CEA as a Tumor Marker in Predicting Pelvic and Para-aortic Lymph Node Metastasis in Squamous Cell Carcinoma Cervix
Download
Abstract
Background: Carcinoma cervix is the major cause of death from gynecological malignancies. Isolated paraaortic lymph node metastasis detected on the initial diagnosis of cervical cancers could be addressed via extended paraaortic lymph node irradiation. Serum Carcinoembryonic antigen (CEA) is useful in detecting early Para aortic lymph node (PALN).
Materials and Methods: Fifty patients of histologically proven squamous cell carcinoma of cervix have been recruited into the study. We assessed pelvic and Para aortic lymph node status via CT or MRI scans. Serum CEA ranges had been evaluated in all from stage I to IV before starting the treatment.
Results: We observed that high pretreatment CEA values were associated with the pelvic and paraaortic lymph node metastasis 65.2% of the patients with high pretreatment CEA value had PALN metastasis (p=0.002). 47.8% of the patients with high CEA had pelvic lymph nodal metastasis (p=0.077) and 70% of the patients with high pretreatment CEA had both Pelvic and PALN metastasis which was statistically significant (p=0.020).
Conclusion: Carcinoembryonic antigen levels should help to prognosticate the Carcinoma Cervix patients and predict the presence of Para-aortic and Pelvic lymph nodes. This may be effective tool for detecting early failures in patients with Carcinoma Cervix
Introduction
Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 [1]. Most common histologic subtype of cervical cancer is squamous cell Carcinoma [2]. Carcinoembryonic antigen (CEA) is a glycoprotein found in colorectal carcinomas [3] that has also been studied in other mucin-producing tissues such as the uterine cervix. Serum CEA was quantified in cervical neoplasms by Rutanen et al. [4] in 1978, who found increased values in 10% of squamous carcinomas. In the literature, overall sensitivity has been described from 39 to 69% and there was an association between percentage of detection and clinical stage of disease, increasing from 26% of cases diagnosed of stage I disease to 71% of stage III disease and 100% of stage IV disease [5-8]. Studies have focused on CEA for over 40 years, suggesting it’s a useful tool in describing the prognosis of cancer [9]. A tremendous correlation has been stated between pre-treatment CEA levels and extent of the disease. High pre-treatment values had been related to bad prognosis [10].
Lymph node (LN) metastasis is an independent prognostic factor for cervical cancer patients [11]. The rate of para-aortic lymph node (PALN) metastases was found to be 5-45 per cent in locally advanced cervical cancer (LACC) [12]. PALN positivity primarily depends on pelvic lymph nodal involvement. The other key factors increasing the probability of PALN involvement are tumor size, parametrial and/or uterine corpus involvement [13]. PALN involvement has been proven to be a detrimental factor in the overall survival of cervical cancer patients irrespective of primary tumor size [14].
The 2018 FIGO (International Federation of Obstetrics and Gynecology) staging system has incorporated lymph nodal involvement; hence, the importance of accurate lymph nodal assessment is compounded and has direct implications on the mode of management [15].
For patients at suspicion of Para-aortic and Pelvic lymph node involvement imaging for metastatic workup is recommended. Extended Pelvic lymph node dissection ought to be taken into consideration accompanied by extended field external beam radiation therapy (EBRT) for patients with para-aortic lymph nodes [16]. Lymphadenopathy can’t be judged clinically, and it requires radiological investigations like CT scan, MRI scan and PET-CT scan.
CEA is a reliable tumor marker in patients with carcinoma of the cervix.[17] The presence of lymph node metastases has extra stated impact on the CEA values than the primary tumor.[18] There has been a lack evidence in the literature comparing pretreatment CEA values and its correlation with lymph node metastasis. Hence this study was conducted to correlate the pretreatment CEA values and presence of lymph node metastasis in Carcinoma Cervix.
Materials and Methods
50 patients of biopsy proven carcinoma cervix of any age were prospectively recruited into the study after taking approval from the institutional ethical committee. A written informed consent has been obtained from all the patients. They were clinically staged using FIGO staging system. In the evaluation of Para-aortic and pelvic lymph node involvement, CT scan or MRI scan was performed in all patients. The criterions for positive node involvement were based on the axial diameter of the lymph node larger than 1 cm. Patients with distant metastasis were excluded from the study. Pretreatment Serum CEA levels were evaluated in all the patients.
Statistical Methods
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean, Standard Deviation (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5 % level of significance. The following assumptions on data is made, 1. Dependent variables should be normally distributed, 2. Samples drawn from the population should be random, and Cases of the samples should be independent. Student t test (two tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (Inter group analysis) on metric parameters. Leven`s test for homogeneity of variance has been performed to assess the homogeneity of variance. Chi-square/ Fisher Exact test has been used to find the significance of study parameters on categorical scale between two or more groups, non-parametric setting for Qualitative data analysis.
Results
The total of 50 patients of carcinoma cervix were included in the study. Age of patients ranged between 21-70 years with the mean age of 51 years. Most of the patients in the study were aged between 40 -60 years and majority of them were in advanced stages i.e. IIB, IIIB, IVB.
Para-aortic lymph node was detected with CT scan or MRI scan in 42 % of patients and 50 % of patients there was pelvic lymph node involvement. In a total of 50 patients, 21 patients were Paraaortic node positive, 25 patients were pelvic node positive, 10 patients were both pelvic and paraaortic node positive. CEA value < 3 ng/ml was taken as normal.
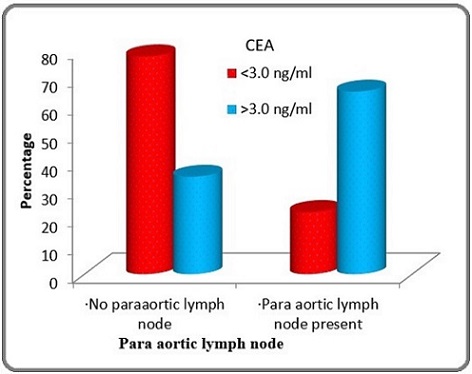
On Comparing CEA levels in Para aortic node positive patients, out of 21, 15 patients had CEA >3 ng/ml and 6 patients had CEA <3ng/ml which was statistically significant (p-0.002). (Table 1 and Figure 1).
| PALN | CEA ng/ml) | Total | |
| <3.0 (n=27) | >3.0 (n=23) | (n=50) | |
| Present | 6 (22.2%) | 15 (65.2%) | 21 (42%) |
| Absent | 21 (77.8%) | 8 (34.8%) | 29 (58%) |
Figure 1. Comparison of Para aortic Lymph Node According to CEA levels of Patients Studied (p=0.002).
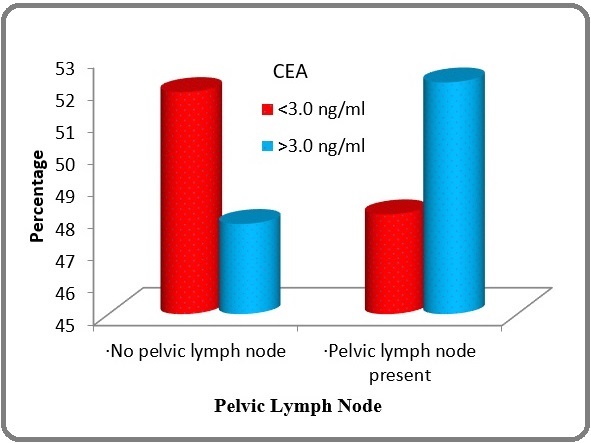
In Pelvic Lymph node positive patient’s node positive patients, out of 25, 12 patients had CEA >3 ng/ml and 13 patients had CEA <3ng/ml which was not statistically significant (p-0.777) as mentioned in Table 2 and Figure 2.
| Pelvic Lymph Node | CEA (ng/ml) | Total | |
| <3.0 (n=27) | >3.0 (n=23) | (n=50) | |
| Absent | 14 (51.9%) | 11 (47.8%) | 25(50%) |
| Present | 13 (48.1%) | 12 (52.2%) | 25(50%) |
Figure 2. Comparison of Pelvic Lymph Node According to CEA levels of Patients Studied (p=0.777).
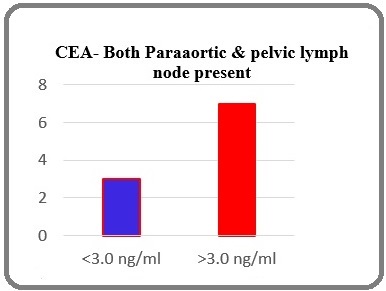
On comparing CEA values when both Para aortic and pelvic lymph node were involved, 70% of the patients with both pelvic and Para aortic node positive cases had increased CEA values and in Para-aortic lymph node present & pelvic lymph node absent cases 72.7% patients had increased CEA values which were highly significant (p-0.020) as mentioned in Table 3 and Figure 3.
| CEA (ng/ml) | Para-aortic & pelvic lymph node present (p-0.020) |
| <3.0 | 3 (30%) |
| >3.0 | 7 (70%) |
Figure 3. Comparison of CEA Levels when both Pelvic and Para Aortic Nodes are Present (p-0.020).
Discussion
Concurrent chemo-radiotherapy (CCRT) is a proven treatment for locally advanced cervical cancer [19-24]. Lymph node involvement is the most important prognostic parameter for patients with cervical cancer. The presence of lymph node metastases significantly influences patient’s outcome and therapeutic modalities more than any other clinical or pathological feature [25]. The latest FIGO staging system has incorporated lymph nodal involvement; hence, the importance of accurate lymph nodal assessment is compounded and has direct implications on the mode of management [15].
For patients at suspicion of Para-aortic and Pelvic lymph node involvement imaging for metastatic workup is recommended. There is no universally accepted diagnostic modality for the detection of PALN metastases. Positron-emission tomography-contrast-enhanced computed tomography (PET-CECT) and surgical staging via open/laparoscopic approach have failed to show a significant survival advantage [26,27]. Similarly, prophylactic irradiation of non-enlarged para-aortic nodes has a doubtful survival benefit [28].
Para-aortic lymph node (PALN) recurrence is not uncommon. The incidence of isolated PA nodal recurrences is 2-12 per cent, in radically treated cases of cervical cancer [29]. The approach to reduce PALN recurrence is a crucial problem for the treatment of locally advanced cervical cancer. Treatment options mainly include irradiating the PA chain to 45-50 Gy with or without a boost to the node or treatment of the gross node alone with stereotactic body radiation therapy (SBRT) [30]. Surgery may also be an option for those with previous irradiation to the PA region. Treatment of such patients could be an area of future investigation. Morris et al. conducted a randomized trial to examine entire pelvic CCRT versus extended field radiotherapy (EFRT). They located that CCRT improved overall survival and disorder-free survival, in addition to reduced loco regional and distant failure than EFRT. However, the 5-yr recurrence rate of Para-aortic lymph node (PALN) turned into 7% and 4% (p = 0.15) (Morris et al. 1999) [19].
CEA is a reliable tumor marker in patients with carcinoma of the cervix [17]. The presence of lymph node metastases has extra stated impact on the CEA values than the primary tumor [18]. The main aim of our study was to correlate the pretreatment CEA values and its association with Lymph node metastasis.
Disaia et al. did a study on a group of patients with carcinoma of the cervix and found that there was a progressive increase in the percentage of patients with positive CEA values correlating with advancing stage of the disease from 26% in stage I to 88% in stage II [31]. Incidentally 85% of the recurrent cases showed positive CEA values. Pre-treatment levels over 5 ug/l are highly suggestive of metastatic disease as they are associated with metastases in pelvic or Para-aortic lymph nodes in 50% of patients with stage IB disease. Also in advanced stages such as III and IV, 48% of patients had a pre-therapy value exceeding 5 ug/l [32]. Similarly in our study, we compared pretreatment CEA values with the incidence of lymph node metastasis which revealed 65.2% of the patients with high pretreatment CEA value had PALN metastasis.47.8% of the patients with high CEA had pelvic lymph nodal metastasis and 70% of the patients with high pretreatment CEA had both Pelvic and PALN metastasis which was statistically significant. Our results suggest that high CEA has direct correlation with the lymph nodal metastasis.
Patients with occult PALN micro metastasis are at risk for PALN recurrence if they undergo pelvic CCRT for CT-negative PALN metastasis. Hence, paraaortic lymphadenectomy can confirm subclinical PALN micro metastasis. Minimal complications are acceptable in modern laparoscopic technique. A large study (n = 253) reports that for 17.9% of patients with pathology confirmed PALN metastasis, their metastases were not detected in the CT scan at initial diagnosis of cervical cancer [33]. The false negative rate of CT detection was 23% in patients with laparoscopic extended para-aortic lymphadenectomy. Taken together, the false negative CT detection rates of around 20% are compatible with the current incidence of PALN recurrence (18.6%). This finding implies that PALN recurrence may result from suboptimal pelvic CTRT without addressing Para aortic nodes. Hence, we suggest that screening for PALN metastasis in high-risk patients should be a priority, where CEA can act as an adjunctive procedure in addition to imaging and surgical procedures. The main limitation of our study was the smaller sample size. Larger prospective randomized studies with larger population are needed for strong evaluation of efficacy and to draw inferences about the pretreatment CEA and its correlation with the Lymph nodal metastasis in Carcinoma Cervix patients.
In Conclusion, CEA has the capability of prognosticating Carcinoma cervix patients with the association of pelvic and Para-aortic nodes. In our Study, we found that CEA could be a useful marker in detecting nodal involvement in Carcinoma Cervix. This may be effective tool for detecting early failures, may help to salvage the unfortunate patients and give them a chance to prolong their survival with treatment like surgical debulking of para-aortic region and extended field radiotherapy.
Authors’ contribution
All authors worked on the conception of the article.
All authors reviewed and vouched for content.
Acknowledgements
Nil
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing interests
There was no conflict of interests.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers Sturgeon CM , Duffy MJ , Stenman U, Lilja H, Brünner N, Chan DW , Babaian R, et al . Clinical Chemistry.2008;54(12). CrossRef
- Demonstration Of Tumor-Specific Antigens In Human Colonic Carcinomata By Immunological Tolerance And Absorption Techniques Gold P, Freedman SO . The Journal of Experimental Medicine.1965;121(3). CrossRef
- Carcinoembryonic antigen in malignant and nonmalignant gynecologic tumors: circulating levels and tissue localization Rutanen EM , Lindgren J, Sipponen P, Stenman UP , Saksela E, Seppäla M. Cancer.1978;42(2). CrossRef
- Immunological study of gynecological cancer, Doctoral Thesis, University of Barcelona Balasch J. 1980.
- Immuno- logical aspeers of gynecological malignancies Disaia PJ , Sinkovics JG , Rutleclge FN , Smith JP . C'ynecol Onco.1975;3. CrossRef
- Carcinoembryonic antigen in patients with gynecologic malignancies DiSaia PJ , Haverback BJ , Dyce BJ , Morrow CP . American Journal of Obstetrics and Gynecology.1975;121(2). CrossRef
- Carcinoembryoinc antigen in cervical and vulvar cancer patients. Serum levels and disease progress DiSaia PJ , Morrow CP , Haverback BJ , Dyce BJ . Obstetrics and Gynecology.1976;47(1).
- The prognostic significance of carcinoembryonic antigen determinations in patients with adenocarcinoma of the cervix Kjorstad KE , Orjasaeter H. Gynecologic Oncology.1984;19(3). CrossRef
- Predictive factors of para-aortic lymph nodes metastasis in cervical cancer patients: a retrospective analysis based on 723 para-aortic lymphadenectomy cases Han X, Wen H, Ju X, Chen X, Ke G, Zhou Y, Li J, et al . Oncotarget.2017;8(31). CrossRef
- The role of lymphadenectomy in cervical cancer patients: the significance of the number and the status of lymph nodes removed in 526 cases treated in a single institution Ditto A, Martinelli F, Lo Vullo S, Reato C, Solima E, Carcangiu M, Haeusler E, et al . Annals of Surgical Oncology.2013;20(12). CrossRef
- Para-aortic lymph node involvement revisited in the light of the revised 2018 FIGO staging system for cervical cancer Ayhan A, Aslan K, Öz M, Tohma YA , Kuşçu E, Meydanli MM . Archives of Gynecology and Obstetrics.2019;300(3). CrossRef
- Lymph node assessment in cervical cancer: prognostic and therapeutic implications Gien LT , Covens A. Journal of Surgical Oncology.2009;99(4). CrossRef
- Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging Gouy S, Morice P, Narducci F, Uzan C, Martinez A, Rey A, Bentivegna E, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(24). CrossRef
- Revised FIGO staging for carcinoma of the cervix uteri Bhatla N, Berek JS , Cuello Fredes M, Denny LA , Grenman S, Karunaratne K, Kehoe ST , et al . International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics.2019;145(1). CrossRef
- Lymphadenectomy in locally advanced cervical cancer study (LiLACS): Phase III clinical trial comparing surgical with radiologic staging in patients with stages IB2-IVA cervical cancer Frumovitz M, Querleu D, Gil-Moreno A, Morice P, Jhingran A, Munsell MF , Macapinlac HA , et al . Journal of Minimally Invasive Gynecology.2014;21(1). CrossRef
- The prognostic value of CEA determinations in the plasma of patients with squamous cell cancer of the cervix Kjorstad KE , Orjasaester H. Cancer.1982;50(2). CrossRef
- Squamous cell carcinoma antigen and carcinoembryonic antigen levels as prognostic factors for the response of cervical carcinoma to chemotherapy Meier W, Eiermann W, Stieber P, Fateh-Moghadam A, Schneider A, Hepp H. Gynecologic Oncology.1990;38(1). CrossRef
- Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer Morris M, Eifel PJ , Lu J, Grigsby PW , Levenback C, Stevens RF , Rotman M, Gershenson DM , Mutch DG . The New England Journal of Medicine.1999;340(15). CrossRef
- Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix Peters WA , Liu PY , Barrett RJ , Stock RJ , Monk BJ , Berek JS , Souhami L, Grigsby P, Gordon W, Alberts DS . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2000;18(8). CrossRef
- Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma Keys HM , Bundy BN , Stehman FB , Muderspach LI , Chafe WE , Suggs CL , Walker JL , Gersell D. The New England Journal of Medicine.1999;340(15). CrossRef
- Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer Rose PG , Bundy BN , Watkins EB , Thigpen JT , Deppe G, Maiman MA , Clarke-Pearson DL , Insalaco S. The New England Journal of Medicine.1999;340(15). CrossRef
- Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis Green JA , Kirwan JM , Tierney JF , Symonds P, Fresco L, Collingwood M, Williams CJ . Lancet (London, England).2001;358(9284). CrossRef
- Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(35). CrossRef
- Clinical importance of lymph node density in predicting outcome of prostate cancer patients Cai T, Nesi G, Tinacci G, Giubilei G, Gavazzi A, Mondaini N, Zini E, Bartoletti R. The Journal of Surgical Research.2011;167(2). CrossRef
- MRI and PET/CT for triaging stage IB clinically operable cervical cancer to appropriate therapy: decision analysis to assess patient outcomes Pandharipande PV , Choy G, Carmen MG , Gazelle GS , Russell AH , Lee SI . AJR. American journal of roentgenology.2009;192(3). CrossRef
- Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB-IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study Marnitz S, Tsunoda AT , Martus P, Vieira M, Affonso Junior RJ , Nunes J, Budach V, et al . International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2020;30(12). CrossRef
- Prophylactic extended-field irradiation with concurrent chemotherapy for pelvic lymph node-positive cervical cancer Oh J, Seol KH , Lee HJ , Choi YS , Park JY , Bae JY . Radiation Oncology Journal.2017;35(4). CrossRef
- Para-aortic lymph node recurrence after curative radiotherapy for cervical cancer Cho WK , Kim YI , Park W, Yang K, Kim H, Cha H. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society.2019;29(7). CrossRef
- Comparison of salvage therapies for isolated para-aortic lymph node recurrence in patients with uterine cervical cancer after definitive treatment Kubota H, Tsujino K, Sulaiman NS , Sekii S, Matsumoto Y, Ota Y, Soejima T, Yamaguchi S, Sasaki R. Radiation Oncology (London, England).2019;14(1). CrossRef
- Carcinoembryonic antigen in cancer of the female reproductive system. Serial plasma values correlated with disease state DiSaia PJ , Morrow CP , Haverback BJ , Dyce BJ . Cancer.1977;39(6). CrossRef
- The significance of paraaortic node status in carcinoma of the cervix and endometrium Manetta A, Delgado G, Petrilli E, Hummel S, Barnes W. Gynecologic Oncology.1986;23(3). CrossRef
- Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix Huang E, Huang Y, Chanchien C, Lin H, Wang C, Sun L, Tseng C, et al . Radiation Oncology (London, England).2012;7. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details