Laparoscopic Sentinel Lymph Node Mapping Using NIR Fluorescence with ICG in Early Endometrial Cancer: Experience at a Tertiary Care Oncology Centre
Download
Abstract
Objective: To determine the detection rates and location of sentinel nodes as well as to determine intraoperative and postoperative surgico-pathological outcomes in endometrial cancer patients who underwent laparoscopic staging with sentinel node biopsy using ICG dye.
Materials and Methods: This retrospective study was conducted in a tertiary care oncology centre, Amrita Institute of medical sciences, Kochi from April 2021 to March 2023. All cases of biopsy proven early stage endometrial carcinoma that underwent laparoscopic staging with SLN mapping using the ICG dye were included. All histological types were included, as long as the disease was confined to the uterus, clinically and on MRI.
Results: A total of 80 patients were found eligible for the study. Only the patients with apparent uterine confined disease on preoperative MRI were selected for sentinel mapping, 80 % of whom had IA disease on MRI, and 20 % had IB, 13.5 % were high grade histology.The overall SLN detection rate was 93.75%, and bilateral detection rate was 92.5 %. Most common location was external iliac in 40.8% cases. Only 1 patient was found to harbour nodal micro metastasis on ultrastaging. Final histopathology identified stage IA in 63 (78.75%) patients, IB in 14 (17.5%), Stage II in 2 (2.5%) and Stage IIIC1 in 1 (1.25%) patient.
Conclusion: Laparoscopic staging with SLNB using ICG is a practicable approach for uterine limited disease on preoperative evaluation. It extends the benefits of minimally invasive surgery to these patients, while overcoming the limitations of prohibitive cost or availability of expensive robotic equipment.
Introduction
Sentinel lymph node (SLN) mapping in endometrial cancer (EC) is now a widely accepted surgical staging strategy in uterine confined disease that offers accurate staging and tailored treatment strategies ,simultaneously reducing the surgical extent and thereby minimizing the associated complications [1,2].
The sentinel node mapping technique along with open, laparoscopic and robotic approaches to it have undergone refinement over past decade facilitating the use of NIR imaging in sentinel node assessment [3,4].
In gynaecologic oncology, SLN mapping with the use of indocyanine green (ICG) has been reported, largely using the robotic systems. Consistent emphasis on the technique of the sentinel procedure and adherence to the sentinel algorithm is of paramount importance while recommending its use in properly selected cases, to reduce the complications as well as to impart adequate nodal staging information. Same principles apply to laparoscopic SLN mapping using ICG. It is a viable option, especially in LMICs where access to robot or its prohibitive cost are limiting factors. In the low resource settings, SLN mapping in EC with ICG, using the laparoscopic platform has been reported by only one institution [5].
We conducted a retrospective analysis of our endometrial cancer patients who underwent laparoscopic staging with sentinel node biopsy using ICG dye with the aim to assess the detection rates and location of sentinel nodes, and the intraoperative and postoperative surgicopathological outcomes.
Materials and Methods
This retrospective study was conducted in a tertiary care oncology centre, Amrita Institute of Medical Sciences, Kochi from April 2021 to March 2023. IRB approval was sought and obtained (IEC-AIMS-2023-GYNC-146).
Inclusion criteria
All cases of biopsy proven, apparent early stage (uterine confined) endometrial carcinoma that underwent laparoscopic staging with SLN mapping using the ICG dye, during April 2021 to March 2023, were included. All histological types were included, as long as the disease was confined to the uterus, clinically and on MRI.
Prep workup
All patients underwent MRI abdomen and pelvis to rule out any extra uterine disease, apart from routine preoperative workup.
Surgical methods, including dye details
SLN mapping was performed with intraoperative cervical ICG injection. ICG was injected into the cervix submucosally (2-3 mm), and deep (1 cm) in the stroma at 3 o’ clock and 9 o’ clock positions. ICG dilution used was 1% (1 mg/ml). For obtaining this dilution, One vial of 25 mg ICG powder AUROGREEN (Figure 1) was constituted with 4 ml sterile water, then 1 ml of this solution was diluted with 4 ml sterile water and 2 ml was used for superficial injection and deep injection each.
Figure 1. Aurogreen Dye.

Laparoscopic port placement was done. Laparoscopic surgery was performed with Stryker 1588 AIM high definition camera system, which has got an inbuilt NIR camera (Figure 2a).
Figure 2. (a), Stryker 1588AIM Camera Head. (b), L10 Light source sytem.
L10 light source enables to visualize standard white light as well as endoscopic Near Infrared wavelengths. SLN mapping was performed after indocyanine green (ICG) injection followed by fluorescence detection using Near Infrared Imaging (NIR) with enhanced real time visual assessment when ENV mode (Endoscopic near Infrared Visualization) is activated (Figure 2b).
Surgical staging began with peritoneal wash cytology and a thorough visualization of the pelvis and abdominal cavity to rule out any gross extra uterine disease. Further, SLN mapping was done after opening the paravesical and pararectal spaces (Figure 3a).
Figure 3. (a) Pelvic Spaces, Arrows Pointing at Sentinel Node Identified in Obturator Fossa. (b) Fluorescent Tract leading to sentinel node. (c) Excised Sentinel node.
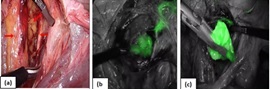
The sentinel nodes were identified as the nodes located nearest to the uterus that showed a fluorescent green colour on the NIR camera (Figure 3b,3c). If a lymphatic channel leading up to a node was found but the node itself did not light up, it was still considered a sentinel node. Enlarged/suspicious looking nodes were excised irrespective of dye uptake. The para aortic and paracaval area was inspected for any dye uptake.
All identified sentinel nodes were excised and retrieved in a glove bag. This was followed by Total Laparoscopic Hysterectomy (TLH) and bilateral salpingo-oophorectomy (BSO), with or without omentectomy/ omental biopsy as indicated. Side specific pelvic lymphadenectomy was performed in case of failed sentinel mapping as recommended by the NCCN SLN algorithm [6].
Excised SLN were sent to permanent histopathological analysis where a complete ultra staging was performed in all cases following Memorial Sloan-Kettering Cancer Centre’s protocol (MSKCC):i.e. cutting two adjacent 5-μm sections at each of two levels, 50-μm apart, from each paraffin block lacking metastatic carcinoma on routine H&E. At each level, one slide is stained with H&E and with immunohistochemistry (IHC) using anti-cytokeratin AE1/AE3 [7].
Demographic and clinicopathological details like age, BMI, ASA, preoperative biopsy, MRI findings, along with perioperative details like duration of surgery, details of mapped sentinel nodes, final HPR , duration of hospital stay and postoperative complication details ; were collected from the prospectively maintained electronic medical records (EMR )system.
Statistical analysis
Demographic and clinicopathological characteristics were evaluated using the basic descriptive statistics. Overall and bilateral detection rates of the SLN mapping were calculated. To calculate overall detection rate the number of cases in which at least one SLN was identified was divided by the total number of procedures performed, and for bilateral detection rate number of procedures in which at least one SLN was identified on each side of the pelvis was divided by the total number of procedures performed. Statistical analyses-were performed using MS Excel software using mean, median and average tools.
Results
A total of 194 cases of endometrial cancer were operated during the study period.
Out of these, 32 (16.4%) underwent Open staging surgery, 82 (42.2%) underwent Robotic staging surgery, 80 (41.2%) underwent laparoscopic staging surgery, and met the inclusion criteria for the present study.
Mean age in the present study population was 56.3 years (range 29-79 years). Mean BMI was 29.09 kg/m2 ranging from 15.9 kg/m2 to highest being 41 kg/m2. On preoperative anaesthesia assessment 4 (5%) patients belonged to ASA (American Society of Anaesthesiologists) III, and 56 (70%) belonged to ASA II (Table 1).
| Variable | N=80 |
| Mean age in years (range) | |
| 56.3 | |
| (29-79) | |
| Mean BMI kg/m 2 (range) | |
| 29.09 | |
| (15.9-41) | |
| ECOG performance status | |
| 0 | 73 (91.2) |
| 1 | 6 (7.5) |
| 2 | 1 (1.25) |
| ASA score | |
| I | 20 (25) |
| II | 56 (70) |
| III | 4 (5) |
| Co morbidities | |
| Hypertension | 27 (33.75) |
| Diabetes | 30 (37.5) |
| RHD/CAD | 3 (3.75) |
| CVA/Hemiparesis | 2 (2.5) |
| CLD/Hepatitis B | 1 (1.25) |
| Post covid | 2 (2.5) |
| Asthma | 3 (3.75) |
| EPTB | 1 (1.25) |
| FIGO Stage | |
| IA | 63 (78.75) |
| IB | 14 (17.5) |
| II | 2 (2.5) |
| III | 1 (1.25) |
| Pathology Type | |
| Endometrioid adenocarcinoma | 70 (83.75) |
| Serous carcinoma | 1 (1.25) |
| Clear cell carcinoma | 1 (1.25) |
| De differentiated/poorly differentiated | 2 (2.5) |
| Carcinosarcoma | 2 (2.5) |
| Endometrioid carcinoma with p53 positivity | 4 (5) |
| Grade | |
| 1 | 52 (65) |
| 2 | 17 (21.25) |
| 3 | 11 (13.75) |
| P53 positive | 11 (13.75) |
| Final prognostic risk groups | |
| Low risk | 54 (67.5) |
| Intermediate risk | 3 (3.75) |
| High intermediate | 9 (11.25) |
| High risk | 14 (17.5) |
Based on the preoperative MRI, 64 (80%) were stage IA and 16 (20%) were stage IB.
Preoperative endometrial biopsy was low grade endometrioid carcinoma in 70 (87.5%) patients. Among the remaining 10 patients, we found grade 3 Endometrioid histology in 4 (5%), carcinosarcoma in 2 (2.5%) clear cell carcinoma in 1 (1.25%), serous carcinoma in 1 (1.25%), poorly differentiated in 2 (2.5%) (Table 1).
Based on P53 positivity, type II endometrial cancer was found in 11 (13.75%) patients preoperatively. Thus, 16 (20%) belonged to high risk group based on preoperative assessment (Table 1).
Overall detection rate was 93.75 % (75/80) and bilateral detection rate was 92.5 % (74/80). At least one SLN was detected in 95.4% of cases (Table 2).
| Variable | N= 80 (%) | ||
| SLN detection rate | |||
| Bilateral detection rate | 74/80 (92.5) | ||
| Overall detection rate | 75/80 (93.75) | ||
| SLN mapping failure | |||
| Bilateral | 5 (6.25) | ||
| Unilateral | 1 (1.25) | ||
| Median number of SLN harvested per patient | 3 (0-11) | ||
| Location of SLN | N = 147 (%) | ||
| Right hemi pelvis | Left hemi pelvis | ||
| Obturator | 35 (23) | 15 | 20 |
| Internal iliac | 47 (31.9) | 27 | 20 |
| External iliac | 60 (40.8) | 33 | 27 |
| Common iliac | 5 (3.4) | 3 | 2 |
| Para aortic | 0 | 0 | 0 |
| Unusual sites | 0 | 0 | 0 |
| Number of patients with SLN metastasis | 1 (1.25) | ||
| Empty packets found | 3 (3.75) |
BMI, Body mass index; ECOG, Eastern Cooperative Oncology Group; FIGO, Federation of Gynaecology and Obstetrics; ASA score, American Society of Anaesthesiologists score
Bilateral sentinel mapping failure was observed in 5 patients and 1 had unilateral failure. Among the patients with failed mapping, 5 were obese with BMI over 32. One patient had history of infertility and previous pelvic surgery, with extensive fibrosis around bilateral pelvic wall, probably leading to failed mapping.
Out of 6 patients with sentinel mapping failure, 2 belonged to High risk group, rest all were low risk group. Both had high grade lesion with <50% MI on preop assessment, and were converted to laparotomy.
All of these underwent side specific PLND and final HPR did not show nodal involvement.
The median number of SLNs removed was three (range 0–11).Total six patients underwent side specific PLND (Table 3).
| Mean blood loss (ml) | 50 (10 -200) |
| Mean duration of surgery (min) | 88.5 (45-120) |
| Mean duration of hospital stays (days) | 2 days (1-5 ) |
| Median number of SLNs removed | 3 (0-11) |
| Overall sentinel detection rate N (%) | 75/80 (93.75) |
| Bilateral sentinel detection rate N (%) | 74/80 (92.5) |
| Intraoperative complications N (%) | |
| Bladder injury | 1 (1.25) |
| Conversion to laparotomy | 2 (2.5) |
| Postop complications | |
| Urinary spasm | 1 (1.25) |
Most common location of SLN was external iliac (40.8%). In 6.25 % (5/80) of cases, additional SLNs were identified in the common iliac region; whereas no isolated para-aortic SLN was detected (Table 2).
Mean blood loss was 20 ml (10-100 ml). Mean duration of surgery was 88.5 min (40-180 min) (Table 3). No ICG injection-related complications were noted.
One intraoperative complication occurred in form of bladder injury in a patient with previous multiple caesarean sections, which was identified intraoperatively and repaired. One patient had difficulty in micturition in immediate postoperative period attributed to urethral spasm which was managed with antispasmodics. No vascular/ nerve injuries occurred intraoperatively. Two patients had to be converted to laparotomy in view of obesity and inability to tolerate the trendelenberg position, making the laparoscopic surgery technically challenging (Table 3).
Mean duration of hospital stay was 2 days (range 1-4 days).
Postop final histopathology identified stage IA in 63 (78.75%) patients, IB in 14 (17.5%), Stage II in 2(2.5%) and Stage IIIC1 in 1 (1.25%) patient (Table 1).
Nodal micro metastasis was detected in one patient on ultra staging which was Endometrioid histology, grade 2, type I; with >50% MI on preop assessment.
On postoperative prognostic risk group assignment, the majority were in low risk group. 14 (17.5%) belonged to high risk group, 9 (11.25%) to High Intermediate risk group, 3 (3.75%) to Intermediate group (Table 1).
Only one patient who had grade 3 carcinoma in preoperative biopsy, was changed to grade 1 and risk stratified as low risk based on final HPR. One patient was upgraded from grade 1 to grade 3; high intermediate group based on final HPR.
Discussion
The results of the present study suggest that laparoscopic SLN biopsy by use of cervical ICG injection can be effectively used after validating institutional sensitivity and false negative rates. This study was conducted after having validated the technique of SLN mapping using ICG and NIR imaging in our institution, in an earlier study [8]. The sensitivity of the procedure was 100 % and there were no false negatives. In another study in our institution, the average detection rate in our institution with radio colloid technetium 99m labelled sulphur colloid was 75 % with a sensitivity of 50 % [9]. ICG injection followed by NIR mapping gives a superior detection rate and sensitivity as shown in multiple studies, and is now the recommended mapping technique [6].
Some of the advantages conferred by ICG are higher sensitivity of SLN detection, low toxicity, and longer duration of visualization as it is mostly retained in vascular compartment without staining the parametrial tissue which is seen with blue dyes. Furthermore it does not require prior planning as with Tc 99 which also inherits risk of radiation exposure [10]. Although, a thorough knowledge of retroperitoneal anatomy and opening of avascular spaces remains a crucial step in SN biopsy, ICG allows the surgeon to visualize the lymph nodes through the peritoneum and thus may help in avoiding a wide dissection [11].
Mean age in the present study population was 56.3 years (29-79 years) and mean BMI was 29.09 kg/m2 ranging from 15.9 kg/m2 to 41 kg/m2. Further, one third of patients (33.75 %) had a BMI >30 kg/m2 (obese) in the present study. In a study by Andrea Papadia et al [4] BMI ranged from (17.4–47) median being 27.2 kg/m2. Similarly, in a Japanese prospective study which aimed to analyse the detection rate and feasibility of SLNB in endometrial cancer, the mean age of patients taken for laparoscopy was 57.1 ± 11.2 and mean BMI being 23.7 ± 4.6 kg/m2 [1]. These findings suggest that laparoscopic route is able to extend the benefit of MIS to patients with obesity, which is commonly found in endometrial cancer patients.
The concentration of ICG used in this study was 1%, higher than the usual concentration of 0.5 % used in robotic surgery. Visualization of sentinel nodes during laparoscopy was found to be better with 1 % concentration, in our experience. There is no specific recommendation for the concentration of ICG, although according to a recent meta-analysis , a lower concentration of ICG may provide better performance of the SLN mapping [12].
The overall SLN detection rate was found to be 93.75 % (75/80), and bilateral detection rate was 92.5 % (74/80) in the present study (Table 2).
Overall DR of SLN ranging from 80% to 100% reported by Bedynska etal [13], and similarly in a meta-analysis of 26 studies performed by Kang et al found a DR of 78% [11].
These results of this study are in keeping with other studies using laparoscopy suggesting that the detection rate depends on the performance of the dye rather than the robot [4].Following a meticulous injection technique for the deep and specifically the superficial injection is of utmost importance in accurate mapping [14,15]. Distribution of sentinel lymph nodes in the present study is comparable to other studies, most common location being External iliac in 40.8% cases, Internal iliac in 31.9 %, Obturator 23 %, Common iliac 3.4 % (Table 2).
The location of the sentinel nodes of our series was compared with the FIRES trial; The findings were as follows external iliac (40.8 %) vs. (38%), obturator 23% versus 25%, common iliac (3.4% vs. 8%), internal iliac (31.9% vs. 10%)(14) (Table 2). No presacral or paraaortic sentinel nodes were identified.
SLN mapping following MSKCC algorithm has a high rate of SLN detection, a high sensitivity for detection of metastasis and a low false-negative rate [13]. The importance of following the algorithm accurately, including removal of suspicious nodes irrespective of mapping, and complete lymphadenectomy in the event of failed mapping, cannot be emphasized enough. In an earlier study published from our institution, we also found that SLN mapping alone seems to have a limitation in detecting positive nodes especially in the high risk subtypes [2].
In our study, 87.5 % (n=70) patients had low grade endometrioid histology on preop endometrial biopsy. 13.5 % (n=11) were found to have high grade histology, most of whom were high grade Endometrioid histology.
Only the patients with apparent uterine confined disease on preoperative MRI were selected for sentinel mapping, 80 % of whom had IA disease on MRI, and 20 % had IB. All patients have an IHC analysis on endometrial biopsy samples in our institution, which includes p53, and MSI. This helps us in a better preoperative selection of patients, excluding patients who might benefit from a complete lymphadenectomy. Eleven patients were found to have type II EC, preoperatively.
Rossi EC et al in SENTI ENDO trial has included 13%, Ballester M et al in FIRES trial 28% and Persson J et al in SHREC trial has included 49 % patients with high grade histological subtypes [14,16,17]. Results of the present study add to this literature.
The SHREC trial, which studied the high risk group, demonstrated a sensitivity to identify pelvic node metastasis of 100% with 95% bilateral mapping rate and 98% sensitivity [17].
In present study only one patient was found to harbour micro metastasis in pelvic nodes, highlighting the importance of ultra staging. Out of 80 patients, 77 (96.25%) were found to be stage I on final HPE. 12 (15%) patients fell into high risk category.
To identify patients with high grade EC with small volume metastases which can be done effectively with SLNB is crucial; since PORTEC 3 trial results have shown that these patients derive a survival benefit on adding adjuvant chemotherapy [18]. The incidence of low volume metastatic disease (LVMD) by ultra staging varies approximately from 3.8% to 19.7% [19]. However, the LVMD detected by ultra-staging accounts for almost 50% of all lymph node metastases [20].
No ICG related adverse events were reported in present study. No nerve or vascular injury happened pertaining to laparoscopic nodal dissection. Intra and postoperative details as shown in the table are in concordance with laparoscopic surgery .This study demonstrated that SLNB with laparoscopy is associated with a shorter OR time and a lower complication rate compared with conventional nodal staging as shown in other studies [4].
Although the endometrial cancer staging using SLN mapping via mainly the robotic, as well as the laparoscopic route has been in practice for many years in the developed countries, in the low resource settings (LMICs), SLN mapping in EC with ICG, using the laparoscopic platform has been reported by only one institution [5]. Thus, the present study adds significantly to the existing literature regarding this aspect.
Strengths and Limitations
All the surgeries were done by a single surgeon,specializing in gynae oncology, allowing for uniformity .
The surgical technique using the robotic platform, and the ultra-staging technique had already been in use in our institution for many years, hence it was a smooth transition to make from the robotic to the laparoscopic route. The study is limited by its retrospective nature, and relatively smaller numbers.
In conclusion, SLN mapping is now established as an alternative to systematic lymphadenectomy for low grade, apparent uterine confined uterine cancer. Its scope continues to widen, with studies showing very high detection rates, and accurate staging information even in high risk histologies, when strict adherence to sentinel algorithm, and ultra staging protocols is followed. Long term survival data should be able to establish SLNB firmly even in high risk early stage EC as a standard of care.
Most centres in our country, as well as internationally, continue to use the robotic platform for performing SLN mapping. However, the lack of widespread availability of robotic equipment limits its use, especially in LMICs like India, and a number of other countries across the world. Our study aims to highlight that laparoscopy can enable the implementation of SLN mapping just as effectively, by adhering to meticulous injection technique, and the sentinel algorithm.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
The authors declare no conflict of interest.
References
- The detection of sentinel lymph nodes in laparoscopic surgery can eliminate systemic lymphadenectomy for patients with early stage endometrial cancer Tanaka T, Terai Y, Fujiwara S, Tanaka Y, Sasaki H, Tsunetoh S, Yamamoto K, Yamada T, Ohmichi M. International Journal of Clinical Oncology.2018;23(2). CrossRef
- A prospective evaluation of the sentinel node mapping algorithm in endometrial cancer and correlation of its performance against endometrial cancer risk subtypes Rajanbabu A, Agarwal R. European Journal of Obstetrics, Gynecology, and Reproductive Biology.2018;224. CrossRef
- Sentinel node mapping using indocyanine green and near-infrared fluorescence imaging technology for endometrial cancer: A prospective study using a surgical algorithm in Indian patients Somashekhar SP , Arvind R, Kumar CR , Ahuja V, Ashwin KR . Journal of Minimal Access Surgery.2021;17(4). CrossRef
- Laparoscopic Indocyanine Green Sentinel Lymph Node Mapping in Endometrial Cancer Papadia A, Imboden S, Siegenthaler F, Gasparri ML , Mohr S, Lanz S, Mueller MD . Annals of Surgical Oncology.2016;23(7). CrossRef
- ICG-Based Sentinel Lymph Node Biopsy in Endometrioid Endometrial Carcinoma—Results of a Prospective Study from a Single Tertiary Cancer Center of India Madhunarayana B, Iyer R, Chyau Patnaik S, Raju K, S Murthy S, Kodandapani S, et al . Indian J Gynecol Oncol.2021;:19.
- uterine.pdf [Internet]. [cited 2023 May 1] Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf..
- Sentinel Lymph Node Mapping in Patients with Endometrial Carcinoma: Less Can Be More Leitao MM . Current obstetrics and gynecology reports.2016;5(4). CrossRef
- Detection of Sentinel Lymph Nodes in Endometrial Cancer with Intracervical Indocyanine Green Injection and Robotically Assisted Near Infrared Imaging: A Feasibility Study in Indian Setting Anupama R, Murali V, Nataraj Y, Vijaykumar D. Indian Journal of Gynecologic Oncology.2015;13. CrossRef
- Sentinel Node Mapping in Endometrial Cancer with Radioactive Colloid Injection: A Feasibility Study Kumar DKV, N R , Rajanbabu A, P SS , R AB . Res Rev J Oncol Hematol.2019;4(2):13-16.
- How to perform a laparoscopic pelvic sentinel lymph node dissection using near-infrared fluorescence with indocyanine green in gynecological cancers Vésale E, Azaïs H, Belghiti J, Nikpayam M, Uzan C, Canlorbe G. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2019;29(2). CrossRef
- Sentinel lymph node biopsy in endometrial cancer: meta-analysis of 26 studies Kang S, Yoo HJ , Hwang JH , Lim MC , Seo SS , Park SY . Gynecologic Oncology.2011;123(3). CrossRef
- Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance Xiong L, Gazyakan E, Yang W, Engel H, Hünerbein M, Kneser U, Hirche C. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology.2014;40(7). CrossRef
- Sentinel lymph node mapping using indocyanine green in patients with uterine and cervical neoplasms: restrictions of the method Bedyńska M, Szewczyk G, Klepacka T, Sachadel K, Maciejewski T, Szukiewicz D, Fijałkowska A. Archives of Gynecology and Obstetrics.2019;299(5). CrossRef
- A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study Rossi EC , Kowalski LD , Scalici J, Cantrell L, Schuler K, Hanna RK , Method M, et al . The Lancet. Oncology.2017;18(3). CrossRef
- Development of a surgical competency assessment tool for sentinel lymph node dissection by minimally invasive surgery for endometrial cancer Moloney K, Janda M, Frumovitz M, Leitao M, Abu-Rustum NR , Rossi E, Nicklin JL , et al . International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2021;31(5). CrossRef
- Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, Querleu D, Golfier F, Leblanc E, Rouzier R, Daraï E. The Lancet. Oncology.2011;12(5). CrossRef
- Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging Persson J, Salehi S, Bollino M, Lönnerfors C, Falconer H, Geppert B. European Journal of Cancer (Oxford, England: 1990).2019;116. CrossRef
- Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial Boer SM , Powell ME , Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB , et al . The Lancet. Oncology.2018;19(3). CrossRef
- Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging Kim CH , Soslow RA , Park KJ , Barber EL , Khoury-Collado F, Barlin JN , Sonoda Y, Hensley ML , Barakat RR , Abu-Rustum NR . International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2013;23(5). CrossRef
- Ultrastaging of Sentinel Lymph Nodes in Endometrial Carcinoma According to Use of 2 Different Methods Euscher E, Sui D, Soliman P, Westin S, Ramalingam P, Bassett R, Malpica A. International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists.2018;37(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details