Correlation of PDL-1 Expression with Tumour Budding and Tumour Infiltrating Lymphocytes in Colorectal Cancers
Download
Abstract
Objective: To study and correlate PDL-1 expression with Tumour budding and Tumour Infiltrating Lymphocytes in Colorectal Carcinoma.
Background: Colorectal cancer (CRC) is third most common cancer with a high mortality. Many attempts have been made to raise overall survival of CRC patients. The immune system plays an important role in clearing the unhealthy cancer cells. Programmed death 1 (PD1) is a regulatory molecule which dampens the immune response when bound to one of its complementary ligands (PDL1). Its expression is related to the response of immunotherapy in CRC treatment which has been exploited in recent times. However, its prognostic value is still controversial, and the distribution of PD-L1 on tumour Cells or Immune Cells has not been comprehensively analysed.
Method: A total of 30 patients diagnosed with CRCs were included who underwent surgical intervention. Cases who took preoperative neoadjuvant chemotherapy or radiotherapy were excluded. IHC analyses of PDL1 was done and was correlated with tumour budding and Tumour Infiltrating Lymphocytes (TILs) and statistical significance was assessed.
Results: 11 cases showed low bud count at the invasive front out of which only 3 cases showed PDL1 positivity. The rest 19 cases had high bud count out of which 18 were PDL 1 positive. This difference was highly significant (p = 0.002). In Low Bud / High TILs, 75% cases showed no PDL1 expression in tumour cells, whereas 62.5% cases showed PDL1 positivity in TILs whereas in High Bud / Low TILs group, all the cases (100%) showed PDL1 expression in tumour cells whereas only 75% cases showed PDL1 positivity in TILs, again being statistically significant (p <0.001).
Conclusion: This study showed an inverse correlation between PDL1 in tumour buds and immune cells, thus emphasising the role of tumour microenvironment. Our study reiterates the fact that high expression of PDL1 in tumour cells suppresses antitumor response whereas its high expression in TILS correlates with a better prognosis.
Introduction
Colorectal cancer (CRC) is third most common cancer in terms of recognition (6.1%) and second in terms of mortality (9.2%) [1]. Recent data show that CRC incidence rates in India have increased by 20% making it the fourth most common cause of death in the country due to cancer after those of lung, stomach, and liver [2].
Many attempts were made to raise overall survival of CRC patients. FDA approved targeted molecular therapy like cetuximab and panitumumab in combination with chemotherapeutic agents such as oxaliplatin or leucovorin proved to be associated with better and progression free treatment outcome [3].
The immune response plays an important role in protecting us from disease and clearing the body’s own unhealthy and ailing cells and so it has been a question of great interest in a wide range of fields such as cancer therapies and anti-tumour immunity through checkpoint inhibitors. The first FDA approved antibody designed for immune checkpoint blockade was Ipilimumab which is an anti-CTLA4 antibody [4]. Following this, blockade of other immune checkpoint proteins, such as PDL-1, indicated other ways of amplifying immune responses against tumours like melanoma, renal cell carcinoma, and non-small cell lung cancer. Programmed death ligand-1 (PD-L1) is a regulatory molecule expressed in T cells which has immunoregulatory function. PD-1 ligand (PD-L1) also referred to as CD279/ B7-H1, is a 33-kDa type 1 transmembrane glycoprotein that contains 290 amino acids [5]. It is usually expressed by macrophages, some activated T cells and B cells, Dendritic Cells, and some epithelial cells, particularly under inflammatory conditions. Binding of PD-L1 to its receptor suppresses T cell migration, proliferation, and secretion of cytotoxic mediators, and restricts tumour cell killing [6]. Thus, inhibitors of PD-1 and PD-L1 disrupt PD-1 axis thereby enhancing endogenous antitumor immunity leading to antitumor responses for patients with a wide range of cancers. Some malignancies express PD-L1 on their cell surfaces leading to the inhibition of the cytotoxic T cells, thus evading the immune response against them [7].
Recently, there seemed to be some evidence from various studies which indicated that PD-L1 expressing MSI tumours show more signals of anti-tumour activity during PD-1 targeting therapy [8-10], following which the Food and Drug Administration (FDA) approved the combination Ipilimumab and Nivolumab for the treatment of some patients with metastatic colorectal cancer who were treated previously with standard chemotherapy drugs [11].
The present study was thus undertaken to evaluate the status of PDL 1 expression on both, tumour cells and immune cells in order to assess the potential utility of its targeted therapies for colorectal carcinoma.
Materials and Methods
The study was an observational cross-sectional study that included 30 prospective and retrospective cases of CRC. Cases who underwent preoperative neoadjuvant chemotherapy or radiotherapy were excluded from the study. Approval by the institutional ethics committee was taken.
Complete resected specimens of all patients were obtained and standard protocol for surgical grossing of the specimens was followed. All sections from 30 cases were reviewed independently by two pathologists. Histopathological examination was done in detail.
•Tumour buds (1-5 cell clusters) were identified at the invasive front of the tumour in scanner view. The invasive front of the tumour was defined as the most progressed cancer cells on the advanced edge of the tumour. The possibility of mimickers of tumour buds like macrophages, multinucleated giant cells, fibroblasts, endothelial cells, smooth muscle cells and artifacts (floaters) were excluded. Number of tumour buds (TB)were counted in 10 high power fields (hpf) (40X objective). Low budding was defined as TB ≤ 5 budding/10 hpf and high budding as >5 budding/10 hpf.
Tumour infiltrating lymphocytes (TILS) were determined as a percentage of mononuclear inflammatory cells in the total intra-tumoral or stromal area as counted in 5 hpf (magnification, ×200-400), in the invasive front with the exception of tumour areas with crush artifacts, necrosis or regressive hyalinization. For statistical analysis, three levels of infiltration in the stroma TILs were determined: 1, weak (0–10% of stromal TILs); 2, moderate (20–40% of stromal TILs); and 3, strong (50–90% of stromal TILs).
Immunohistochemistry was done in all cases for PDL1 with Rabbit monoclonal primary antibody (RBT-PDL1) from Bio SB.
Criteria for positive immunohistochemical staining PD LI
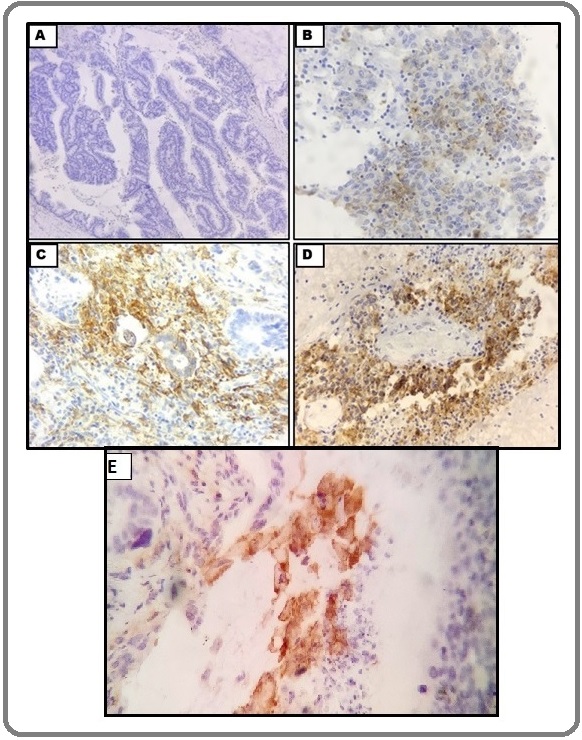
PDL-1 expression in tumour cells was evaluated in the cytoplasm and membrane of tumour cells. Cytoplasmic expression level (intensity) was scored as 0 (absent), 1 (weak), 2 (moderate) or 3 (strong), and membrane expression level was scored as 0 (absent) or 1 (present; if distinct membrane staining above cytoplasmic staining level existed). If staining difference was observed across multiple tumour cores in each case, intensity of predominant staining pattern in tumour component was recorded. The overall tumour PDL-1 expression score was the sum of the cytoplasmic and membrane scores, ranging from 0 to 4 (Figure 1).
Figure 1. Micrographs Showing PDL-1 IHC Scoring in Tumour Cells. (A) Cytoplasmic and Membranous staining both negative - Score 0 [100x]. (B) Cytoplasmic staining -Score 1 [400x]; (C) Cytoplasmic staining- Score 2 [400x]. (D) Cytoplasmic staining -Score 3 [400x]; (E) Membranous staining -Score 1 [400x].
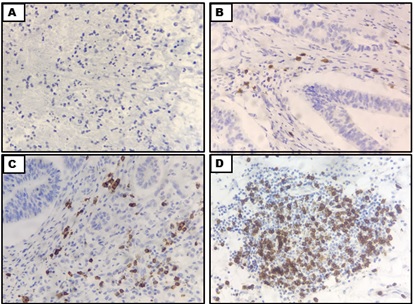
PDL1 positivity in TILs was scored in the same manner as scoring in H&E: 0- Negative; 1- weak (0–10% of stromal TILs); 2- moderate (20–40% of stromal TILs); and 3- strong (50–90% of stromal TILs) (Figure 2).
Figure 2. Microphotographs Showing PDL1 Scoring in TILS (A) Negative (B) Weak(C) Moderate (D) Strong [IHC – PDL1; 40x].
A correlation between PDL1 expression and histomorphological parameters was done with special emphasis on tumour budding and TILS as based on these cases were divided into four subgroups: Low Bud / Low TILs; Low Bud / High TILs; High Bud / Low TILs; High Bud / High TILs with particular focus on Low Bud / High TILs and High Bud / Low TILs entities. Results were analysed.
Statistics
The data was tabulated in Microsoft excel sheet and parameters in different groups were compared. Chi square test was applied and P value of < 0.05 was considered as significant.
Results
Mean age of the patients was forty-nine years with a majority of cases in fourth and fifth decade of life. Male to female ratio was 2:1. Majority of the patients presented with bleeding PR (62%) followed by constipation (32%). Distal colon being the most affected site (64%) followed by proximal colon and rectum. Most of the cases i.e. (64%) were Moderately Differentiated Adenocarcinoma (Grade 2).
PDL1 expression correlation with clinical and histomorphological parameters showed a statistically significant association with gender, LVI and tumour budding (Table 1).
| S.NO | Parameter | Negative For PDL-1 (n=30) | Positive For PDL-1 (n=30) | P-value | |
| 1 | Gender | Male | 3 | 17 | 0.02† |
| Female | 6 | 4 | |||
| 2 | Site | Proximal Colon | 3 | 6 | 0.75 |
| Distal Colon | 5 | 14 | |||
| Rectum | 1 | 1 | |||
| 3 | Histological Subtype | Well Differentiated Adenocarcinoma | 1 | 4 | 0.1 |
| Moderately Differentiated Adenocarcinoma | 3 | 15 | |||
| Poorly Differentiated Adenocarcinoma | 3 | 0 | |||
| Mucinous Adenocarcinoma | 1 | 1 | |||
| Signet Ring Cell Carcinoma | 1 | 0 | |||
| Adenosquamous Carcinoma | 0 | 1 | |||
| 4 | pT Stage | T1 | 4 | 0 | 0.1 |
| T2 | 3 | 9 | |||
| T3 | 2 | 11 | |||
| T4 | 0 | 1 | |||
| 5 | LVI Status | Present | 1 | 10 | 0.03† |
| Absent | 9 | 10 | |||
| 6 | PNI Status | Present | 1 | 6 | 0.2 |
| Absent | 9 | 14 |
† Significant p value
Reviewing tumour budding alone, of the total 30 resected specimens, 11 (36.66%) cases showed low bud count at the invasive front out of which 3 (27.2%) case s showed PDL1 positivity. However, 18/19 (94.7%) cases of high budding showed PDL1 expression. In the Low Bud / High TILs subgroup, 75% cases showed no PDL1 expression in tumour cells, whereas 62.5% cases showed PDL1 positivity in TILs. However, in High Bud / Low TILs subgroup, all the cases (100%) showed PDL1 expression in tumour cells whereas only 75% cases showed PDL1 positivity in TILs (Table 2).
| Bud /TILs groups | n | TC PDL1 status (%) | IC PDL1 status (%) | p Value | ||
| (+) | (-) | (+) | (-) | |||
| Low Bud / Low TILs | 3 | 0 (0) | 3 (100) | 0 (0) | 3 (100) | - |
| Low Bud / High TILs | 8 | 2 (25) | 6 (75) | 5 (62.5) | 3 (37.5) | <0.0001† |
| High Bud / Low TILs | 12 | 12 (100) | 0 (0) | 3 (25) | 9 (75) | <0.0001† |
| High Bud / High TILs | 7 | 6 (85.70) | 1 (14.20) | 4 (57.10) | 3 (42.80) | - |
† Significant p value
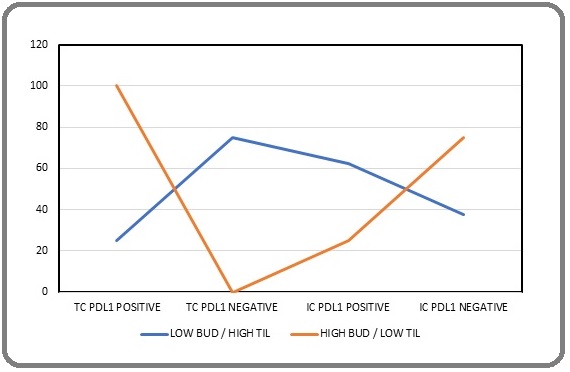
Thus, an inverse correlation was established between PDL1 expression in tumour buds and immune cells which came out to be statistically significant (p value - <0.001) (Figure 3).
Figure 3. Line Diagram Showing High Bud / Low TIL and Low Bud / High TIL PDL1 Expression on Tumour Cells and Immune Cells.
Discussion
Our study aimed to investigate the role of PDL1 in colorectal carcinomas by evaluating its expression on tumour cells and tumour infiltrating lymphocytes and its correlation with other histopathological prognostic markers.
Tumour budding was first mentioned in the history for colorectal cancers itself for the staging of tumour.
In recent years, numerous studies have demonstrated its vital role in various other cancers including Gastric, Breast and Oral cancers. Tumour budding, as a morphologic sign of epithelial-mesenchymal transition, is associated with higher tumour stage, higher nodal status, venous invasion and lymphatic vessel infiltration, local tumour recurrence, distant metastasis, and higher tumour aggressiveness [12].
Tumor infiltrating lymphocytes (TILs) are considered as reflection of primary host immune response against cancer. It is noticed that tumor infiltration by activated CD8+ cytotoxic T lymphocytes correlates with better survival of CRC patients as it is significantly associated with differentiation, staging and microsatellite instability [13].
Evasion of the immune system is thus essential for cancer development, progression, and resistance to treatment. Advances in understanding the role of immune checkpoints in suppression of T-cell activation led to the development of immune checkpoint inhibitors in the treatment of cancer [14]. Since the first use of nivolumab in humans in 2006, many clinical trials using other PD-1/ PD-L1 inhibitors like Pembrolizumab, Atezolizumab, Durvalumab, and Avelumab for the treatment of various refractory cancers such as melanoma and lung cancer have been conducted which resulted in a significant survival benefit [15].
Our study thus called attention to the importance of PD-L1 expression in tumour microenvironment in CRC and correlating it with tumour budding and density of infiltrating immune cells showing PDL1 positivity.
Talking about the 30 resected specimens we received 94.7% cases of high budding showed PDL1 expression. This was statistically significant and was very much in consistency with the studies conducted earlier [12, 16-18]. A highly significant association was seen between PDL1 with other clinical and histomorphological markers such as gender, age, LVI and pT stage which were also congruous with past studies [19-21].
To study the correlation of PDL1 expression on tumour cells and TILs with tumour budding, we divided our cases in four subgroups as mentioned earlier. An inverse correlation was established between PDL1expression in tumour budding and immune cells that came out to be statistically significant (p value - <0.001).
Consistent with these findings, low expression of PDL1 on tumour cells with high expression on immune cells, have been considered as a good prognostic factor for some other types of cancer like squamous cell carcinoma, cholangiocarcinoma, urothelial carcinoma and gastric carcinoma [22-25].
However, not many studies have shown similar findings when it came to colorectal carcinoma. Few studies to analyse the correlation of PDL1 expression on tumor cells with various prognostic markers including TILs were undertaken [16, 18, 19, 26-30] (Table 3).
| S.NO | Study with Year | Parameter | PDL (+) Tumour Cells (%) | PDL (-) Tumour Cells(%) | PDL (+) TILS (%) | PDL (-) TILS (%) | ||
| 1 | Wang et al 2016 [26] | Differentiation | High | 33.3 | 66.7 | |||
| Moderate | 19.6 | 80.4 | ||||||
| Poor | 22.4 | 77.6 | NA | |||||
| pT stage | II | 33.3 | 66.7 | |||||
| III | 22.2 | 77.8 | ||||||
| IV | 17.7 | 82.8 | ||||||
| 2 | Masugi et al 2017 [28] | Overall | 89 | 11 | 5 | 95 | ||
| 3 | Koganemaru et al [27] | Stage IIIa | 0 | 100 | 18.36 | 81.63 | ||
| Stage IIIb | 6.38 | 93.6 | 17 | 83 | ||||
| Stage IIIc | 22.2 | 77.7 | 6.6 | 93.3 | ||||
| 4 | Valentini et al 2018 [19] | TB absent | 6.25 | 21.28 | 16.33 | 21.43 | ||
| TB Present | 93.75 | 78.72 | 83.67 | 78.57 | ||||
| Tumour Grade 1 and 2 | 0 | 70.21 | P < 0.001 † | 46.94 | 71.43 | |||
| Tumour Grade 3 | 100 | 29.79 | P < 0.001 † | 53.06 | 28.57 | |||
| 5 | Martinez et al 2019 [16] | Low TB | 0 | 100 | 100 | 0 | ||
| High TB | 40 | 60 | - | - | ||||
| 6 | Elfishawy et al 2020 [29] | Grade 1 | 0 | 100 | P < 0.01 † | 0 | 100 | |
| Grade 2 | 8.8 | 91.1 | P < 0.01 † | 40 | 60 | |||
| Grade 3 | 0 | 100 | P < 0.01 † | 45.5 | 54.5 | |||
| 7 | Mohamed et al 2021 [18] | Tumour Bud Intensity | Low TB | 38 | 62 | 62 | 38 | |
| Intermediate TB | 63 | 37 | 66 | 34 | ||||
| High TB | 21 | 79 | 78 | 22 | ||||
| Stage | Early | 68 | 32 | 36 | 64 | |||
| Advanced | 37 | 63 | 63 | 37 | ||||
| Low Grade | 42 | 58 | 52 | 48 | ||||
| 8 | Schwaz et al 2021 [31] | Low Bud / High TILs | 14 | 86 | 41.9 | 58.1 | ||
| Low Bud / Low TILs | 2.4 | 97.6 | 20.5 | 79.5 | ||||
| High Bud / High TILs | 7.1 | 92.9 | 23.8 | 76.2 | ||||
| High Bud / Low TILs | 2.1 | 97.9 | 12.5 | 87.5 | ||||
| 9 | Zeynep et al 2023 [30] | Low CD8+ Peritumoral lymphocytes | 11.3 | 88.7 | 49.3 | 50.7 | ||
| High CD8+ Peritumoral lymphocytes | 13.2 | 86.8 | 7.5 | 24.5 |
† Significant p value
But they were not able to prove the reverse causation due to various limitations. A recent study conducted on 340 patients of CRC by Schwarz et al in 2021 however concluded that PD-L1 positivity significantly correlated with TILs > 5% and MMR deficiency, and PD-L1-positive cases showed significantly longer overall survival. Also, the low budding/high TIL group showed longer disease-free survival and longer Overall Survival in PD-L1-positive cases [31]. This study is the closest to what we have tried to analyse in our cohort.
Our evidence presented thus supports the idea that immune profile of a tumour microenvironment is of great value for detecting biomarkers of response immune checkpoint inhibitors.
In conclusion, PDL1 expression in immune cells in general has been considered to have a favourable prognosis. Studies focussing on PD-L1 immunohistochemistry in CRC are very heterogeneous and therefore the prognostic value of PD-L1 is still controversial, and the distribution of PD-L1 on Tumour Cells or Immune Cells has not been comprehensively analysed in CRC. This study supports the fact that high expression of PDL1 in TILs correlates with a better prognosis and hence better overall survival whereas high expression in tumour cells in cases with high bud count leads to a bad prognosis.
Therefore, scoring conventions and PDL1 expression analysis in CRC needs to be standardised moving forward. Once done, it may be possible to assess thresholds in clinical trials to determine if PDL1 can select CRC patients who will respond to anti-PD-1 therapy.
Acknowledgements
None
Ethical Declaration
Ethical approval was obtained from the Institutional Ethical Committee headed by Principal and Dean of the Institute.
Statement of Transparency and Principals:
· Author declares no conflict of interest
References
- A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE . Cancers.2021;13(9). CrossRef
- Trends in colorectal cancer incidence in India. Mathew Thomas V, Baby B, Wang K, Lei F, Chen Q, Huang B, Mathew A. Journal of Clinical Oncology.2020;38(15_suppl). CrossRef
- A Comprehensive Review of Clinical Trials on EGFR Inhibitors Such as Cetuximab and Panitumumab as Monotherapy and in Combination for Treatment of Metastatic Colorectal Cancer Yazdi MH , Faramarzi MA , Nikfar S, Abdollahi M. Avicenna Journal of Medical Biotechnology.2015;7(4).
- The foundations of immune checkpoint blockade and the ipilimumab approval decennial Korman AJ , Garrett-Thomson SC , Lonberg N. Nature Reviews. Drug Discovery.2022;21(7). CrossRef
- Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation Sanmamed MF , Chen L. Cancer Journal (Sudbury, Mass.).2014;20(4). CrossRef
- Immune checkpoint inhibitors of PD-L1 as cancer therapeutics Akinleye A, Rasool Z. Journal of Hematology & Oncology.2019;12(1). CrossRef
- PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM , Hashemy SI . Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2019;110. CrossRef
- Antagonists of PD-1 and PD-L1 in Cancer Treatment Lipson EJ , Forde PM , Hammers HJ , Emens LA , Taube JM , Topalian SL . Seminars in oncology.2015;42(4). CrossRef
- Regulation and Function of the PD-L1 Checkpoint Sun C, Mezzadra R, Schumacher TN . Immunity.2018;48(3). CrossRef
- Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: a key role of exosomal PD-L1 Yin Z, Yu M, Ma T, Zhang C, Huang S, Karimzadeh MR , Momtazi-Borojeni AA , Chen S. Journal for Immunotherapy of Cancer.2021;9(1). CrossRef
- Advances in immunotherapy for colorectal cancer: a review Golshani G, Zhang Y. Therapeutic Advances in Gastroenterology.2020;13. CrossRef
- Budding and tumor-infiltrating lymphocytes - combination of both parameters predicts survival in colorectal cancer and leads to new prognostic subgroups Lang-Schwarz C, Melcher B, Haumaier F, Lang-Schwarz K, Rupprecht T, Vieth M, Sterlacci W. Human Pathology.2018;79. CrossRef
- Intratumoral infiltrating lymphocytes correlate with improved survival in colorectal cancer patients: Independent of oncogenetic features Nazemalhosseini-Mojarad E, Mohammadpour S, Torshizi Esafahani A, Gharib E, Larki P, Moradi A, Amin Porhoseingholi M, Asadzade Aghdaei H, Kuppen PJK , Zali MR . Journal of Cellular Physiology.2019;234(4). CrossRef
- Regulation of PD-L1: a novel role of pro-survival signalling in cancer Chen J, Jiang CC , Jin L, Zhang XD . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2016;27(3). CrossRef
- Immuno-oncology in GI tumours: Clinical evidence and emerging trials of PD-1/PD-L1 antagonists Stein A, Moehler M, Trojan J, Goekkurt E, Vogel A. Critical Reviews in Oncology/Hematology.2018;130. CrossRef
- Low miR200c expression in tumor budding of invasive front predicts worse survival in patients with localized colon cancer and is related to PD-L1 overexpression Martinez-Ciarpaglini C, Oltra S, Roselló S, Roda D, Mongort C, Carrasco F, Gonzalez J, et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2019;32(2). CrossRef
- Association of Tumor Budding With Immune Evasion Pathways in Primary Colorectal Cancer and Patient-Derived Xenografts Guil-Luna S, Mena R, Navarrete-Sirvent C, López-Sánchez LM , Khouadri K, Toledano-Fonseca M, Mantrana A, et al . Frontiers in Medicine.2020;7. CrossRef
- The role and relationship between programmed death ligand 1 and cytotoxic T lymphocyte-associated antigen-4 immunohistochemical expression in colorectal carcinoma patients: an impact on outcome El Dein Mohameda AS , El-Rebey HS , AboElnasr LSA , Abdou AG . Ecancermedicalscience.2021;15. CrossRef
- PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments Valentini AM , Di Pinto F, Cariola F, Guerra V, Giannelli G, Caruso ML , Pirrelli M. Oncotarget.2018;9(9). CrossRef
- Diagnostic and pathologic value of programmed death-ligand 1 expression in colonic carcinoma Helmy DO , Hussein MTE , Negm MS , Onsy ME . Egyptian Journal of Pathology.2020;40(2). CrossRef
- PD-1/PD-L1-dependent immune response in colorectal cancer Payandeh Z, Khalili S, Somi MH , Mard-Soltani M, Baghbanzadeh A, Hajiasgharzadeh K, Samadi N, Baradaran B. Journal of Cellular Physiology.2020;235(7-8). CrossRef
- PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients Kim HR , Ha SJ , Hong MH , Heo SJ , Koh YW , Choi EC , Kim EK , et al . Scientific Reports.2016;6. CrossRef
- Application of PD-1 Blockade in Cancer Immunotherapy Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X. Computational and Structural Biotechnology Journal.2019;17. CrossRef
- High PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor in urothelial carcinoma Zhong Q, Shou J, Ying J, Ling Y, Yu Y, Shen Z, Zhang Y, et al . Future Oncology (London, England).2021;17(22). CrossRef
- PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for patients with intrahepatic cholangiocarcinoma Mocan LP , Craciun R, Grapa C, Melincovici CS , Rusu I, Al Hajjar N, Sparchez Z, et al . Cancer immunology, immunotherapy: CII.2023;72(4). CrossRef
- Significance of Programmed Death Ligand 1 (PD-L1) Immunohistochemical Expression in Colorectal Cancer Wang L, Ren F, Wang Q, Baldridge LA , Monn MF , Fisher KW , Sheng W, Zhou X, Du X, Cheng L. Molecular Diagnosis & Therapy.2016;20(2). CrossRef
- Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, Tomizawa K, et al . Cancer Science.2017;108(5). CrossRef
- Tumour CD274 (PD-L1) expression and T cells in colorectal cancer Masugi Y, Nishihara R, Yang J, Mima K, Silva A, Shi Y, Inamura K, et al . Gut.2017;66(8). CrossRef
- Immunohistochemical Expression of Programmed Death Ligand-1 (PDL-1) in Colorectal carcinoma and Its Correlation with Stromal Tumor Infiltrating Lymphocytes Elfishawy M, Abd-ELaziz SA , Hegazy A, El-Yasergy DF . Asian Pacific journal of cancer prevention: APJCP.2020;21(1). CrossRef
- PD-L1 and PD-L2 expression in colorectal cancer Zeynep O, Funda C, Evrim Y, Deniz A, Bülent Y, Fatih YN . Indian Journal of Pathology & Microbiology.2023;66(1). CrossRef
- Programmed death ligand 1 (PD-L1) in colon cancer and its interaction with budding and tumor-infiltrating lymphocytes (TILs) as tumor-host antagonists Lang-Schwarz C, Melcher B, Hartmann A, Bertz S, Dregelies T, Lang-Schwarz K, Vieth M, Sterlacci W. International Journal of Colorectal Disease.2021;36(11). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details