Epidemiology and Management of Immune Thrombocytopenia in Adult Patients in Algeria: A Non-interventional, Longitudinal, Nationwide Estimation Study
Download
Abstract
Introduction: Globally, immune thrombocytopenia (ITP) affects more women than men with a higher incidence in older patients. However, data on the epidemiology and treatment regimen for ITP is limited and varies across different countries. In this non-interventional, longitudinal, nationwide estimation study, we present incidence (by age, sex, and diagnosis stage), prevalence, characteristics, and treatment regimens of ITP in Algeria.
Methods: Patients ≥16 years of age with prevalent (diagnosed before September 3, 2017) or incident (diagnosed between September 3, 2017–August 30, 2018) ITP were involved in this study. Patient data were collected from public hospitals and the incidence and prevalence estimates were made using Poisson distributions (95% confidence interval [CI]).
Results: Of 1,746 patients from 16 hematology departments, 1,159 were included in the study; 173 (14.9%) were incident and 986 (85.1%) were prevalent patients with ITP. The median (quartiles) age of patients at diagnosis was 36 (25, 50) years; 895 (77.2%) were women. At inclusion, 88.2% of patients were asymptomatic and 3.8% had severe bleeding. The national incidence was 0.85 (0.75–0.96) and prevalence was 5.65 (5.39–5.93) per 100,000 population. The incidence of ITP in women (1.18 [1.02–1.37]/100,000 population) was higher versus men (0.54 [0.43–0.67]/ 100,000 population). The incidence of ITP was four times higher in the ≥75 years cohort (2.37 [1.60–3.51]/100,000 population) versus that observed in the 15–35-years age cohort (0.54 [0.43–0.68]/100,000 population). First-line treatments included corticosteroids (80.8%), intravenous immunoglobulin (3.4%), and rituximab (1.3%); 17.4% of patients underwent splenectomy as a third-line procedure.
Conclusion: The overall incidence rate of ITP in Algeria was low. However, the incidence trends are similar to those reported globally, with high incidence reported in women and older patients. Corticosteroids were the most prescribed therapy, and few patients were prescribed thrombopoietin receptor agonists before opting for splenectomy.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by a transient or persistent decrease in platelet count (<100×103/mm3) [1-3]. The annual incidence of ITP is estimated at 1.9–6.4 per 100,000 children and 3.3 per 100,000 adults [4]. Among the adult patient population, the incidence of ITP is higher in women than in men, with the incidence rate increasing with age. Studies from the United Kingdom (UK), France, Taiwan, and Korea reported an incidence of 3.03–6.0/100,000 person-years among women and 2.3–4.5/100,000 person- years among men, with the highest incidence observed in the age range of 60–84 years [5-8]. A similar trend was reported from Denmark, Sweden, and Norway, where 57.8% of patients were women and the incidence of chronic ITP was the highest in patients aged 70 years and above [9]. Frequently, patients with ITP are asymptomatic and are diagnosed incidentally following a routine blood examination. In other cases, ITP is diagnosed following moderate or, more rarely, severe bleeding [10]. Since there are no confirmatory tests for ITP, the diagnosis is made only after the exclusion of secondary causes of thrombocytopenia, such as viral infections or autoimmune infections [1, 11].
The 2019 American Society of Hematology (ASH) guidelines suggest that corticosteroid treatment for ITP should be initiated if the platelet count falls below 30× 103/mm3 and if the patient has minor mucocutaneous bleeding or no symptoms [12]; however, little clinical evidence is available to support this strategy. For patients with a platelet count greater than 30×103/mm3, the ASH 2019 guidelines strongly recommend against corticosteroid therapy and support an observational or “watch-and-wait” approach. In contrast, recommendations by the World Health Organization (WHO) for grade III and grade IV ITP treatment are based on bleeding symptoms, regardless of the platelet count [13]. While patients with severe thrombocytopenia (platelet count of <30×103/mm3) may be offered treatment, a monitoring strategy could also be considered in the absence of hemorrhagic signs.
The optimal treatment strategy for ITP requires various factors to be taken into consideration such as bleeding history, patient age, treatment side effects, and patient preference [14]. Current therapeutic strategy for ITP aims to prevent bleeding; additionally, a better understanding of the pathophysiology of ITP has expanded treatment options in recent years. Corticosteroids are still the standard first-line treatment for newly diagnosed patients with ITP. However, in cases needing a rapid increase in platelet count (e.g., severe bleeding or before surgery), intravenous immunoglobulins (IVIGs) are usually given along with corticosteroids. Before the introduction of thrombopoietin receptor agonists (TPO-RAs), the second- line therapy for ITP included splenectomy and rituximab (an anti-CD20 antibody). Notably, rituximab does not have marketing authorization to be used as a treatment for ITP. Current recommendations, based on phase III studies, state that TPO-RAs can be offered as the second-line treatment [3] to patients with chronic ITP as they are effective, regardless of the patients’ splenectomy status [15] or age [16]. TPO-RAs act by stimulating platelet production and the proliferation and differentiation of megakaryocytes in the bone marrow [17]. While clinical trial data show that patients administered romiplostim achieved a platelet count of ≥50×103/mm3) within a mean duration of 13.8 weeks [18], there is no known predictive biomarker indicating the clinical response towards the treatment. Two TPO-RAs currently approved in the European Union are romiplostim and eltrombopag [10, 14]. Although TPO-RAs are not approved as a first-line therapy for ITP, they may be considered for treatment in patients with life-threatening bleeding [14]. Other immunosuppressive drugs, such as azathioprine or cyclosporine, can be used as third-line treatment.
To date, very little has been published regarding the real-life management of patients with ITP in Algeria. This study presents data from a national multicenter survey undertaken from September 3, 2017, to August 30, 2018, to describe the clinical, biological, and therapeutic characteristics of ITP in Algeria and to assess its national incidence and prevalence.
Materials and Methods
Study design
This was a national, non-interventional, longitudinal, multicenter study involving patients diagnosed with ITP who were treated at public hospitals across Algeria. Patients were diagnosed with ITP if they had a platelet count ≤100×103/mm3 and/or the presence of petechiae, bruises, intraoral hemorrhagic bullae, epistaxis, gingival bleeding, gross hematuria, and retinal bleeding. Data from pathological tests used for ITP diagnosis included, hemogram, myelogram, tricyclic antidepressant test, prothrombin time, fibrinogen, D-dimer, blood cultures for patients with fever, liver function test, hemolysis tests (lactic dehydrogenase, haptoglobin, and Coomb’s test), viral serologies (human immunodeficiency virus [HIV], hepatitis A, B, C, cytomegalovirus, Epstein-Barr virus, COVID-19), and immunological tests (antinuclear antibody, rheumatoid factor, antiphospholipid antibody).
All patient data were collected using a specially designed questionnaire that included questions on patient history, sex, age, clinical signs of disease at the time of diagnosis, diagnosis stage and classification, diagnostic work-up, platelet counts, and parameters relating to the progression of ITP and the type of treatment received for ITP. Patient data also included bleeding symptoms, which were categorized according to the WHO bleeding scale. The WHO bleeding scale is a five-point scale that can be used to classify investigator-determined bleeding as follows: grade 0 for no bleeding; grade 1 for petechiae; grade 2 for mild blood loss; grade 3 for gross/significant blood loss; and grade 4 for debilitating/severe blood loss [19]. For this study, bleeding was assessed by the attending physician at the medical center where the patient received care. The study concluded on March 30, 2020, with 18 months of patient follow-up marked from the date of patient inclusion, that is, September 3, 2017. The study protocol, case report form, and the informed consent form were approved by an independent ethics committee (CHU Beni Messous Comite d’Ethique pour les Essais Cliniques) and the Ministry of Health of Algeria.
Study population
All patients ≥16 years of age irrespective of the date of diagnosis during the inclusion period, who were diagnosed with ITP, treated in hematology departments in Algeria, and who provided their informed consent were included in the study. Diagnosis data collected for this study included date of diagnosis, age at diagnosis, bleeding score, diagnosis stage (asymptomatic, easy bruising or severe bleeding), complete blood count, and peripheral blood smear. To ensure a representative patient cohort, all available data from patients diagnosed with ITP based on clinical symptoms and with a platelet count ≤100×103/mm3 were included in the study. Additionally, to avoid duplication of data, a coding system was generated for patients who received care at more than 1 hematology department. Patients were divided into two groups: the prevalent group included patients who were diagnosed and treated with ITP before September 3, 2017, and the incident group comprised of patients newly diagnosed with ITP within the inclusion period from September 3, 2017, to August 30, 2018.
Endpoints
The primary endpoint of the study was estimation of the number of eligible (≥16 years of age) patients diagnosed with ITP in Algeria during the 12-month study duration. The key secondary endpoints included estimation of the number of newly diagnosed patients with ITP in Algeria according to age, sex, and diagnosis stage; number of existing patients with ITP in Algeria during the study (prevalent patients); number of patients based on treatment, risk factors, and comorbidities.
Statistical analysis
The results stated in this study are for descriptive purposes only. The SAS software (SAS 9.4 and SAS/STAT 14.3) was used to estimate the incidence and prevalence of ITP per 100,000 population and 95% confidence interval (CI) using Poisson distribution. Population data for Algeria for the year 2017, available on the Office National des Statistiques (ONS) website [20], were used to estimate the national incidence and prevalence of ITP.
Results
Data for 1,746 patients with ITP were collected over a period of 12 months from 16 hematology departments across Algeria. Of the 1,746 patients, 587 refused to take part in the study or did not visit the study site during the inclusion period; as a result, they could not be assessed in this study. Five patients were excluded from the study due to major deviations from the study protocol including death, a history of hepatitis C, and diagnosis of Raynaud’s disease, multiple sclerosis, and familial thrombocytopenia. Finally, 1,159 patients were included and assessed in the study. The median (quartiles) age of patients at the time of diagnosis was 36 (25, 50) years, with 88.7% (1,028/1,159) of patients aged ≤65 years; 77.2% (895/1,159) of the patients were women. All health regions were represented in the patient cohort; 50.0% (579/1,159) of the patients were from the center of the country, 34.0% (394/1,159) from the east, 13.0% (151/1,159) from the west, and 3.0% (35/1,159) from the south. At the time of inclusion in the study, 14.9% (173/1,159) of the patients were incident while 85.1% (986/1,159) were prevalent patients with ITP (Table 1).
| Characteristic | Incident cases | Prevalent cases | Total cases |
| n=173 | n=986 | N=1,159 | |
| Sex, n (%) | |||
| Male | 53 (30.6) | 211 (21.4) | 264 (22.8) |
| Female | 120 (69.4) | 775 (78.6) | 895 (77.2) |
| Age at inclusion (years) | |||
| Mean (range) | 44.7 (15–94) | 43.7 (16–88) | 43.9 (15–94) |
| ≤65 years, n (%) | 152 (87.9) | 876 (88.8) | 1,028 (88.7) |
| >65 years, n (%) | 13 (7.5) | 80 (8.1) | 93 (8.0) |
| >75 years, n (%) | 8 (4.6) | 30 (3.0) | 38 (3.3) |
| Disease manifestation at diagnosis, n (%) | |||
| Nose bleeding | 4 (2.3) | 5 (0.5) | 9 (0.8) |
| Gingival bleeding | 22 (12.7) | 15 (1.5) | 37 (3.2) |
| Gastrointestinal bleeding | 2 (1.2) | 0 | 2 (0.2) |
| Vaginal bleeding | 6 (3.5) | 14 (1.4) | 20 (1.7) |
| Platelet counts (×10 3 /mm 3 ), n (%) | |||
| Normal* | 31 (17.9) | 219 (22.2) | 250 (21.6) |
| Abnormal without symptoms | 50 (28.9) | 328 (33.3) | 378 (32.6) |
| Abnormal with symptoms | 82 (47.4) | 385 (39.0) | 467 (40.3) |
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 10 (5.8) | 53 (5.4) | 63 (5.4) |
*Normal platelet counts are defined as 150–450×103/mm
Of the 1,159 patients, 83.3% (966/1,159) had no comorbidity that could increase the bleeding risk, while 16.7% (193/1,159) had comorbidities including 91.7% (177/193) of patients with a history of diabetes or 21.8% (42/193) with high blood pressure.
Etiologies tested before ITP diagnosis
Etiologies present before ITP diagnosis (Table 2) included viral infections in 2.6% (30/1,159) of patients, where 66.7% (20/30) of patients had influenza-like illness. Viral serology tests for hepatitis C were done for 73.3% (850/1,159) of patients, of whom 0.6% (5/850) tested positive.
| Etiology | Incident cases | Prevalent cases | Total cases |
| n=173 | n=986 | N=1,159 | |
| Viral infections preceding thrombocytopenia, n (%)* | |||
| Missing | 0 | 6 (0.6) | 6 (0.5) |
| Not done | 167 (96.5) | 956 (97.0) | 1,123 (96.9) |
| Yes | 6 (3.5) | 24 (2.4) | 30 (2.6) |
| Sore throat | 2 (33.3) | 2 (8.3) | 4 (13.3) |
| Influenza-like illness | 2 (33.3) | 18 (75.0) | 20 (66.7) |
| Other | 2 (33.3) | 4 (16.7) | 6 (20.0) |
| Viral hepatitis serologies, n (%)* | |||
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 32 (18.5) | 276 (28.0) | 308 (26.6) |
| Yes | 141 (81.5) | 709 (71.9) | 850 (73.3) |
| Negative | 140 (99.3) | 705 (99.4) | 845 (99.4) |
| Positive | 1 (0.7) | 4 (0.6) | 5 (0.6) |
| HIV serology, n (%)* | |||
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 32 (18.5) | 278 (28.2) | 310 (26.7) |
| Yes | 141 (81.5) | 707 (71.7) | 848 (73.2) |
| Negative | 140 (99.3) | 703 (99.4) | 843 (99.4) |
| Positive | 1 (0.7) | 4 (0.6) | 5 (0.6) |
| Helicobacter pylori infection, n (%) | |||
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 161 (93.1) | 836 (84.8) | 997 (86.0) |
| Yes | 12 (6.9) | 149 (15.1) | 161 (13.9) |
| Negative | 5 (41.7) | 60 (40.3) | 65 (40.4) |
| Positive | 7 (58.3) | 89 (59.7) | 96 (59.6) |
| Autoimmune disease, n (%) | |||
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 34 (19.7) | 199 (20.2) | 233 (20.1) |
| Yes | 139 (80.3) | 786 (79.7) | 925 (79.8) |
| Lymphoid hemopathy, n (%) | |||
| Missing | 0 | 1 (0.1) | 1 (0.1) |
| Not done | 18 (10.4) | 261 (26.5) | 279 (24.1) |
| Yes | 155 (89.6) | 724 (73.4) | 879 (75.8) |
*Incidence rates of prior etiologies were calculated from the number of patients tested for the disease. HIV, human immunodeficiency virus; ITP,immune thrombocytopenia
Of the 73.2% (848/1,159) of patients who took the test, 0.6% (5/848) tested positive for human immunodeficiency virus (HIV) infection. Tests for Helicobacter pylori infection were performed for 13.9% (161/1,159) of patients, of whom 59.6% (96/161) tested positive (Table 2). A total of 79.8% (925/1,159) of patients were tested for autoimmune diseases and 75.8% (879/1,159) of patients for lymphoid hemopathies. Lymphoid hemopathies included non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, chronic lymphocytic leukemia, and multiple myeloma. The underlying causes for secondary ITP were primarily linked to viral infections (2.6%), infections due to Helicobacter pylori (59.6%), and HIV infection (0.6%). Lastly, incidental findings led to the diagnosis of ITP in 21.0% (243/1,159) of the cases. A myelogram test was performed in 72.0% (834/1,159) of patients, of whom 33.9% (283/834) underwent a bone marrow biopsy to exclude other hematological conditions such as lymphoma.
Categorization of the patient population based on ITP symptoms and platelet counts
At inclusion, 88.2% (1,022/1,159) of the patients were asymptomatic (including 914 prevalent and 108 incident patients with ITP), 8.0% (93/1,159) had ecchymosis or easy bruising, and 3.8% (44/1,159) had severe bleeding, whereas at diagnosis, 34.7% (60/173) of the patients had severe bleeding (Figure 1).
Figure 1. Categorization of Patients with ITP According to Symptoms at Diagnosis. Patients within the incident cohort (n=173) were categorized based on clinical symptoms observed at diagnosis. Asymptomatic patients were defined as those with no bleeding or visible signs of ITP. Ecchymosis/easy bruising was defined as occurrence of superficial bruises that may develop from minor trauma or even from movement, and severe hemorrhage was defined as severe internal bleeding that may rarely occur intracranially. ITP, immune thrombocytopenia.
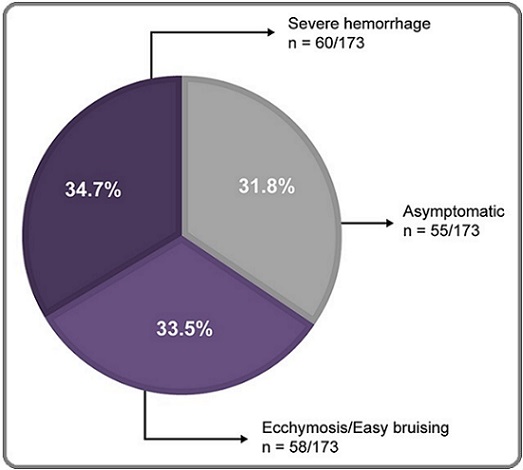
The overall incidence of asymptomatic patients was 0.37 (95% CI, 0.31–0.45) per 100,000 people, those with easy bruising was 0.14 (95% CI, 0.10–0.19) per 100,000 people, and those with severe hemorrhage was 0.09 (95% CI, 0.06–0.13) per 100,000 people in Algeria. The severity of bleeding was assessed according to the WHO bleeding scale in incident patients at diagnosis: 28.9% (50/173) of patients had no bleeding and were at grade 0, 13.9% (24/173) had petechiae and were assigned grade I, 19.7% (34/173) had mild bleeding and were at grade II, 30.1% (52/173) had significant blood loss and were assigned grade III, and 7.5% (13/173) had severe debilitating bleeding and were assigned grade IV on the bleeding scale (Figure 2).
Figure 2. Grading of Bleeding Symptoms According to the WHO Bleeding Scale. Clinical manifestation of ITP in incident patients (n=173) was categorized according to the WHO bleeding scale. Grade 0 represents asymptomatic patients, grades I and II represent mild forms of the disease, and grades III and IV represent severe disease states. ITP, immune thrombocytopenia; WHO, World Health Organization.
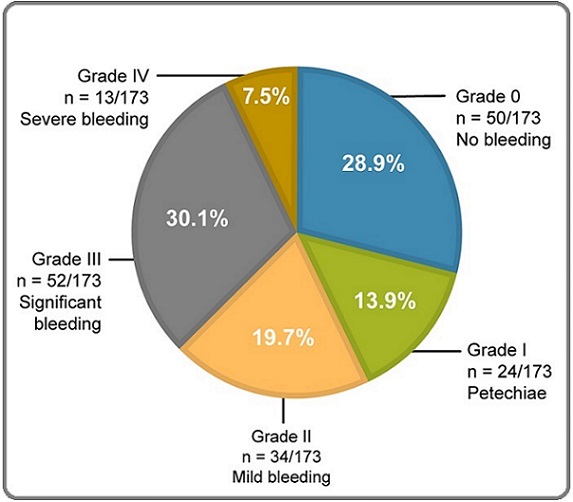
At the time of diagnosis, the platelet count was 0–30×103/mm3 in 71.7% (124/173) of patients, 30–50×103/mm3 in 11.6% (20/173) of patients, 50–100×103/mm3 in 16.2% (28/173), and more than 100×103/mm3 in 0.6% (1/173) of patients within the incident population. In the overall cohort, 32.6% (378/1,159) of patients had thrombocytopenia with no clinical signs of bleeding, while 40.3% (467/1,159) of patients had thrombocytopenia associated with signs of bleeding (Table 1).
Incidence and prevalence of ITP in Algeria
The incidence and prevalence of ITP were assessed over a 12-month period in patients ≥16 years of age. The national incidence of ITP was 0.85 (95% CI, 0.75– 0.96)/100,000 population, while the prevalence was 5.65 (95% CI, 5.39–5.93)/100,000 population. Additionally, disease incidence increased with age, from 0.54 (95% CI, 0.43–0.68)/100,000 population in the 15–35-year age group to 2.37 (95% CI, 1.60–3.51)/100,000 population in patients aged 75 years and above. The incidence of ITP also varied according to the clinical status at the time of diagnosis and on the sex of the patient, being higher in women (1.2 [95% CI, 1.02–1.37]/100,000 population) than in men (0.54 [95% CI, 0.43–0.67]/100,000 population). A similar trend was observed in the prevalence of ITP, which increased from 3.19 (95% CI, 2.78–3.67) in the 15–25-year age group to 11.25 (95% CI, 9.68–13.08) in the 65–75-year age group and dropped to 7.60 (95% CI, 4.79–12.06)/100,000 population in patients aged 85 years and above (Figure 3).
Figure 3. Incidence and Prevalence of ITP in Algeria by Age. Incidence and prevalence of ITP were assessed per 100,000 persons in the overall patient cohort categorized with respect to age. ITP, immune thrombocytopenia.
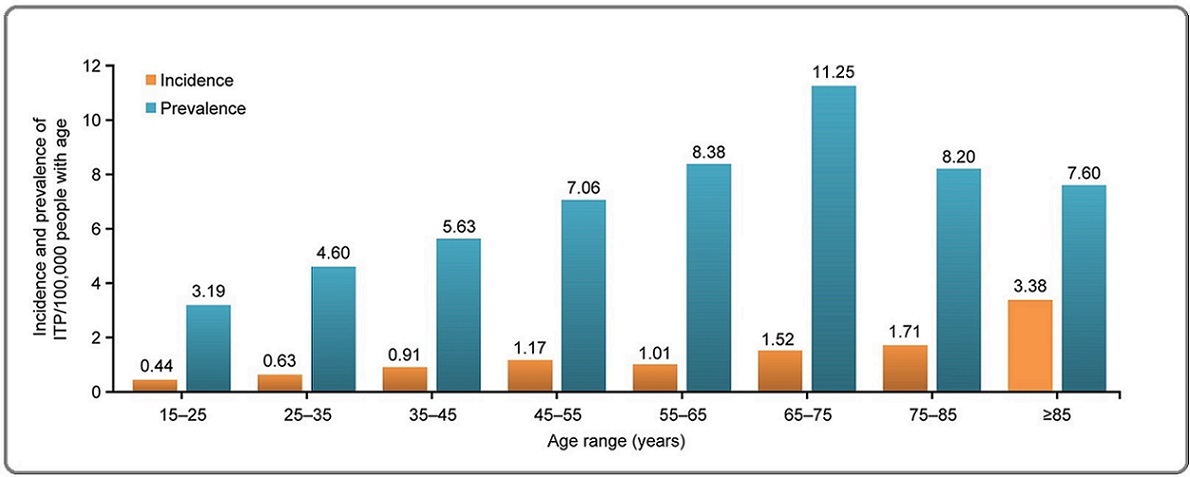
Similarly, the prevalence of ITP was higher in women (8.80 [95% CI, 8.33–9.29]/100,000 population) than in men (2.58 [95% CI, 2.34–2.85]/100,000 population).
Therapeutic strategies used to treat ITP in Algeria
Treatment strategies for ITP differed depending on the patient’s platelet count at the time of diagnosis. In the overall patient cohort, 19.6% (227/1,159) of patients were recommended the watch-and-wait approach. For patients with severe thrombocytopenia and platelet counts ranging from 0 ×103/mm3 to 10 ×103/mm3, corticosteroids (prednisone) at a dose of 1mg/kg/day were administered orally for three weeks as the first-line therapy (80.8% [937/1,159]), followed by IVIGs (3.4% [39/1,159]). Other first-line treatments included rituximab (1.3% [15/1,159]) and anti–Helicobacter pylori (HP) therapy (0.2% [2/1,159]). Corticosteroids were administered to 78.6% (136/173) of incident and 80.6% (795/986) of prevalent patients for up to 6 months. The last or ongoing treatments for ITP for prevalent patients were primarily corticosteroids administered to 72.2% (712/986) of patients, rituximab as second or third-line treatment to 5.7% (56/986) and IVIG to 3.0% (30/986) of patients, TPO-RA (romiplostim) as third-line treatment to 1.9% (19/986), and anti-HP therapy to 0.4% (4/986) of patients. Among prevalent patients, 17.4% (172/986) of patients underwent a splenectomy, mainly as a third-line procedure (Figure 4).
Figure 4. Last/ongoing Treatments for ITP. Data for the last/ongoing treatment were collected from the prevalent patient cohort (n=986) who were undergoing treatment for ITP. HP, Helicobacter pylori; ITP, immune thrombocytopenia.
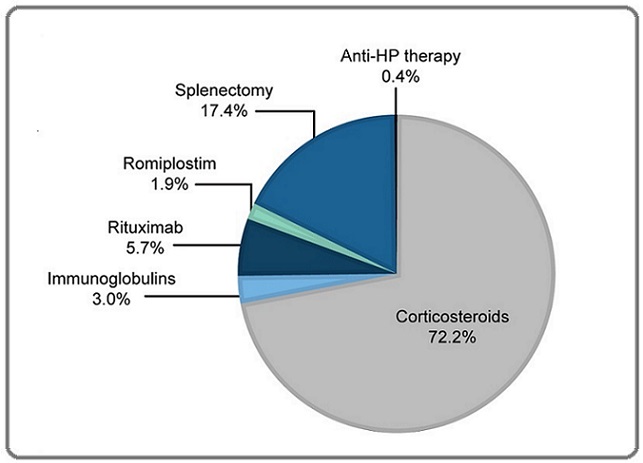
The median (quartiles) duration between ITP diagnosis and splenectomy was 19 (10.9, 35.6) months.
In addition, therapeutic strategies differed slightly depending on the age of the patient. Younger (≤65 years) and older (>65 years) patients received the same first-line treatment; younger patients received rituximab (5.7% [56/986]) as second-line treatment or underwent a splenectomy (17.4% [172/986]) as third-line treatment more often than older patients.
Discussion
This is the first of its kind study conducted on a national scale presenting clinical, biological, and epidemiological data on ITP in Algeria. Therapeutic data from the study reflects the reality of ITP management in Algeria. Our cohort represents a typical population of patients with ITP where most patients were diagnosed with primary ITP (72.9%) and incidental findings were the main reason for ITP diagnosis. At the time of diagnosis, 16.7% of new patients had platelet counts above 50×103/mm3, and 71.7% of patients had very severe thrombocytopenia with platelet counts of 0–30×103/mm3. Among these patients, ITP was most often diagnosed due to hemorrhagic bleeding, characterized by petechiae and bruising.
Epidemiologically, our results show that the median age at diagnosis was 36 years, with 88.7% of the patients aged 65 years or below and a clear predominance of women (77.2%). These data are consistent with that reported in the literature [21], except for a lower age in Algeria, which is because of the overall lower average age of the Algerian population [19]. National incidence (0.85/100,000) as well as national prevalence (5.65/100,000) of ITP are considerably lower than those reported in the literature, where an incidence of 2.68–6.1/100,000 persons was reported from Denmark, France, and the USA and a prevalence of 50.29/100,000 persons was reported from the UK [7, 22-24]. This difference could be due to the high number of incidental ITP diagnoses in Europe and the USA [7, 24], and may also reflect difficulties in the diagnosis of primary ITP in Algeria, which include technical hindrances in immunological or biological examinations (viral serologies and antibody assays) in some medical facilities across the country.
Corticosteroids, immunoglobulins, rituximab (off-label), splenectomy, and other therapies were frequently used to treat ITP. A recently introduced TPO-RA (romiplostim) was used in a small number of patients in Algeria. Thus, all patients either received corticosteroids or, in keeping with current recommendations, received no therapy (watch-and-wait approach) as a first-line treatment [11, 14]. Furthermore, patients with severe thrombocytopenia (platelet count 20–30×103/mm3) who were at a high risk of bleeding were given first-line treatment (mainly corticosteroids) more frequently than those who had a higher platelet count. Additionally, most patients were administered corticosteroids as the last or ongoing therapy for ITP, making corticosteroids the main therapeutic choice for ITP.
Splenectomy was considered the preferred third-line therapy for many years. However, splenectomy may be limited by the risk of surgical complications, an increased risk of thrombosis, and long-term infectious complications [25]. Currently, with the introduction of TPO-RAs, the use of splenectomy has declined and is generally reserved for patients who do not respond to corticosteroids, have profound thrombocytopenia, and have a high risk of bleeding [26]. In our study, splenectomy was performed in 17.4% of prevalent patients, which is comparable with that in other studies where 15–25% of patients with ITP underwent this treatment [26] but is higher than the 5% rate reported in Germany [27].
The use of TPO-RAs has broadened the therapeutic choice for the management of ITP, and clinical studies report promising results with romiplostim and eltrombopag [28]. Romiplostim was used in approximately 2% of the patients in our study due to the late introduction of the drug and was restricted to patients with chronic ITP who had failed three or four lines of treatment, suffered from the disease even after a splenectomy, or were third-line patients for whom splenectomy was contraindicated. Retrospective studies have shown that the use of TPO-RAs in the treatment of incident patients with ITP in daily clinical practice is as effective and safe as in chronic ITP [29, 30]. An ongoing clinical trial is currently assessing this line of treatment in children (NCT03939637). While the current marketing authorization for TPO-RAs in Algeria is limited to splenectomized patients with refractory ITP, changes in the ITP management strategy are expected in the future [31] and further real-life studies are needed to confirm the effect of these changes.
The strengths of our study lie in the large patient cohort and the geographical distribution of patients throughout the country, which ensures a broad representation of all patients within the population. However, the study is limited by its partly retrospective nature, that is, inclusion of prevalent patients with ITP, and unavoidable biases in data collection and patient selection. We included all available data from patients diagnosed with ITP and with a platelet count of ≤100×103/mm3 to minimize bias. Additionally, some health centers refused to participate in the study and patients who had a follow-up in public or private establishments across the country could not be included in the study; hence the total number of patients with ITP could be higher than that reported in this study. Future studies with a longer observation period will be conducted to provide more information on the occurrence of bleeding events in patients with ITP.
Our study describes the therapeutic strategy used in Algeria based on current international guidelines. We will use the data generated by this survey to provide recommendations for the management of ITP in Algeria in keeping with international recommendations [32] in terms of diagnosis and the recruitment profile of our patients. We will also provide suggestions for ITP therapy including a rational approach to use TPO-RAs, which will undoubtedly help in improving the management of patients with ITP in Algeria.
In conclusion, the trends in the epidemiology of ITP in Algeria were similar to those seen globally. Analysis of data from all geographical locations of the country indicate that the incidence and prevalence of ITP were higher in women than in men, and both increased with age. Corticosteroids were the primary therapy and were also the last/ongoing treatment for most of the patients with ITP. Second-line treatments like TPO-RAs were the last ongoing treatment for a minority of the patient population before undergoing third-line treatments such as splenectomy. This study highlights the need for a reduction in the duration of corticosteroid treatment and for an improvement in treatment options like TPO-RAs for adult patients with ITP in Algeria. Additionally, this study necessitates further research exploring the reasons behind lower incidence and prevalence of ITP in Algeria than in occidental countries, the need to establish national guidelines for the diagnosis and treatment of adult patients with ITP, and integration of novel therapies (like TPO-RAs) for the management of ITP in the Algerian population.
Acknowledgments
Editorial support was provided by Utkarsha A Singh, PhD, of Cactus Life Sciences (part of Cactus Communications Pvt Ltd), whose work was funded by Amgen Inc. We thank Susanna Mac, MD, PhD, of Amgen Inc. for providing feedback on the manuscript.
Funding
This work was funded by Amgen Inc.
Conflict of Interest
RT and HS are employees and stakeholders of Amgen. All remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability Statement
Restrictions apply to the datasets: The datasets presented in this article are not readily available because the data may contain information that could compromise research participants’ privacy or consent. Requests to access the datasets should be directed to Prof. Mohamed Amine Bekadja.
Ethics Statement
This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines of the International Conference on Harmonisation (ICH-GCP E6, 7/17/96), and according to the laws and regulations of Algeria. The study protocol, case report form, and the informed consent form were approved by an independent ethics committee and the Ministry of Health of Algeria.
References
- The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA . Blood.2011;117(16). CrossRef
- Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM , Bussel JB , et al . Blood.2009;113(11). CrossRef
- Immune Thrombocytopenia - Current Diagnostics and Therapy: Recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI Matzdorff A, Meyer O, Ostermann H, Kiefel V, Eberl W, Kühne T, Pabinger I, Rummel M. Oncology Research and Treatment.2018;41 Suppl 5. CrossRef
- The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports Terrell DR , Beebe LA , Vesely SK , Neas BR , Segal JB , George JN . American Journal of Hematology.2010;85(3). CrossRef
- Incidence of immune thrombocytopenia in Taiwan: a nationwide population-based study Hung G, Lee C, Yen H, Lin L, Horng J. Transfusion.2018;58(11). CrossRef
- Epidemiology and management of primary immune thrombocytopenia: A nationwide population-based study in Korea Lee JY , Lee J, Lee H, Kang B, Kim J, Kim SH , Lee J, et al . Thrombosis Research.2017;155. CrossRef
- Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France Moulis G, Palmaro A, Montastruc J, Godeau B, Lapeyre-Mestre M, Sailler L. Blood.2014;124(22). CrossRef
- Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database Schoonen WM , Kucera G, Coalson J, Li L, Rutstein M, Mowat F, Fryzek J, Kaye JA . British Journal of Haematology.2009;145(2). CrossRef
- Chronic immune thrombocytopenia in Denmark, Sweden and Norway: The Nordic Country Patient Registry for Romiplostim Christiansen CF , Bahmanyar S, Ghanima W, Risbo N, Ekstrand C, Stryker S, Acquavella J, et al . EClinicalMedicine.2019;14. CrossRef
- Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review Neunert C, Noroozi N, Norman G, Buchanan GR , Goy J, Nazi I, Kelton JG , Arnold DM . Journal of thrombosis and haemostasis: JTH.2015;13(3). CrossRef
- Updated international consensus report on the investigation and management of primary immune thrombocytopenia Provan D, Arnold DM , Bussel JB , Chong BH , Cooper N, Gernsheimer T, Ghanima W, et al . Blood Advances.2019;3(22). CrossRef
- American Society of Hematology 2019 guidelines for immune thrombocytopenia Neunert C, Terrell DR , Arnold DM , Buchanan G, Cines DB , Cooper N, Cuker A, et al . Blood Advances.2019;3(23). CrossRef
- Reporting results of cancer treatment Miller AB , Hoogstraten B, Staquet M, Winkler A. Cancer.1981;47(1). CrossRef
- Immunthrombozytopenie (ITP) Onkopedia Matzdorff A , Holzhauer S , Kühne T , Meyer O , Ostermann H , Pabinger-Fasching I , et al . https://www.onkopedia.com/de/onkopedia/guidelines/immunthrombozytopenie-itp/@@guideline/html/index.html (Accessed November 23, 2023)..2021.
- Safety and efficacy of romiplostim in splenectomized and nonsplenectomized patients with primary immune thrombocytopenia Cines DB , Wasser J, Rodeghiero F, Chong BH , Steurer M, Provan D, Lyons R, et al . Haematologica.2017;102(8). CrossRef
- Efficacy and safety of the thrombopoietin receptor agonist romiplostim in patients aged ≥ 65 years with immune thrombocytopenia Michel M, Wasser J, Godeau B, Aledort L, Cooper N, Tomiyama Y, Khellaf M, Wang X. Annals of Hematology.2015;94(12). CrossRef
- Emerging Concepts in Immune Thrombocytopenia Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG , Jansen AJG . Frontiers in Immunology.2018;9. CrossRef
- Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial Kuter DJ , Bussel JB , Lyons RM , Pullarkat V , Gernsheimer TB , Senecal FM , Aledort LM , et al . Lancet (London, England).2008;371(9610). CrossRef
- Selective validation of the WHO Bleeding Scale in patients with chronic immune thrombocytopenia Fogarty PF , Tarantino MD , Brainsky A, Signorovitch J, Grotzinger KM . Current Medical Research and Opinion.2012;28(1). CrossRef
- Demographie Algerienne 2017 Alger: Office National des Statistiques (2017). http://www.ons.dz/IMG/pdf/Demographie_Algerienne_2017.pdf (Accessed March 20, 2023) .
- Characteristics and management of primary and other immune thrombocytopenias: Spanish registry study Palau J, Sancho E, Herrera M, Sánchez S, Mingot ME , Upegui RI , Rodríguez Salazar MJ , et al . Hematology (Amsterdam, Netherlands).2017;22(8). CrossRef
- Prevalence of diagnosed adult immune thrombocytopenia in the United Kingdom Bennett D , Hodgson ME , Shukla A , Logie JW . Advances in therapy.2011;28(12). CrossRef
- The incidence of idiopathic thrombocytopenic purpura in adults increases with age Frederiksen H, Schmidt K. Blood.1999;94(3).
- Primary immune thrombocytopenia in US clinical practice: incidence and healthcare burden in first 12 months following diagnosis Weycker D, Hanau A, Hatfield M, Wu H, Sharma A, Bensink ME , Chandler D, Grossman A, Tarantino M. Journal of Medical Economics.2020;23(2). CrossRef
- Splenectomy as a curative treatment for immune thrombocytopenia: a retrospective analysis of 233 patients with a minimum follow up of 10 years Vianelli N , Palandri F , Polverelli N , Stasi R , Joelsson J , Johansson E , Ruggeri M , et al . Haematologica.2013;98(6). CrossRef
- Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years Palandri F, Polverelli N, Sollazzo D, Romano M, Catani L, Cavo M, Vianelli N. American Journal of Hematology.2016;91(4). CrossRef
- Disease management of patients with immune thrombocytopenia-results of a representative retrospective survey in Germany Kubasch AS , Kisro J, Heßling J, Schulz H, Hurtz H, Klausmann M, Ehrnsperger A, Willy C, Platzbecker U. Annals of Hematology.2020;99(9). CrossRef
- Eltrombopag versus romiplostim in treatment of adult patients with immune thrombocytopenia: A systematic review incorporating an indirect-comparison meta-analysis Zhang J, Liang Y, Ai Y, Li X, Xie J, Li Y, Zheng W, He R. PloS One.2018;13(6). CrossRef
- Efficacy and safety of eltrombopag in persistent and newly diagnosed ITP in clinical practice González-López TJ , Fernández-Fuertes F , Hernández-Rivas JA , Sánchez-González B , Martínez-Robles V , Alvarez-Román MT , Pérez-Rus G , et al . International journal of hematology.2017;106(4). CrossRef
- Romiplostim in adult patients with newly diagnosed or persistent immune thrombocytopenia (ITP) for up to 1 year and in those with chronic ITP for more than 1 year: a subgroup analysis of integrated data from completed romiplostim studies Kuter DJ , Newland A, Chong BH , Rodeghiero F, Romero MT , Pabinger I, Chen Y, et al . British Journal of Haematology.2019;185(3). CrossRef
- Comparison of treatments for persistent/chronic immune thrombocytopenia: a systematic review and network meta-analysis Arai Y, Matsui H, Jo T, Kondo T, Takaori-Kondo A. Platelets.2019;30(8). CrossRef
- International consensus report on the investigation and management of primary immune thrombocytopenia Provan D, Stasi R, Newland AC , Blanchette VS , Bolton-Maggs , Bussel JB , Chong BH , et al . Blood.2010;115(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details