Staining Intensity of P16INK4a and Ki-67 Determine the Grade of Cervical Lesion: An Experience from Single Tertiary Care Centre
Download
Abstract
Background: Various non-neoplastic and neoplastic lesions occur in cervix. HPV infection plays a major role in genesis of cervical lesions. Although most HPV infections are cleared out, infections with high risk HPVs may persist resulting in cervical dysplastic lesions. Using p16, a surrogate marker for HPV infection and Ki-67, a proliferation marker, along with histopathology, help improve the diagnostic accuracy of these lesions. The aim of the study was to evaluate the expression of p16 and Ki-67 in cervical lesions and association of their staining intensity with the histologic grading. Also to determine the predictive value of these markers in association with the cervical lesions.
Methodology: A cross sectional study was carried out in 122 cases which were diagnosed histopathologically and then evaluated for the immunohistochemical expression of p16 and Ki-67 and their staining intensities.
Results: The dysplastic lesions comprised of 83.7% cases [29.5% LSIL, 5.7% HSIL, 43.5% SCC and 4.9% adenocarcinoma] and 16.3% had non dysplastic lesions. p16 and Ki-67 expression were seen in 64.7% and 68% cases respectively. The intensity of p16 and Ki-67 expression was scored according to Galgano et al (2010). An increasing intensity of p16 and Ki-67 expression with higher grades of the cervical lesions was noted and this association was found to be statistically significant. (χ2 = 43.46 and p value < 0.0001).
Conclusion: Though histopathology is the gold standard, the role of p16 and Ki-67 have emerged as useful adjuncts in detecting the true nature of the cervical lesions. They aid in the proper diagnosis, classification and distinction from non-dysplastic lesions, helping the clinicians in taking prompt action for management of the cases.
Introduction
The lesions encountered in cervix vary from mere non neoplastic inflammatory lesions to dysplastic lesions, Squamous Intraepithelial Lesion and carcinoma of uterine cervix and they occur mainly due to sexual and behavioural factors, socio-economic factors (education and income), smoking, diet, oral contraceptives, hormones, other infections like herpes simplex virus (HSV), human immunodeficiency virus (HIV) [1]. According to Walboomers (1999) infection with Human Papilloma Virus (HPV), mainly subtypes 16 and 18 are implicated in cervical neoplasm [2,3]. The imbalance between persistence of HPV infections and clearing it off results in most HPV associated cervical lesions. Most HPV infections are self limiting or regress if treated early. However, infection with high-risk HPVs may progress into much severe forms like dysplasia, Squamous Intraepithelial Lesion and later into cervical cancer if the HPV infection persists. But carcinoma cervix can be 100% preventable as its rate of progression is very slow with a long preinvasive stage. Also, with the presence of diagnostic procedures like cervical cytology and histopathology and use of several biomarkers, prompt early diagnosis makes it readily treatable giving the patients a long survival period [4].
Also, cervical cancer can be prevented by use of vaccines. Two vaccines licensed globally are available in India against cervical cancer. Cervarix, a bivalent vaccine against serotypes 16 & 18 and Gardasil, a quadrivalent vaccine against serotypes 16, 18, 6 & 11. The recommended age to initiate vaccination is 9–12 years. Catch-up vaccination can be done up to the age of 26 years. A total of three doses at 0, 2 and 6 months are recommended for Gardasil or 0, 1 and 6 months for Cervarix. The vaccines provide protection against cervical cancer and genital warts as well [5].
Cancer of uterine cervix is the fourth most common cancer recorded in women worldwide. It is also the fourth leading cause of cancer deaths among women worldwide with an estimated 570,000 cases and 311,000 deaths in 2018. In lower Human Development Index setting, cervical cancer ranks as second in both incidence and mortality [6]. Cervical cancer accounts for 17% of all cancer deaths amongst women aged 30-69 years of age [7,8]. Poor living standards, a higher prevalence of HPV (10% in women above the age of 30 years) and due to lack of screening are the causes of cervical cancer [8].
In Indian scenario, carcinoma of cervix is the second most common cancer in women [6]. India has a burden of cervical carcinoma accounting nearly 1/3rd of global carcinoma of cervix [9]. Among the women of lower socio-economic status, the burden of cervical cancer is much higher. It has been reported that every 7 minutes an Indian woman dies of cervical cancer [10].
p16INK4a is a tumor-suppressor protein. It works by blocking cyclin dependent kinases 4/6-mediated pRb phosphorylation and inhibits E2F-dependent transcription and cell-cycle progression [11]. The E7 protein of high- risk HPVs inactivates pRB, hence there is resulting overexpression of p16INK4a which may be used as a good marker for infection with high risk HPV types. Immunostaining of p16INK4a allows precise identification of even small CIN or cervical cancer lesions in biopsy sections. Thus it can help reduce inter-observer variation in the histopathological interpretation of cervical biopsy specimens [12,13].
Ki67 is used as an indicator of cellular proliferation frequently. Ki67 is expressed more significantly in malignant than in normal tissues. Ki67 increases with decreasing tissue differentiation, and it correlates with the clinical stage of tumors and presence of occult metastasis. [14,15].
The p16/Ki-67 co-expression implies deregulation of the cell cycle induced by HR-HPV and detection of p16/Ki-67 co-expression can serve as a marker to predict the cell transformation by HR-HPV and the presence of high-grade CIN lesions [16].
As the cell cycle controls are overruled by HPV infections, the detection of cell proteins p16 and ki67 which are differentially expressed in HPV infected cells are currently being considered for cervical cancer screening and as prognostic markers [13].
The study was carried out to evaluate the expression of p16 and Ki-67 in cervical lesions and to see the association of the staining intensity of the markers with the histologic grading. Also to determine the predictive value of these markers in association with the cervical lesions.
Materials and Methods
A hospital based cross sectional study was conducted for a period of one year from July 2021 to June 2022 in the Department of Pathology in collaboration with Department of Obstetrics and Gynaecology, GMCH. The approval was given by Institutional ethics committee having vide letter no. 190/2007/Pt-II/July-2021/TH-10.
Inclusion Criteria
Cervical biopsies and surgically resected specimens of cervix sent to the Department of Pathology, Gauhati Medical College and Hospital.
Exclusion Criteria
1. Inadequate biopsy, ulcerated and necrotic tissue.
2. Endocervical polyps.
3. Secondary carcinomas of cervix.
4. Cases undergoing chemotherapy/radiotherapy.
5. Patients who did not give consent.
A total of 122 cases were collected following the inclusion and exclusion criteria from Gynaecology OPD who presented with complaints of bleeding PV, white discharge, irregular menses, etc and underwent biopsy and hysterectomy. All the demographic as well as the clinical data were entered in MS Excell sheet.
Nature of specimen received were: Punch Biopsy: 102, Amputated cervix: 1, Hysterectomy: 18, Modified Radical hysterectomy: 1. Specimens were collected in 10% Neutral Buffered Formalin and the specimens were grossed, processed and then stained as per standard protocols [17]. Diagnosis was made based on the morphologic changes noted in the lining epithelium of the cervix along with the stromal reaction. For reporting, we followed the standard pathology textbook (Rosai and Ackerman’s surgical pathology) [18]. For tumors, we followed WHO classification of Tumours of Female Reproductive Organs- Tumours of the uterine cervix (2022) [19]. The cases were then evaluated for p16 and Ki67 immunostain on representative blocks of paraffin embedded tissue. Immunohistochemistry procedure was carried out according to standard protocols as follows: [17] All the paraffin-embedded tissue blocks were cut at 4 μM thickness. Two sections were made ready from each block of which one was placed on albuminized slides for routine H&E stain and the other set was mounted on poly L–lysine coated slides for immunostain. The poly L –lysine coated slides were placed in the oven for 10 minutes after which deparaffinization was done by passing the sections through xylene. Subsequently, sections were rehydrated in graded alcohol of decreasing concentration i.e. 100 %, 70% and 50% for five minutes each. The sections were then rinsed in running water for five minutes. Antigen retrieval was performed using the microwave method (also known as Heat Induced Epitope Retrieval, HIER) is used in our IHC set up, where, the slides were immersed in a bowl containing TRIS-EDTA buffer (6.05g of TRIS and 0.744g of EDTA dissolved in 1 litre of distilled water to set the pH at 9). A microwave oven set at 600 watt for 3 heating cycles of 5 minutes each was used. The sections were then allowed to cool at room temperature for 20 minutes.
Then the sections were washed with running water and rinsed with tris-buffer saline (TBS). Endogenous peroxidase blocking was done by applying 3% hydrogen peroxide in 100 ml methanol for 10 minutes and then washed thoroughly twice by TBS 3 minutes each. Primary antibody, antibody to p16 (mouse monoclonal anti-p16 INK4a antibody from BioGenex) and antibody to Ki67 (SP6 rabbit monoclonal antibody from BioGenex) respectively were added to slides and incubated for 60 minutes at room temperature. Again slides were washed with TBS twice for 30 minutes each. Secondary antibody, conjugated with HRP (Horseradish Peroxidase) were applied for 30 minutes and are washed again with TBS twice for 30 minutes each. DAB (3-3’ Diaminobenzidine) chromogen was added and incubated for 3-10 minutes and washed instantly by distilled water.
The sections were counterstained with haematoxylin for 30-45 seconds before rinsing with running water for three minutes and dehydrated in increasing alcohol concentration and mounted. All the staining pattern were compared with the result of positive control and negative control in each set of immuno-stain.
Interpretation of p16 immunostaining: The scoring of p16 generally includes both nuclear and cytoplasmic staining which are graded according to Galgano et al, 2010 [20] as: 0: no staining, 1: rare singly dispersed cells staining, 2: patchy but strong staining, often not continuous from basement membrane and 3: strong and diffuse staining, usually continuous staining from the basement membrane and extending upward in proportion to lesion grade.
Interpretation of Ki67 immunostaining: To determine the grade of Ki-67 expression, nuclei of 200 epithelial cells located across the whole epithelial layer has been examined in a high-power field.
The ki-67 index is defined as the percentage of Ki-67 positive cells. According to Galgano et al, 2010 (20), it will be graded as: 1+ (5%), 2+ (5-30%), 3+ (>30%) depending upon the percentage of Ki-67 positive cells (the Ki-67 index) as stated by Galgano et al., 2010.(20)
The scoring of Ki-67 includes nuclear staining only and scored as: 0 (no staining), 1 (1-2 layers of basal/ parabasal staining), 2 (diffuse staining confined to the bottom third or superficial staining but with skip areas usually between parabasal and upper zones), 3 (continuous staining of greater than the lower third of the epithelium).
Statistical Analysis
The data collected was analyzed using SPSS version 20. The association between histopathological diagnosis and each biomarker was statistically calculated by using the Chi- square (χ2) test of significance by adopting the statistical software SPSS, P values less than 0.5 was considered statistically significant . Sensitivity, specificity, and predictive values were calculated using 2×2 tables and standard formula.
Results
Histological diagnosis of the 122 cases were as follows: 43 cases had squamous intraepithelial lesions of which 29.5% (36/43 cases) had LSIL and 5.7% (7/43 cases) had HSIL, 59 cases had cervical cancer of which 43.5% (53/59 cases) were squamous cell carcinoma and 4.9% (6/59 cases) had adenocarcinoma. 20 cases were non dysplastic of which 9.8% (12/20) cases had chronic cervicitis and 6.6% (8/20) cases had metaplastic change. It was found in our study that 79/122 cases (64.7%) showed p16 immunoexpression whereas 43/122 cases (35.3%) were negative for p16. It was also found that p16 immunoexpression was highest in cervical carcinoma cases [55/122 cases (45.1%)], followed by 22/122 cases of SIL and 2/122 cases of non-dysplastic lesions, which indicated that the p16 expression increased with the increasing grades of the cervical lesion. A significant association was observed between routine histologic grading and p16 immunoexpression, Chi- square value is 50.70 and p value is <0.001.
The intensity of p16 expression was scored according to Galgano et al [20]. Among the 20 non-dysplastic lesions, 2 metaplastic lesions showed mild (grade 1) p16 positivity. Out of 15/36 LSIL cases that were p16 positive, 8/15 cases showed grade 1, 6/15 cases showed grade 2 and 1/15 cases showed grade 3 positivity. All the 7 cases of HSIL, 51/53 cases of cervical squamous cell carcinoma and 4/6 cervical adenocarcinoma cases showed p16 positivity. Of the 7 HSIL cases, 1/7 cases showed Grade 1, 2/7 cases showed grade 2 and 4/7 cases showed grade 3 positivity for p16. Of the cervical SCC cases, 51/53 showed p16 positivity of which 2/51 cases showed grade 1, 4/51 cases showed grade 2 and 45/51 cases showed grade 3 positivity.
Of the 4/6 cervical adenocarcinoma cases that were positive for p16, 1/4 cases showed grade 2 positivity while 3/4 cases showed grade 3 positivity. An increasing trend of p16 expression intensity from focal positivity in low grade lesions to diffuse intensity in higher grade lesions was found which was statistically significant. [chi square value= 76.87 and p value <0.0001] (Table 1).
| Cervicitis | Metaplasia | LSI L | HSIL | SCC | Adeno Ca | Total cases (%) | d.f | Chi square value | P value | |
| Negative | 12 | 6 | 21 | 0 | 2 | 2 | 43 (35.2) | 15 | 76.87 | <0.0001 |
| (Grade 0) | ||||||||||
| Mild | 0 | 2 | 8 | 1 | 2 | 0 | 13 (10.7) | |||
| Positivity | ||||||||||
| (Grade 1) | ||||||||||
| Moderate | 0 | 0 | 6 | 2 | 4 | 1 | 13 (10.7) | |||
| Positivity | ||||||||||
| (Grade 2) | ||||||||||
| Intense | 0 | 0 | 1 | 4 | 45 | 3 | 53 (43.4) | |||
| Positivity | ||||||||||
| (Grade 3) | ||||||||||
| Total | 12 | 8 | 36 | 7 | 53 | 6 | 122 (100) |
[Grade 0 (no staining), grade 1 (rare singly dispersed cells staining), grade2 (patchy but strong, often not continuous from basement membrane) and grade 3 (strong and diffuse staining, usually continuous staining from basement membrane and extending upward in proportion to lesion grade)]
Similarly, 83/122 cases (68%) were positive for Ki-67 and 39/122 cases (32%) were negative for Ki-67 immunoexpression. We also observed that Ki-67 immunoexpression was highest in cervical carcinoma cases, 56/122cases (46%) followed by 25/122 cases (20.4%) of SIL and 2/122 cases (1.6%) of non-dysplastic lesions, which indicated that the ki67 expression increased with the increasing grades of the cervical lesion. A significant association was observed between routine histologic grading and Ki-67 immunoexpression, Chi square value is 52.51 and p value is <0.001
The intensity of ki67 expression was scored according to Galgano et al. [20] of the 18/36 LSIL cases positive for ki67, 12/18 cases had score 1 and 6/18 cases had score 2. Of the 7/7 HSIL cases positive for KI67, 1/7 case had score 1, 3/7 cases had score 2 and 3/7 cases had score 3. Of the 51/53 SCC cases positive for KI67, 5/51 had score 2, 20/51 cases had score 2 and 26/51 cases had score 3. Of the 5/6 adenocarcinoma cases positive for ki67, 1/5 cases had a score 1, 1/5 cases had score 2 and 3/5 cases had score 3 (Table 2).
| Cervicitis | Metaplasia | LSIL | HSIL | SCC | ADENO Ca | Total cases (%) | d.f | Chi square | P value | |
| Score 0 | 12 | 6 | 18 | 0 | 2 | 1 | 39 (32) | 15 | 67.14 | <0.0001 |
| Score 1 | 0 | 1 | 12 | 1 | 5 | 1 | 20 (16.4) | |||
| Score 2 | 0 | 1 | 6 | 3 | 20 | 1 | 31 (25.4) | |||
| Score 3 | 0 | 0 | 0 | 3 | 26 | 3 | 32 (26.2) |
[score 0, no staining; score 1, showed only 1-2 layers of basal/parabasal staining; score 2, diffuse staining confined to bottom third or superficial staining but with skip areas usually between para basal and upper zones of the epithelium; score 3, continuous staining of greater than lower third of the epithelium]
There was an increasing trend in the staining intensity of Ki-67 with increasing grades of cervical lesions which was found to be statistically significant [chi square = 67.14, p value <0.0001].
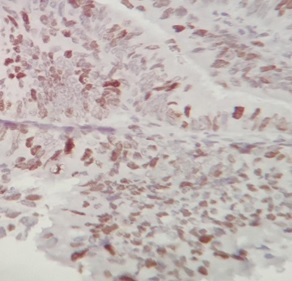
The diagnostic value of p16INK4a and Ki-67 immuno-histochemical markers on cervical biopsy were evaluated by calculating sensitivity, specificity, PPV and NPV of p16 and Ki-67 which was found to be 75.49%, 90%, 97.47% and 41.86% respectively for p16 and 79.41%, 90%, 97.59% and 46.15% respectively for Ki-67. The sensitivity and specificity of both stains together is 98.21% and 100% respectively (Table 3), (Figure 1 and 2).
Figure 1. Diffuse Staining Pattern (grade 3) of p16 in Cervical Cancer A (10X) and B (40X).
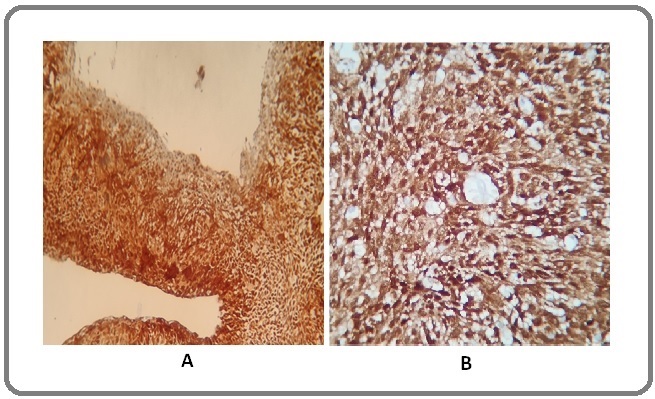
Figure 2. Diffuse Staining Pattern of Ki-67 ( score 3) in Cervical Cancer (40X).
| Variables | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| p16 and routine histology | 75.49 | 90 | 97.47 | 41.86 |
| (65.98 to 83.47) | (68.30 to 98.77) | (91.14 to 99.31) | (33.20 to 51.05) | |
| Ki-67 and routine histology | 79.41% | 90 | 97.59 | 46.15 |
| (70.27 to 86.78) | (68.30 to 98.77) | (91.55 to 99.34) | (36.30 to 56.32) | |
| p16 and Ki-67 | 98.21 | 100 | 100 | 75 |
| (90.45 to 99.95) | (29.24 to 100) | (29.24 to 100) | (30.07 to 95.44) |
Discussion
A total number of 122 histopathologically diagnosed cases of cervical lesions underwent immunohistochemical examination for p16 and Ki67 in the Department of Pathology. The results and observations are compared with observations of the literatures by other workers in similar studies from different regions of the world with a detailed discussion.
The present study aimed to study p16 and Ki-67 expression and their staining intensity in cervical lesions. It was found in several studies that the normal cervical epithelium did not express p16, but intensity of p16 and Ki-67 expression increased with degree of histologic atypia in the cervical lesions. Diffuse and strong p16 and Ki-67expression is observed in high grade dysplastic lesions.
Aslani et al (2013) recommended the use of these two markers as complementary tests for differentiating between dysplastic and non-dysplastic lesions [21]. In our study as well, the markers were positive in dysplastic lesions but negative in most non dysplastic lesions.
Our study showed that along with the high grade cervical lesions, p16 and Ki-67 were positive in CIN [I, II, III] as well. Majority of CIN-I lesions and a few of CIN-II lesions have the capacity to regress spontaneously. Therefore, predicting the development of CIN is also an important issue for cervical cancer prevention and treatment [22].
p16/ Ki-67 co-expression showed a strong association with CIN II+ lesions which is due to persistence of HPV infections, especially with HPV16/18. Hence, p16/Ki-67 is considered as a suitable biomarker for cervical cancer screening [23,24]. In our study, we found that diagnosis using p16 has high specificity (90.0%), but the sensitivity is poor (75.49%). But when combined with Ki-67, sensitivity (98.21%) and specificity (100%) were both at a high level.
The combined use of p16 and Ki-67 immunostain can be used as an auxiliary tool in diagnosis of carcinomas of cervix as seen in the present study and in other studies like Quin Shi et al., 2019 [25] Ding et al. in their study, provided an insight in using these markers to identify CIN patients, who are at a higher risk of malignant progression, facilitating more prompt and cost-effective, efficient interventions [26].
Studies by Klaes et al [27], Agoff et al [28], Kanthiya et al [29], Izadi et al [30] and Sarma et al [24] reported an increase in p16 and Ki-67 expression along with increased staining intensity with higher grades of cervical lesions. In the present study as well, similar findings were noted.
The present study aimed to evaluate the expression of the markers p16 and Ki-67 in cervical lesions. An ascending pattern of p16 and Ki-67 immunoexpression was seen with increasing grades of cervical lesion and it was statistically significant. Also, their staining intensity showed an increasing score with higher grades of the cervical lesions and it was statistically significant. Thus it can be said that p16 and Ki-67 over expression can be associated with dysplastic or neoplastic lesions.
In conclusion, though histopathology is the gold standard, the role of p16 and Ki-67 have emerged as useful adjuncts in predicting the true nature of the cervical lesions. The staining pattern of p16 and Ki-67 in different histologic grades of cervical lesions have justified its usefulness in confirmation of a histologic diagnosis and its biological behaviour. Positive p16 immunostain of the low grade dysplastic lesions may predict their aggressive behaviour and their propensity to turn to a higher grade or malignant lesion. Ki-67 being a proliferation marker, helps in detecting the proliferation potential of the lesions, but it can show positivity in reparative and inflammatory conditions as well. Hence it is imperative to use Ki-67 along with p16, as complementary markers, to differentiate between the dysplastic and non-dysplastic cervical lesions. Thus, the present study eshtablishes the importance of using p16 and Ki-67 immunostains in routine histopathology to detect the true nature of the cervical lesions.
Strength of the Study
1. Data generated from the study has evaluated the utility of the biomarkers p16 and Ki-67 in predicting the nature of the cervical lesions.
2. There is a future scope of molecular or genetic study from the samples that were included in the study. HPV status can be determined in the cases that stained positive for p16 in our study. It may detect the persistence of HPV infection and help in successful management of the cases.
Limitations of the Study
1. A smaller sample size and shorter duration of the study.
2. It was a cross sectional study, hence the cases could not be followed up. The expression of only two markers was studied. Application of more markers would have been beneficial to predict the other parameters of cervical cancer [CD31, CD105, Factor VIII, E- cadherin, P53, BCl2 etc]
Acknowledgements
We would like to extend our gratitude to the Department of Obstetrics and Gynaecology for collaborating with us for the study. We would also like to thank the technical staffs of the Department of Pathology, GMCH, for helping us with the work.
References
- [Etiology and pathogenesis of precancerous lesions and invasive cervical carcinoma] Panjković M, Ivković-Kapicl T. Medicinski Pregled.2008;61(7-8). CrossRef
- Epidemiology of cancer of the cervix: global and national perspective Shanta V, Krishnamurthi S, Gajalakshmi CK , Swaminathan R, Ravichandran K. Journal of the Indian Medical Association.2000;98(2).
- Human papillomavirus is a necessary cause of invasive cervical cancer worldwide Walboomers JM , Jacobs MV , Manos MM , Bosch FX , Kummer JA , Shah KV , Snijders PJ , et al . The Journal of Pathology.1999;189(1). CrossRef
- Cervical cancer in women with comprehensive health care access: attributable factors in the screening process Leyden WA , Manos MM , Geiger AM , Weinmann S, Mouchawar J, Bischoff K, Yood MU , Gilbert J, Taplin SH . Journal of the National Cancer Institute.2005;97(9). CrossRef
- Cervical cancer in India and HPV vaccination Kaarthigeyan K. Indian Journal of Medical and Paediatric Oncology: Official Journal of Indian Society of Medical & Paediatric Oncology.2012;33(1). CrossRef
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- The Challenge Ahead: Progress and Setbacks in Breast and Cervical Cancer [Internet]. Institute for Health Metrics and Evaluation. 2014 [cited 2022 Jul 3] Available from: https://www.healthdata.org/policy-report/challenge-ahead-progress-and-setbacks-breast-and-cervical-cancer..
- Effective screening programmes for cervical cancer in low- and middle-income developing countries Sankaranarayanan R, Budukh AM , Rajkumar R. Bulletin of the World Health Organization.2001;79(10).
- Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 Ferlay J, Shin HR , Bray F, Forman D, Mathers C, Parkin DM . International Journal of Cancer.2010;127(12). CrossRef
- Cervical Cancer Kills One Indian Women Every 7 Minutes Sarathi S, Hemavathy V, Vijayalakshmi R. International Journal of Innovative Research and Development.2015.
- p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors | Oncogene [Internet]. [cited 2022 Jul 17] Available from: https://www.nature.com/articles/onc2010614..
- Introduction of p16INK4a as a surrogate biomarker for HPV in women with invasive cervical cancer in Sudan Sarwath H, Bansal D, Husain NE , Mohamed M, Sultan AA , Bedri S. Infectious Agents and Cancer.2017;12. CrossRef
- p16INK4a Expression in Cervical Lesions Correlates with Histologic Grading - a Tertiary Level Medical Facility Based Retrospective Study Sarma U, Biswas I, Das A, Das GC , Saikia C, Sarma B. Asian Pacific journal of cancer prevention: APJCP.2017;18(10). CrossRef
- The Ki-67 protein: from the known and the unknown Scholzen T, Gerdes J. Journal of Cellular Physiology.2000;182(3). CrossRef
- Ki67 is a promising molecular target in the diagnosis of cancer (review) Li LT , Jiang G, Chen Q, Zheng JN . Molecular Medicine Reports.2015;11(3). CrossRef
- Interpretation of p16(INK4a) /Ki-67 dual immunostaining for the triage of human papillomavirus-positive women by experts and nonexperts in cervical cytology Allia E, Ronco G, Coccia A, Luparia P, Macrì L, Fiorito C, Maletta F, et al . Cancer Cytopathology.2015;123(4). CrossRef
- Bancroft's Theory and Practice of Histological Techniques. 2019 .
- Rosai and Ackerman’s Surgical Pathology - PDF Drive [Internet]. .
- Female Genital Tumours [Internet] Board WC of TE . Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020..
- Using biomarkers as objective standards in the diagnosis of cervical biopsies Galgano MT , Castle PE , Atkins KA , Brix WK , Nassau SR , Stoler MH . The American Journal of Surgical Pathology.2010;34(8). CrossRef
- Evaluation of Ki67, p16 and CK17 Markers in Differentiating Cervical Intraepithelial Neoplasia and Benign Lesions Sari Aslani F, Safaei A, Pourjabali M, Momtahan M. Iranian Journal of Medical Sciences.2013;38(1).
- Natural history of cervical intraepithelial neoplasia: a critical review Ostör AG . International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists.1993;12(2).
- Diagnostic value of p16INK4A, Ki-67, and human papillomavirus L1 capsid protein immunochemical staining on cell blocks from residual liquid-based gynecologic cytology specimens Yu L, Wang L, Zhong J, Chen S. Cancer Cytopathology.2010;118(1). CrossRef
- Predictive Value of Marker of Proliferation Ki-67 and Cell Cycle Dependent Protein kinase Inhibitor P16INK4a in Cervical Biopsy to Determine Its Biological Behaviour Sarma U, Das GC , Sarmah B. Asian Pacific journal of cancer prevention: APJCP.2021;22(7). CrossRef
- Ki-67 and P16 proteins in cervical cancer and precancerous lesions of young women and the diagnostic value for cervical cancer and precancerous lesions Shi Q, Xu L, Yang R, Meng Y, Qiu L. Oncology Letters.2019;18(2). CrossRef
- Predictive value of p16INK4a, Ki-67 and ProExC immuno-qualitative features in LSIL progression into HSIL Ding L, Song L, Zhao W, Li X, Gao W, Qi Z, Wang J. Experimental and Therapeutic Medicine.2020;19(4). CrossRef
- Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al . International Journal of Cancer.2001;92(2). CrossRef
- p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types Agoff SN , Lin P, Morihara J, Mao C, Kiviat NB , Koutsky LA . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2003;16(7). CrossRef
- Expression of the p16 and Ki67 in Cervical Squamous Intraepithelial Lesions and Cancer Kanthiya K, Khunnarong J, Tangjitgamol S, Puripat N, Tanvanich S. Asian Pacific journal of cancer prevention: APJCP.2016;17(7).
- Potential diagnostic value of P16 expression in premalignant and malignant cervical lesions Izadi-Mood N, Asadi K, Shojaei H, Sarmadi S, Ahmadi SA , Sani S, Chelavi LH . Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences.2012;17(5).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details