since a sizable section of the world’s population is made up primarily of young and middle-aged people [9]. Younger people are more likely to access medical care than older ones, therefore it could represent a referral bias. Due to the anatomical proximity and continuity of the colon and rectum, there is also a chance of misclassification with over diagnosis. The greater accessibility of diagnostic colonoscopies could be another factor contributing to the rise in rectal cancer incidence that we observed [3].
Gender
Our analysis found that males had a higher sex distribution for rectal cancer than females, which is consistent with the male preponderance reported in the prior literature [10,11].
Location of Tumour
Most patients in our study presented with low-lying rectal tumours, which was similar to the study by Deo. SS et al. [12], 2021 where the predominant location of the tumour was in the lower rectum (58%) followed by the middle and upper rectum of their patients. In another study, 71% of patients with rectal cancer had tumour growth within 5 cm of the anal margin [13].
Role of Dwi-Mri and Dce-Mri in Rectal Cancer
Now, in the current study, the diagnosis of rectal cancer was conclusive in 95% of patients with DWI and 97.5% with contrast-enhanced MRI. A study by Rao SX et al [14] 2008 on 45 patients with rectal cancer showed that the sensitivity in detecting the primary lesion improved from 82–84% to 93%–96% by adding the DWI technique together with T2-weighted imaging [14]. In 2014, Attenbergeret al. demonstrated that multi-parametric MRI in rectal cancer in 54 patients showed a significant difference between cT2 and cT3 tumours and also predicted tumour grade using perfusion MRI [15]. A study by Sun H et al [16] 2018 showed that DWI MRI is useful in tumour grading and staging as well as the detection of extramural vascular invasion. Albert et al. 2013 reported DCE MRI as a useful tool for nodal staging but not for tumour staging, CRM involvement, or complete response detection [17]. High nodal staging performance has been reported for DCE MRI with lymph node-specific contrast agents [18]. In contrast to our study, a previous report reported that DWI is less useful for the detection of mucinous rectal tumours because the high mucin content of these tumours shows less restricted diffusion, and the evaluation is limited by T2 effects [19]. After treatment with neo-adjuvant therapy, in our study, we noted improved results in 15% of patients in the T2 stage, 28 patients with no lymph nodal metastasis, a significant difference in the craniocaudal length, extramesorectal lymph nodal involvement in only 1 patient as against 4 patients before treatment. 77.5% of patients showed tumour downgrading. However, the study noted disease progression in 22.5% of patients. Even after treatment, the inconclusive diagnosis according to T2, DWI, and CE MRI was 30%, 17.5%, and 10% respectively of the study population. The results of our study are consistent with those of previous rectal cancer studies [20-23] in which the addition of DWI to conventional MR sequences helped to detect viable tumours after neo-adjuvant CRT. Previous literature supports the addition of DWI to conventional MRI in local restaging after NCRT [24-28]. In a meta- analysis by van der Paardt et al [29] 2013, the pooled sensitivity for predicting response (defined as either ypT0, ypT0-2 or T-down staging compared to primary staging) was significantly higher in studies that included DWI in the MR protocol compared to studies, which did not (83.6% vs. 50.4%). Oberholzer et al. [30] 2013, demonstrated in a large prospective study that DCE curves and semi quantitative perfusion parameters were able to distinguish between good responders and poor responders. In contrast to our results, previous reports suggest that DWI-MRI is futile in predicting nodal response [25,26]. Nie et al [37] 2016, evaluated the pathologic response of rectal cancer after neo-adjuvant treatment in 48 patients using multi-parametric MRI (DWI and DCE) and found better predictive value compared to conventional imaging metrics through a systematic analysis of multi-parametric MRI features. Hotker et al [31] 2016, performed a study on 24 patients using multi-parametric MRI and observed a significant association between histo-pathological grades of tumor regression with DEC and DWI volumetry. However, they did not find an association between functional parameters (ADC, Ktrans) and tumor regression. Attenberger et al’s [32] 2017, research on multi-parametric MRI evaluation of rectal cancer response to treatment after NCRT found significant differences in ADC values about tumor regression with no statistically significant difference in perfusion parameters [32]. Sun et al [16] 2018, observed good interobserver agreement with diffusion and perfusion parameters using multi-parametric MRI. However, it should be noted that the authors did not perform a contrast perfusion technique, which in our opinion is the main limitation of their study [16].
Our study showed that CE-MRI is more conclusive in staging, after treatment than DWI MRI. Similar results were also reported in a study of 51 patients evaluating the value of (DCE-MRI) for predicting pathologic response in rectal cancer. The study reported 100% PPV for ΔK trans DCE-MRI, which was better than DW-MRI PPV. However, the sensitivity of 67% was significantly lower when using DCE-MRI. The lower sensitivity has been attributed to differences in the biological/physiological background of the two imaging techniques, with DW-MRI showing cell density while DCE-MRI reflects tumour vasculature [33]. High nodal staging performance has been reported for DCE MRI with lymph node-specific contrast agents [18]. A retrospective analysis of 328 patients also showed that the contrast-enhanced MRI protocol in predicting the pathologic stage of ypT0-1 tumours was 0.81 (95% CI: 0.77, 0.85), better than the T2-weighted DWI protocol (0.66; 95 % CI: 0.60, 0.71; P < 0.001) and concluded that CE MRI provides accurate discrimination of ypT0-1 from ypT2-4 tumours after NCRT. In 2015, Petrillo et al. [35] investigated the predictive role of MRI volumetry based on signal intensity characteristics on DCE to differentiate between complete and incomplete responders. They showed that the diagnostic performance associated with the volumetric change of DCE-MRI was superior to that of T2-weighted volumes and DW-MRI.
Despite the obvious use of DWI, image interpretation requires a certain level of experience due to certain pitfalls such as suboptimal sequential angulation, misinterpretation of low ADC signal from fibrosis, sensitivity effects, and fluid translucency in the rectal lumen. These hinder the diagnostic performance of inexperienced radiologists [36]. The better results with CEMRI could also be due to the higher artefacts seen in DWMRI, for which a previous study suggested performing a micro-enema before performing rectal MRI in the setting of tumour restaging to reduce the amount of rectal gas to reduce artefacts in DWI MRI [29-37].
In conclusion, oblique axial T2 weighted imaging with minimal spacing and slice thickness along with post contrast T1 images allow the most accurate assessment of residual tumour. Current guidelines do not routinely recommend contrast study but we strongly recommend in all cases. DWI and dynamic contrast imaging also shows accurate results and should be added to routine sequences if possible. Several morphological responses like fibrosis and tumour necrosis are well demonstrated on MRI and are extremely useful for tumour chemo-radiotherapy response. Multi-parametric MRI correlates well with pathological responses and offers superior soft tissue characteristics, that allows for detection of recurrence/ residual tumour and restaging of post-NACRT patients. It plays a crucial role in the staging of rectal cancer post NACRT. Multi-parametric novel pulse sequences aid in predicting the extent of involvement of mesorectal fascia in pre and post treatment, being more sensitive in differentiating post treatment remnant lesion from post treatment fibrosis. The advent in multi-parametric sequences and introduction of techniques like CE-MRI have improved the quality of detection of lymph node positivity.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- https://www.ncdirindia.org/Area_Cancer.aspx .
- Colorectal Cancer in India: An Audit from a Tertiary Center in a Low Prevalence Area Patil PS , Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A, Chopra S, Bal M. Indian Journal of Surgical Oncology.2017;8(4). CrossRef
- Colorectal cancer in young adults in a tertiary care hospital in Chhattisgarh, Raipur Sudarshan V, Hussain N, Gahine R, Mourya J. Indian Journal of Cancer.2013;50(4). CrossRef
- Profile of colorectal cancer in Eastern India Sarkar S, Mukherjee R, Paira SK , Roy B, Banerjee S, Mukherjee SK . Journal of the Indian Medical Association.2012;110(12).
- Colorectal cancer distribution in 220 Indian patients undergoing colonoscopy Peedikayil MC , Nair P, Seena SM , Radhakrishnan L, Sadasivan S, Naryanan VA , Balakrishnan V. Indian Journal of Gastroenterology: Official Journal of the Indian Society of Gastroenterology.2009;28(6). CrossRef
- High frequency of young age rectal cancer in a tertiary care centre of southern Assam, North East India Laskar RS , Talukdar FR , Mondal R, Kannan R, Ghosh SK . The Indian Journal of Medical Research.2014;139(2).
- Rectal cancer in young adults: a series of 102 patients at a tertiary care centre in India Nath J, Wigley C, Keighley MRB , Perakath B. Colorectal Disease: The Official Journal of the Association of Coloproctology of Great Britain and Ireland.2009;11(5). CrossRef
- Colorectal cancer statistics, 2014 Siegel R, Desantis C, Jemal A. CA: a cancer journal for clinicians.2014;64(2). CrossRef
- Colorectal Cancer: Epidemiology, risks and prevention. In :Ahlgren JD, Mc Donald JS (editors): Gastrointestinal Oncology Alabaster O. JB Lipincott: Philadelphia.;1972:243-259.
- National Cancer Registry Programme. Population based cancer registries 2004-2005. New Delhi: Indian Council of Medical Research; 2008 .
- Outcome of patients with acute intestinal obstruction due to colorectal carcinoma Rasool A, Bari S, Rashid S, Wani A, Wani R, Peer G. Internet J Surg.2008;20(1).
- Colorectal Cancers in Low- and Middle-Income Countries-Demographic Pattern and Clinical Profile of 970 Patients Treated at a Tertiary Care Cancer Center in India Deo SVS , Kumar S, Bhoriwal S, Shukla NK , Sharma A, Thulkar S, Das P, et al . JCO global oncology.2021;7. CrossRef
- Colorectal Cancer Profile in a Tertiary Care Centre, Bangalore, India Sailaja Suryadevara , Kv VK , Skm P, Arjun R, Deshmani V, Mukherjee G. Online Journal of Health & Allied Sciences.2014;13(1).
- The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection Rao SX , Zeng MS , Chen CZ , Li RC , Zhang SJ , Xu JM , Hou YY . European Journal of Radiology.2008;65(2). CrossRef
- Multi-parametric MRI of rectal cancer - do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Attenberger UI , Pilz LR , Morelli JN , Hausmann D, Doyon F, Hofheinz R, et al . European Journal of Radiology.2014;83(7). CrossRef
- Intravoxel Incoherent Motion MRI of Rectal Cancer: Correlation of Diffusion and Perfusion Characteristics With Prognostic Tumor Markers Sun H, Xu Y, Song A, Shi K, Wang W. AJR. American journal of roentgenology.2018;210(4). CrossRef
- Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer Alberda WJ , Dassen HPN , Dwarkasing RS , Willemssen FEJA , Pool AEM , Wilt JHW , Burger JWA , Verhoef C. International Journal of Colorectal Disease.2013;28(4). CrossRef
- Performance of gadofosveset-enhanced MRI for staging rectal cancer nodes: can the initial promising results be reproduced? Heijnen LA , Lambregts DMJ , Martens MH , Maas M, Bakers FCH , Cappendijk VC , Oliveira P, et al . European Radiology.2014;24(2). CrossRef
- Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma Nasu K, Kuroki Y, Minami M. Japanese Journal of Radiology.2012;30(2). CrossRef
- Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy Kim SH , Lee JM , Hong SH , Kim GH , Lee JY , Han JK , Choi RI . Radiology.2009;253(1). CrossRef
- Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Sassen S, Booij M, Sosef M, Berendsen R, Lammering G, Clarijs R, Bakker M, et al . European Radiology.2013;23(12). CrossRef
- Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study Lambregts Dm , Vandecaveye V, Barbaro B, Bakers Fc , Lambrecht M, Maas M, Haustermans K, et al . Annals of surgical oncology.2011;18(8). CrossRef
- Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging Song I, Kim SH , Lee SJ , Choi JY , Kim MJ , Rhim H. The British Journal of Radiology.2012;85(1013). CrossRef
- The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the "DISTANCE"? Nougaret S, Reinhold C, Mikhael HW , Rouanet P, Bibeau F, Brown G. Radiology.2013;268(2). CrossRef
- Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting Beets-Tan RGH , Lambregts DMJ , Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, et al . European Radiology.2013;23(9). CrossRef
- Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes Heijnen LA , Lambregts DMJ , Mondal D, Martens MH , Riedl RG , Beets GL , Beets-Tan RGH . European Radiology.2013;23(12). CrossRef
- Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy Park MJ , Kim SH , Lee SJ , Jang KM , Rhim H. Radiology.2011;260(3). CrossRef
- Diffusion weighted MRI: overview and implications for rectal cancer management Boone D, Taylor SA , Halligan S. Colorectal Disease: The Official Journal of the Association of Coloproctology of Great Britain and Ireland.2013;15(6). CrossRef
- Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis Paardt MP , Zagers MB , Beets-Tan RGH , Stoker J, Bipat S. Radiology.2013;269(1). CrossRef
- Rectal cancer: assessment of response to neoadjuvant chemoradiation by dynamic contrast-enhanced MRI Oberholzer K, Menig M, Pohlmann A, Junginger T, Heintz A, Kreft A, Hansen T, et al . Journal of magnetic resonance imaging: JMRI.2013;38(1). CrossRef
- Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters Hötker AM , Tarlinton L, Mazaheri Y, Woo KM , Gönen M, Saltz LB , Goodman KA , Garcia-Aguilar J, Gollub MJ . European Radiology.2016;26(12). CrossRef
- mMRI at 3.0 T as an Evaluation Tool of Therapeutic Response to Neoadjuvant CRT in Patients with Advanced-stage Rectal Cancer Attenberger UI , Ong MM , Rathmann N, Doyon F, Kienle P, Hofheinz RD , Pilz LR , et al . Anticancer Research.2017;37(1). CrossRef
- Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation Intven M, Reerink O, Philippens MEP . Journal of magnetic resonance imaging: JMRI.2015;41(6). CrossRef
- Standardized Index of Shape (SIS): a quantitative DCE-MRI parameter to discriminate responders by non-responders after neoadjuvant therapy in LARC Petrillo A, Fusco R, Petrillo M, Granata V, Sansone M, Avallone A, Delrio P, et al . European Radiology.2015;25(7). CrossRef
- Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching Lambregts DMJ , Heeswijk MM , Delli Pizzi A, Elderen SGC , Andrade L, Peters NHGM , Kint PAM , et al . European Radiology.2017;27(10). CrossRef
- Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment Maas M, Lambregts DMJ , Nelemans PJ , Heijnen LA , Martens MH , Leijtens JWA , Sosef M, et al . Annals of Surgical Oncology.2015;22(12). CrossRef
- Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI Nie K, Shi L, Chen Q, Hu X, Jabbour SK , Yue N, Niu T, Sun X. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2016;22(21). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details