Preparation, Characterization and Cytotoxic Studies of Cisplatin-containing Nanoliposomes on Breast Cancer Cell Lines
Download
Abstract
Objective: Today, cancer is one the most important challenges in modern medicine. Breast carcinoma is one type of cancer that is treated with cisplatin, a chemotherapy drug. By using liposomal nanocarriers, this study seeks to increase the therapeutic efficiency of cisplatin.
Method: The zeta potential, particle size, and drug-release characteristics of nanoliposomal cisplatin were evaluated after it had been synthesized using the reverse phase evaporation technique. The cytotoxicity rate of nanoliposomal cisplatin was then assessed using the T-47D breast cancer cell line.
Results: This study’s liposomal nanoparticles (NPs) had a zeta potential of -24.9 mV and a particle size of 342.3 nm. 3.51% and 79.6%, respectively, were found to be the drug loading level and amount of encapsulated drug. A significant improvement over the free drug was seen in this nanoliposome’s cytotoxic effect on the T-47D breast cancer cell line (P<0.05).
Conclusion: According to what we have discovered, cisplatin liposomal nanocarriers may prove to be a cutting-edge chemotherapy treatment for breast cancer.
Introduction
Breast cancer is the most prevalent type of cancer that is found and is the main cause of cancer-related deaths in women worldwide [1,2]. Breast cancer mortality rates have remained largely stable over the past three decades, despite significant improvements in chemotherapy treatment [3]. Increasing age, active and passive smoking, microbial infections, and genetic predisposition are just a few of the numerous risk factors connected to breast cancer. These multifaceted risk factors have a significant impact on how cancer develops and spreads, which ultimately affects the efficiency and results of cancer therapy [4-7]. It has been acknowledged that the main difficulties or restrictions of chemotherapy treatment are the initial response to the drugs and the ensuing development of resistance, which causes relapse and the metastatic spread to vital organs like the lungs, liver, and bones [8]. A promising approach for delivering chemotherapeutics is to use nanotechnology-based materials as they improve tumor targeting, increase drug penetration into tumors, and decrease side effects [9,10]. Breast cancer is one of many malignancies for which cisplatin is frequently used as a chemotherapeutic agent [11]. The drug significantly affects cancer cells by attaching to the DNA molecule, causing apoptosis and necrosis to occur [12]. Notwithstanding, nephrotoxicity and neurotoxicity are among the side effects of cisplatin administration [13]. The creation of drug delivery systems with the intention of influencing and managing drug distribution within the body has received a lot of attention over the last few decades. One method uses nanoliposomes, which can easily pass through the interendothelial cell gap of newly formed tumor capillaries and collect inside the tumor. These nanoliposomes are packed with anticancer agents. These innate characteristics aid in boosting the concentration of anticancer medications specifically within the tumor, thereby lowering their toxicity in healthy tissues [14,15]. Researchers have had difficulty creating the ideal nanoliposome formulation for the drug cisplatin, despite prior attempts to deliver it using a variety of carriers. Researchers optimized cisplatin nanoliposomes in this study and assessed the toxicity of the nanoliposomes on breast cancer cell lines.
Materials and Methods
Cisplatin, cholesterol, and polyethylene glycol 3350 were obtained from Sigma-Aldrich Co., UK. Lecithin was obtained from Acros Co., Belgium. RPMI 1640 cell culture medium was obtained from Gibco Co., Germany. Additionally, the T-47D cell line was purchased from the cell bank of the Pasteur Institute in Iran.
Nanoliposomal drug preparation
The reverse phase evaporation method was used for producing liposomal nanoparticles. Briefly, 100 ml of 96% ethanol was used to dissolve 10 mg of cisplatin, 150 mg of lecithin, 60 mg of cholesterol, and 72 mg of polyethylene glycol 3350 (with a molar ratio of 55: 40: 5%). For two hours, the mixture was stirred at 140 rpm while being heated to 37 C. A rotary evaporator was used to separate the solvent following complete dissolution. In two separate additions, the resulting thin film was dissolved in 15 cc of phosphate buffer (pH 7.4). The formulations were then placed in an ultrasonic bath (Bandelin Sonorex Digitec, Germany) and sonicated for 5 minutes.
Particle size and zeta potential analysis
Four milliliters of phosphate-buffered saline (PBS) were used to dissolve one milligram of the formulation. Then, a Zetasizer (Nano ZS3600, Malvern Instruments, UK) was used to measure the zeta potential and mean diameter of the nanoliposomes as well as their absorption at 633 nm.
Encapsulation efficiency
40 mg of the formulation were centrifuged for an hour at 4 degrees Celsius and 13,200 rpm to ascertain the rate of drug entrapment. Using a spectrophotometer (UV1800, Shimadzu Co.), the optical absorbance of the supernatant from each formulation was then measured at 220 nm. Then, using Formulas 1 and 2, the rate of drug loading and encapsulation efficiency were calculated.
(1) Encapsulation percent=(PC-CS )/(PC ) x 100
(2) Drug loading percent=(C )/(W ) x 100
In Formula 1, PC: primary cisplatin and CS: cisplatin in supernatant in mg/ml
In Formula 2, C: cisplatin content in the nanoliposomes and W: weight of nanoliposomes in mg/ml
Nanoliposomal drugstability study
For a month, the nanoliposomes were kept in a refrigerator at 4°C. Following this, the above-described procedure was used to determine the nanoliposome properties once more.
Drug release study
A dialysis bag with a cut-off of 10,000 Daltons was filled with 1 ml of the liposomal drug suspension and its control in order to assess the release pattern of nanoliposomal cisplatin. The bag was then submerged in 100 ml of pH 7.4 PBS buffer and stirred for 35 hours at 140 rpm at room temperature. Then, 1 ml of the used PBS buffer was taken out and swapped out for 1 ml of brand-new PBS buffer. The samples’ optical absorbance was determined at 227 nm, and a standard curve was used to determine the concentration of the free drug. The drug release profile was finally plotted versus time.
Cell culture
In RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 g/ml streptomycin, and 100 U/ml penicillin, the T-47D cell line was cultured. At 37°C, 5% CO2, and 90% humidity, the cell culture was kept alive. A 96-well plate was filled with 100 of a cell suspension containing 10,000 cells for each cell culture. The plate was incubated with 5% CO2 at 37°C. The cells’ supernatant was removed after 24 hours. The cells were then treated with various concentrations of the standard cisplatin drug, control, and the nanoliposomal cisplatin formulation. After another 24 hours, this process was repeated.
Cytotoxicity assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to test for cytotoxicity. The cells were exposed for 48 hours before the supernatant was taken out and a 100 µl solution of MTT (0.5 mg/ml) was added. After the cells had been incubated for 3 hours, the culture medium was then added with a 100 µl solution of isopropanol. After 30 minutes, a plate reader (Synergy Multi-Mode Elisa Reader, Bio-Tek, USA) was used to measure the formazan product’s optical absorbance at 570 nm. Finally, the pharm program was used to determine the IC50 value.
Statistical analysis
The SPSS software, version 11, was used to analyze the results. The results of three independent tests, each with two samples, are shown as Mean±SD. The statistical test was performed using the unpaired-samples t-test, and the significance level was set at 0.05.
Results
Particle size and zeta potential analysis
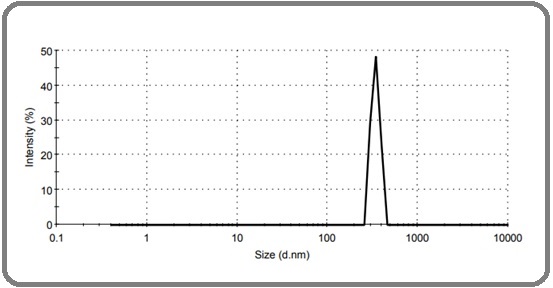
According to measurements, the average size of nanoliposomal cisplatin and the potential of zeta were found to be 342.3 nm and -24.9 mV, respectively, as shown in Figure 1.
Figure 1. The Mean Size of Nanoliposomal Cisplatin.
Encapsulation efficiency and drug loading studies
The drug loading contents and encapsulation efficiency were calculated using Formulas 1 and 2 as 79.6% and 3.5%, respectively.
Nanoliposomal drug stability study
The characteristics of the nanoliposomes at the time of production and after one month of storage were compared, as shown in Table 1.
| Time | Zeta potential (mV) | Size (nm) | Encapsulation efficiency (%) | Drug loading (%) |
| Production time | -24.9±1.7 | 342.3±18.7 | 79.6±3.7 | 3.5±1.1 |
| 1 months after production | -25.2±1.8 | 350.5±19.2 | 70.7±2.9 | 3.15±1.0 |
Data are expressed as mean±SD of three different experiments.
The findings showed that when compared to the time of production, the nanoliposomes stored at 4 °C for a month did not show any appreciable changes in parameters like zeta potential, size, encapsulation efficiency, and drug loading percentage.
In vitro drug release studies
At specific time intervals of 1, 2, 4, 7, 10, 23, 27, 31, and 35 hours, the release of cisplatin into the PBS buffer was measured, as shown in Figure 2.
Figure 2. Cisplatin Nanoliposome Release Characteristics in vitro in PBS Buffer. The results of three different experiments, each of which was carried out in duplicate, are expressed as the mean and SD.
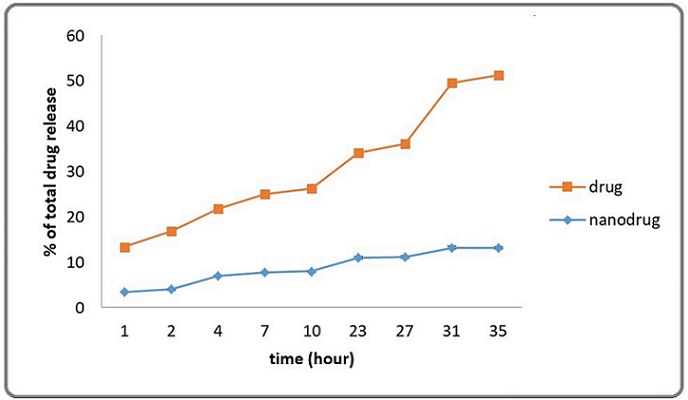
The amount released was calculated using the cisplatin standard curve. The outcomes showed that a maximum of 78.2±2% of the cisplatin contained in the nanoliposomes was released over the course of the 35-hour period.
Cytotoxicity assay
Following exposure of breast cell lines to both free cisplatin and its nanoliposomal formulation, as determined by the MTT assay, the IC50 values showed a significant decrease in a dose-dependent manner, as shown in Table 2.
| Breast Cell Lines | IC50 (µg/ml) of Cisplatin | IC50 (µg/ml) of Nanoliposomal Cisplatin | P value |
| T-47D | 140.8±51.8 | 58.1±22.7 | P<0.05 |
The results of three different experiments, each of which was carried out in duplicate, are expressed as the mean +-SD.
Discussion
Chemotherapy is still the mainstay treatment for various cancers. However, it has run into problems like low tumor selectivity and MDR (multidrug resistance). One creative solution to these problems is the use of nanotechnology materials for targeted drug delivery [16,17]. This study’s goals included improving cisplatin nanoliposomes and determining how toxic they were to breast cancer cell lines. The results of the study showed that cisplatin’s cytotoxic effects were increased in liposomes as opposed to in its free form. In conclusion, nanoliposome synthesis methods have proven to be an invaluable asset in improving therapeutic drugs, particularly in the area of chemotherapy [18]. Nevertheless, it is essential to carefully assess the toxicity and fate of nanoliposomes before using them for pharmaceutical applications [19]. Numerous studies have investigated the effects of different medications formulated in nanoliposomes in various cell lines. In the case of the breast cancer cell line MCF-7, Koohi Moftakhari Esfahani et al. [20] evaluated the cytotoxicity of liposomal paclitaxel. Their analysis of the drug release showed that the maximum amount of paclitaxel, or about 5.53%, was released from liposomes in just 28 hours. Additionally, their research showed that nanoliposomal paclitaxel had more cytotoxic effects than free paclitaxel. Pegylated nanoliposomal artemisinin was investigated by Dadgar et al. [21] as a potential treatment for breast cancer cell lines. They found that over a 48-hour period, the drug release amounted to roughly 5.17%. According to their research, artemisinin’s cytotoxicity displayed a greater effect when it was packaged in pegylated nanoliposomes than when it was used alone. The dialysis tubing method was used to examine the drug release profile of nanoparticles containing cisplatin. The release process first showed rapid diffusion, which was followed by a phase of slower diffusion. According to the results, the majority of drug release happened in the first four hours. It is clear that nanoliposomal cisplatin is more effective at killing breast cancer cells when compared to free cisplatin when looking at their respective cytotoxic effects. This is explained by the fact that it has a phospholipid structure similar to the bilayer membrane found in breast cell lines. Because of their structural similarity, these cells can be penetrated more easily by nanoliposomal cisplatin, which then releases the drug directly into the target cells, killing them [18]. Using the MTT method, we successfully loaded cisplatin onto liposomal nanoparticles in this study and assessed the cytotoxicity of both the free form of cisplatin and its liposomal formulation on a cell line from a human breast carcinoma. We also looked at the produced nanoparticles’ physicochemical characteristics. The ability of the liposomal nanocarrier to lengthen the drug’s half-life in the bloodstream and play a protective role can be credited for the liposomal formulation’s improved performance. The presence of polyethylene glycol during nanoparticle preparation enables them to remain hidden and stay in circulation for longer periods despite the low loading seen in this study. As a result, this prolonged time in circulation increases the effectiveness of the drug [22]. As a result, our research shows that free cisplatin does not have the same cytotoxic effects on breast cell lines as nanoliposomal cisplatin. This suggests that this formulation has the potential to be a potent substitute for upcoming chemotherapeutic strategies for the treatment of breast cancer.
Co-Author Contributions
Authors’ contribution Fateme Mohammadinezhad, Armin Talebi, Elham Saberian, Somayeh Eslami, Parizad Ghanbarikondori, and Zeinab Jabbari Velisdeh performed the experimental tests. Reza Nahavandi, and Maryam Vesal designed the nanoparticle. Ali Habiba did the drug release test. Cell culture was carried out by Azim Akbarzadeh Khiyavi and Paniz mirmoghaddam. Armin Sedighi set up and performed the work with the laboratory devices. Mohammadreza Allahyartorkaman wrote the article.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
References
- Efficacy Comparison of Nanoniosomal and Pegylated Nanoniosomal Cisplatin on A172 Cell Line Babaei M, Ardjmand M, Akbarzadeh A, Seyfkordi A. Tissue Engineering and Regenerative Medicine.2014;11. CrossRef
- Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates Jabbarzadeh Kaboli P, Shabani S, Sharma S, Partovi Nasr M, Yamaguchi H, Hung M. American Journal of Cancer Research.2022;12(4).
- Paclitaxel-loaded niosomes for intravenous administration: pharmacokinetics and tissue distribution in rats Bayindir ZS , Be AB , Yüksel N. Turkish Journal of Medical Sciences.2015;45(6).
- Estimation of Recent Transmission of Mycobacterium Tuberculosis Strains among Iranian and Afghan Immigrants: A Cluster-Based Study Torkaman MRA , Nasiri MJ , Farnia P, Shahhosseiny MH , Mozafari M, Velayati AA . Journal of clinical and diagnostic research: JCDR.2014;8(9). CrossRef
- Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, Amini S, Zakiloo M, Nasiri MJ . Scientific Reports.2019;9(1). CrossRef
- Direct drug susceptibility testing of Mycobacterium tuberculosis using the proportional method: A multicenter study Amini S, Hoffner S, Allahyar Torkaman MR , Hamzehloo G, Nasiri MJ , Salehi M, Sami Kashkooli G, et al . Journal of Global Antimicrobial Resistance.2019;17. CrossRef
- Comparison of loop-mediated isothermal amplification and real-time PCR for detecting Bordetella pertussis Torkaman MRA , Kamachi K, Nikbin VS , Lotfi MN , Shahcheraghi F. Journal of Medical Microbiology.2015;64(Pt 4). CrossRef
- Emerging nanotherapeutic strategies in breast cancer Blanco E, Ferrari M. Breast (Edinburgh, Scotland).2014;23(1). CrossRef
- The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats Hosseinian S, Khajavi Rad A, Hadjzadeh MAR , Mohamadian Roshan N, Havakhah S, Shafiee S. Avicenna Journal of Phytomedicine.2016;6(1).
- Isolation and characterization of a novel GRP78-specific single-chain variable fragment (scFv) using ribosome display method Shabani S, Moghadam MF , Gargari SLM . Medical Oncology (Northwood, London, England).2021;38(9). CrossRef
- Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review Kanamala M, Wilson WR , Yang M, Palmer BD , Wu Z. Biomaterials.2016;85. CrossRef
- Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery Kowapradit J, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Sajomsang W, Opanasopit P. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences.2012;47(2). CrossRef
- Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis Li M, Tang Z, Zhang Y, Lv S, Li Q, Chen X. Acta Biomaterialia.2015;18. CrossRef
- In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer Zhang X, Yang H, Gu K, Chen J, Rui M, Jiang GL . International Journal of Nanomedicine.2011;6. CrossRef
- Cytokine sustained delivery for cancer therapy; special focus on stem cell- and biomaterial- based delivery methods Mehralizadeh H, Nazari A, Oruji F, Roostaie M, Hosseininozari G, Yazdani O, Esbati R, Roudini K. Pathology, Research and Practice.2023;247. CrossRef
- Design and development of niosomal delivery system for Ketoprofen. 2012 Mujoriya R, Babu Bodla R. available at http://hdl.net/123456789/97,2012..
- Evaluation of Inflammasome Activation in Peripheral Blood Mononuclear Cells of Hemodialysis Treated Patients with Glomerulonephritis Hashemi A, Bigdeli R, Shahnazari M, Oruji F, Fattahi S, Panahnejad E, Ghadri A, et al . Iranian journal of pharmaceutical research: IJPR.2021;20(3). CrossRef
- Preparation, characterization, and cytotoxic effects of liposomal nanoparticles containing cisplatin: an in vitro study Poy D, Akbarzadeh A, Ebrahimi Shahmabadi H, Ebrahimifar M, Farhangi A, Farahnak Zarabi M, Akbari A, Saffari Z, Siami F. Chemical Biology & Drug Design.2016;88(4). CrossRef
- In vivo toxicological evaluation of polymeric nanocapsules after intradermal administration Bulcão RP , Freitas FA , Dallegrave E, Venturini CG , Baierle M, Durgante J, Sauer E, et al . European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V.2014;86(2). CrossRef
- Cytotoxicity of liposomal Paclitaxel in breast cancer cell line mcf-7 Esfahani MKM , Alavi SE , Movahedi F, Alavi F, Akbarzadeh A. Indian journal of clinical biochemistry: IJCB.2013;28(4). CrossRef
- Study of toxicity effect of pegylated nanoliposomal artemisinin on breast cancer cell line Dadgar N, Alavi SE , Esfahani MKM , Akbarzadeh A. Indian journal of clinical biochemistry: IJCB.2013;28(4). CrossRef
- Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D Shahbazian S, Akbarzadeh A, Torabi S, Omidi M. Biotechnology Letters.2015;37(7). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details