The Expression of Ki-67 and E-cadherin in Triple Negative Breast Cancer: A Hospital Based Study
Download
Abstract
Introduction: Triple negative breast cancer (TNBC) is a subtype of breast cancer with aggressive nature as it does not respond to targeted chemotherapeutics. Expression of biomarkers is considered as an important factor for selecting treatment strategies and assessing prognosis.
Objective: The aim of this study was to assess the expression of Ki-67 and E–cadherin in TNBC tissue.
Methods: Immunohistochemical staining was done to assess the expression of proliferative marker Ki-67 and junctional complex marker E-cadherin in the Formalin Fixed Paraffin Embedded tissue (FFPE) of 80 TNBC patients. Ki-67 positive cells were identified on the basis of brown staining nuclei and E-cadherin expressed cells were considered positive on the basis of brown staining cell membrane. Ki-67 positive TNBCs were divided into low and high Ki-67 groups.
Results: Ki-67 was expressed in 65% of the TNBC, the median of Ki-67 index was 30.25%. The difference between the medians of Ki-67 expression of low and high groups (7.5% and 53.25% respectively) was highly significant (p = 0.000). E-cadherin was negative in 67.5% of TNBC patients. The mean (±SD) age at diagnosis was 43.79 (±9.10) years. Most of the cancers were invasive ductal cell carcinoma (95%) and of grade II (70%). Lymph node metastasis was present in 73.8% patients and in 51% cases the cancer was in the left breast. Ki-67 expression was significantly associated with lymph node metastasis and E-cadherin expression was more negative in the left sided cancer.
Conclusion: The study signifies that the TNBC has an early age at onset and Ki-67 expression is significantly high and associated with lymph node metastasis pointing the importance of this biomarker in TNBC patients. Negative expression of E-cadherin suggests close monitoring of the left sided TNBC.
Introduction
TNBC is a molecular subtype of breast cancer, where hormone receptors and human epidermal growth factor receptor 2 (Her2) are negative [1]. It occurs relatively in young ages and it does not respond to endocrine therapy or targeted therapy, it is extremely malignant with a high risk of distant metastasis [2]. Biological markers play an important role in choosing therapeutic options and to assess the prognosis of the patients with TNBC.
Various biological markers have been used in cancer patients for evaluating the treatment and assessing the prognosis. Ki-67 and E-cadherin are considered as the most promising markers [3]. Ki-67 is a non-histone nuclear protein discovered by Gerdes and colleagues in 1980 [4]. Its gene is located on the long arm of human chromosome 10q26 [5]. It is associated with cellular proliferation. Though the protein is expressed in all phases of cell cycle except G0 (quiescent) phase, its cellular location and levels of expression varies in different phases of cell cycle. The expression is low in G1 and early S phase, increased in mitosis; it is high in metaphase and is decreased again in anaphase and telophase. Moreover, Ki-67 has short half-life. These properties make it a suitable biomarker for assessing cell cycle phase regulation [3]. The international Ki-67 in Breast Cancer Working Group (IKWG) has suggested Ki-67 for the measurement of proliferation in standard clinical practice and also in research [6]. Researchers have shown a promising role of Ki-67 expression in TNBC for selection of chemotherapeutics, assessing prognosis and prediction of survival [7]. In the laboratory, Ki-67 protein is assessed by immunohistochemistry (IHC). The marker is reported as Ki-67 index which means the percentage of Ki-67 expression in tumor cells.
E-cadherin is a cell adhesion molecule and is expressed in all epithelial cells. It suppresses cell proliferation, invasion and metastasis. It is a transmembrane glycoprotein. It has intracytoplasmic, transmembrane and extracellular domains. The cytoplasmic domain acts as a junctional/ structural protein and also acts as a signal transducer [8]. E-cadherin regulates the cellular motility and invasion during the epithelial-mesenchymal transition process [9]. Loss of E-cadherin is an indicator of cell transition, which is associated with carcinogenesis. Negative or loss of expression of this molecule is associated with large tumor size, high tumor grade, and lymph node metastasis in breast cancer. E-cadherin plays an important role as a predictor of prognosis in the clinical management of patients with breast cancer [9].
Breast cancer occupied the first position (28.3%) among the cancers occurring in females in Bangladesh [10]. A substantial portion of this cancer is TNBC. In a study conducted on Bangladeshi breast cancer patients found about 60% of the breast cancers were of TNBC type [11]. Evaluation of prognostic and predictive factors is very important for TNBC patients for identification of persons who are at high risk of development and recurrence of cancer. For this purpose, research on biomarkers especially Ki-67 and E-cadherin expressions and their role in selecting chemotherapy for TNBC patients and prediction of survival is important. Adequate information regarding the expression of Ki-67 and E-cadherin in Bangladeshi TNBC patients is not available yet. This research was aim to assess the expression of Ki- 67 and E-cadherin in FFPE TNBC tissue of Bangladeshi females for predicting prognosis and to create a database for further research.
Materials and Methods
Sample collections
This cross sectional descriptive study was conducted in the Department of Anatomy of Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka from January 2021 to July 2022. Eighty immunohistochemically diagnosed TNBC patients were selected from the National Institute of Cancer Research and Hospital (NICRH), Dhaka. TNBC patients diagnosed during January 2021 to March 2022 were invited to participate in this study over telephone because of the COVID-19 pandemic. Bengali version of the informed consent form was read out over telephone and the agreed patients who met the inclusion and exclusion criteria were selected for the study. FFPE TNBC tissue blocks of the selected patients were collected from the Department of Histopathology, NICRH. The FFPE blocks were prepared from core-cut biopsy, incisional biopsy or excision of specimen. Cancer related information of the patients was collected by interviewing patients and from hospital records.
Immunohistochemical staining
The tissue blocks were cut into four micrometer (µM) thick slice and taken on adhesive glass slides. The slides were allowed to incubate for 16 hours at 37ºC temperature. Deparaffinization and clearing were performed subsequently. After that, antigen retrieval was done with Dako target retrieval solution in a conventional water bath. In this step, the coplin jars were kept into the water bath at 95°C for 40 minutes. After that the slides were rinsed with TBS (Tris buffer solution) for 5 minutes and placed in a moist box. The slides were incubated in 100-150µL PRB (peroxidase blocking reagent) for 10 minutes and placed again in the moist box. After that the slides were incubated in diluted ready to use antibodies for 30 minutes (FLEX monoclonal Mo A Hu Ki-67 Antigen, MIB-1, Dako, Denmark) in the moist box at room temperature. Staining and counterstaining was done with Dako EnVision + Dual link system-HRP (DAB+) (Dako, Denmark, Expiry date August 31, 2023) and hematoxylin subsequently. Then the slides were dehydrated and repeated clearing were performed. The slides preparation was completed by mounting with DPX.
Assessing the expressions of Ki-67 and E-cadherin
Ki-67 positive cells were identified on the basis of brown staining nuclei (Figure 1).
Figure 1. Photomicrograph of the TNBC Tissue Showing both Positive Expression (marked by green arrows) and Negative Expression (marked by orange arrows) of Ki-67.
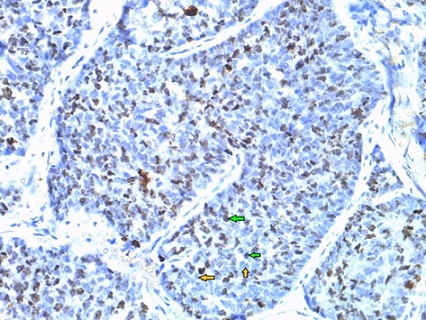
Intensity of the positive staining cell was not relevant. First the slides were observed under x40 and x100 objective lens respectively. Then Ki-67 counting was performed in ‘type writer pattern’ using an ocular grid under x400. The counting was done with two distinct methods, either hot spot (one field with highest Ki-67 stained cells) or global (four representative fields of Ki-67 stained cells) methods. In hot spot method, the area with highest number of ki-67 positive nuclei was selected and at least 500 cancer cell nuclei were counted and the Ki-67 expressed nuclei among these 500 cells were also counted. The Ki-67 score or index was expressed as the percentage of Ki-67 expressed cancer cells. If the hot spot was unavailable, then global method was followed. Ki-67 expressed cells were counted from four selected fields containing highest Ki-67 expressed cells. At least 100 cancer cells in each of the positive hot spots were counted. The Ki-67 positive stained nuclei among these cells were counted and divided by the total number of counted cancer cells under high power magnification x400. Ki-67 index was expressed as the percentage of Ki-67 positive cells. Ki-67 positive cancer patients were divided into high Ki-67 group when the index was >30 and low Ki-67 group when the index was ≤30 [7].
E-cadherin expression was considered positive on the basis of brown color staining of the cell membrane (Figure 2).
Figure 2. Photomicrograph of the TNBC Tissue Showing the Positive Expression of E-cadherin (shown in green arrow) (at x400 magnification)..
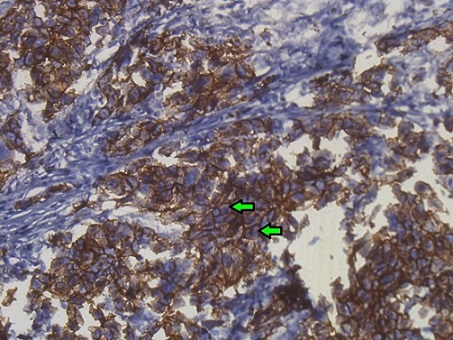
Positive and negative score were dependent on the staining intensities. The scored was done as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining). For each sample the score had obtained by multiplying each case. The final score 0-1 were considered as negative and 2-9 were as positive. Two independent histopathologists were evaluated the percentage of positive expression of Ki-67 and E-cadherin and the average value was used in statistical analysis.
Both of the histopathologists were blinded to the cancer related characteristics.
Data analysis
Statistical analysis was done using IBM SPSS software 26 version (IBM Corporation). Chi-square (χ2) test and Fisher’s exact test were done for analyzing the association between Ki-67 and E-cadherin expressions and with tumor-related characteristics of TNBC patients. Shapiro-Wilk’s normality test was done for assessing the distribution of data. All statistical tests were two sided and p-value ˂ 0.05 considered as statistically significant.
Ethical Implication
All selected patients were informed that their FFPE tissue blocks will be used for research purpose. They were also informed that they have the right to withdraw their participation from the study at any time. The study was approved by the Institutional Review Board of BSMMU and NICRH, Bangladesh. A memorandum of understanding (MOU) was also signed by the concerned persons of the above-mentioned institutions.
Results
Ki-67 and E-cadherin expressions were assessed in 80 TNBC patients (Table 1).
| Biomarker expression status | Number (Percentage) | p value |
| Ki-67 expression | ||
| Positive | 52 (65) | |
| >30% Ki-67 index | 26 (32.5) | |
| Median (range) | 53.25 (31-83) | |
| ≤30%. Ki-67 index | 26 (32.5) | |
| Median (range) | 7.5 (5-30) | 0.00 (SS*) |
| Negative | 28 (35) | |
| E-cadherin expression | ||
| Positive | 26 (32.5) | |
| Negative | 54 (67.5) |
*SS, Statistically significant
Ki-67 was expressed in 52 (65%) TNBC patients; the median of expression was 30.25 (range 5 to 83). The half of the Ki-67 positive patients (26 out of 52) had Ki-67 index >30%. The difference of medians of low and high Ki-67 expressed group (7.5 and 53.25 respectively) was highly significant (p = 0.000).
E-cadherin was negative in 54 (67.5%) patients.
Tumor related characteristics of the TNBC patients were are summarized in Table 2.
| Tumor related characteristics | Number (percentage) |
| Age at diagnosis (years) | |
| 26 - 35 | 14 (17.50) |
| 36 - 45 | 39 (48.75) |
| 46 - 55 | 18 (22.50) |
| 56 - 70 | 9 (11.25) |
| Mean ± SD | 43.79 ± 9.10 |
| Family history of breast cancer | |
| Positive | 25 (31.20) |
| Negative | 55 (68.80) |
| Lymph node metastasis | |
| Yes | 59 (73.75) |
| No | 21 (26.25) |
| Distant metastasis | |
| Yes | 16 (20) |
| No | 64 (80) |
| Histological type | |
| Invasive ductal carcinoma | 76 (95) |
| Other carcinoma | 4 (5) |
| Histological grade | |
| I | 5 (6.25) |
| II | 56 (70) |
| III | 15 (18.75) |
| Not applicable* | 4 (5) |
| Laterality of the TNBC | |
| Right | 38 (47.50) |
| Left | 41 (51.25) |
| Bilateral | 1 (1.25) |
| Outcome of the TNBC patient | |
| Alive | 73 (91.25) |
| Dead | 7 (8.75) |
*Chemotherapy started before grading
The age range of the patients was from 26 to 70 years and the mean (±SD) age at diagnosis was 43.79 (±9.10) years. Most of the cancers (95%) were diagnosed as invasive ductal cell carcinoma and histological grade II was reported in 70% of patients. Most of the patients (73.8%) had lymph node metastasis and distant metastasis was reported in 20% of patients. Left sided breast cancer was reported in 41 patients.
Statistical analysis showed that Ki-67 expression was significantly associated with lymph node metastasis (p = 0.002) (Table 3).
| Tumor related characteristics | Ki-67 expression | p value | |
| Low (≤30% index) | High (>30% index) | ||
| (n = 26) | (n = 26) | ||
| Age at diagnosis (years) | |||
| 26 – 35 | 4 | 2 | |
| 36 – 45 | 15 | 14 | |
| 46 –55 | 5 | 7 | |
| 56 – 65 | 2 | 3 | 0.75 (NS*) |
| Family history of breast cancer† | |||
| Positive | 12 | 6 | |
| Negative | 14 | 20 | 0.72 (NS*) |
| Lymph node metastasis† | |||
| Present | 24 | 14 | |
| Absent | 2 | 12 | 0.002 (SS**) |
| Distant metastasis† | |||
| Present | 7 | 4 | |
| Absent | 19 | 22 | 0.25 (NS*) |
| Histological type† | |||
| Invasive ductal carcinoma | 25 | 23 | |
| Other carcinoma | 1 | 3 | 0.30 (NS*) |
| Histological grade | |||
| I | 1 | 2 | |
| II | 20 | 16 | |
| III | 5 | 5 | 0.29 (NS*) |
| Not graded | 0 | 3 | |
| Laterality of the TNBC† | |||
| Right | 10 | 14 | |
| Left | 16 | 12 | 0.20 (NS*) |
*NS, non-significant; **SS, Statistically significant; †Fisher’s Exact test was done.
E-cadherin expression was significantly (p = 0.02) negative in left sided TNBC than that of the right sided cancer (Table 4).
| Tumor related characteristic | E-cadherin expression | p value | |
| Positive (n = 26) | Negative (n = 54) | ||
| Age at diagnosis (years) | |||
| 26 – 35 | 3 | 11 | |
| 36 – 45 | 13 | 26 | |
| 46 - 55 | 7 | 11 | |
| 56 – 70 | 3 | 6 | 0.79 (NS*) |
| Family history of breast cancer† | |||
| Positive | 11 | 14 | |
| Negative | 15 | 40 | 0.11 (NS*) |
| Lymph node metastasis† | |||
| Yes | 17 | 42 | |
| No | 9 | 12 | 0.18 (NS*) |
| Distant metastasis† | |||
| Yes | 4 | 12 | |
| No | 22 | 42 | 0.34 (NS*) |
| Histological type† | |||
| Invasive ductal carcinoma | 24 | 52 | |
| Other carcinoma | 2 | 2 | 0.39 (NS*) |
| Histological grade | |||
| I | 1 | 4 | |
| II | 18 | 38 | |
| III | 5 | 10 | |
| Not done | 2 | 2 | 0.82 (NS*) |
| Laterality of the TNBC | |||
| Right | 18 | 20 | |
| Left | 8 | 33 | 0.02 (SS**) |
| Bilateral | 1 |
*NS, non-significant; **SS, Statistically significant; †Fisher’s Exact test was done.
No association was observed between Ki-67 and E-cadherin expression (Table 5).
| E-cadherin expression | Ki-67 expression | p value | |
| Positive (n = 52) | Negative (n = 28) | ||
| Positive (n = 26) | 18 | 8 | |
| Negative (n = 54) | 34 | 20 | 0.39 (NS*) |
| Total (n = 80) | 52 | 28 |
Discussion
TNBC is an aggressive type of breast cancer; it has the potential to metastasize to the central nervous system and lung through hematogenous route [12]. Chemotherapy is considered as highly effective treatment modality of this cancer. However, the recurrence rate is high in TNBC even with appropriate chemotherapy, indicates to investigate the special biological nature of this cancer [12]. Study of molecular behavior of TNBC is necessary to improve further treatment efficacy [2]. Several biological markers are used in TNBC to assess the therapeutic response and prognosis of the disease. Among the markers, Ki-67 and E-cadherin are the widely investigated indicators.
Although Ki-67 is used as an indicator of TNBC prognosis, it is still a topic of debate among the cancer researchers. Many scientists conducted studies to investigate the relationship between Ki-67 and chemosensitivity and prognosis of TNBC. Different studies have diverse conclusions which favor the debate is still open and more studies on different populations can contribute in this field. The cutoff point of Ki-67 value is also a topic of discussion. In healthy breast tissue Ki-67 expression index is less than 3%. Breast Cancer Research Foundation (BCRF) established the international Ki-67 breast cancer working group in 2009. BCRF determined and updated the cutoff range of less than 5% to more than 30% [6]. The St. Gallen International Expert consensus panel adopted high ki-67 value as ≥20% [13] . The most of the scientists has considered less than 10% as low, 10-20% as intermediate and >20% index as high [14, 15]. The baseline values of Ki-67 are much higher in TNBC than in the luminal tumors. In TNBC Ki-67 cutoff value is diverse and controversial; it varies from 10% to 61% in the literature. Researchers considered different cutoff values in their researches on Ki-67 in TNBC [7]. For resolving the controversy, Zhu et al. (2020) [7] solved this issue by defining Ki-67high patients when the index is >30% and Ki-67low patients at ≤30% index of Ki-67 expression. In our study, we observed the median value of Ki-67 expression was 30.25 with a range from 5 to 83. We categorized the Ki-67 expressed patients as Ki- 67 low and Ki-67 high groups on the basis of 30% index threshold and the difference of the medians of this high and low groups is highly significant (p = 0.000).
A study conducted on 285 TNBC patients in China found Ki-67 was positive in 53.33% patients and the patients with high Ki-67 expression were more sensitive to chemotherapy and displayed high recurrence rate [2]. In our study, Ki-67 was expressed in 65% of TNBC, which is higher than the above-mentioned study but is lower than another Chinese study that observed 83.3% of Ki-67 index in 24 TNBC [16]. Ricciardi et al. (2015) [3] found ≥ 20% Ki-67 index in 37.7% of 45 TNBC patients and positive expression of this marker is associated with poor outcome. On the other hand Arafah et al (2021) [17] observed the median value of Ki-67 expression was 70% in 51 TNBC patients in Saudi Arabia. They observed a significant correlation of Ki-67 expression with lymph node metastases, tumor invasion, high nuclear grade, clinical stage, adverse survival outcome, and failure to achieve pathological complete response. Nurses’ health study, an ongoing prospective cohort study analyzed 2,555 subtypes of breast tumor and Ki-67 expression score was reported the highest (>20.6%) in the TNBC than the other subtypes [18]. Ki-67 expression is significantly correlated with lymph node involvement [16]. In our study, the median of Ki-67 index of high Ki-67 group is 53.25% and lymph node metastasis is significantly associated with the expression of Ki-67.
A study conducted on 141 TNBC patients in India observed >10% Ki-67 expression index in 63.12% patients and its expression is significantly associated with nuclear grades III tumors [14]. They did not observe any significant association of Ki-67 expression with age, histological types, tumor size, site or laterality, lymph node or distant metastasis. Our study result is almost similar to the Indian study except tumor grade association with Ki-67 expression, but we observed a significant association of Ki-67 expression with lymph node metastasis.
E-cadherin expression was negative in 67.5% patients in our study which is close to the finding of Ricciardi et al., (2015)[3] who observed negative expression of E-cadherin in 58% of TNBC patients. Shen et al. (2016) [19] found reduced expression of E-cadherin in 56% of TNBC patients which also support our observation. Liu et al. (2017) [20] observed E-cadherin loss in 31.6% of TNBC patients which is almost half than that of our finding. Kashiwagi et al., (2010) [21] found low expression of E-cadherin was associated with poor outcome and lymph node metastasis. We observed significantly lower expression of E-cadherin in left sided TNBC than that of the right. We did not find any significant association between the expression of E-cadherin with age at onset of TNBC, histological type and grade of tumor, lymph node or distant metastases. Shetty and Rao (2019)[14] observed E-cadherin loss is associated with lymph node involvement. They did not find any correlation between the expressions of Ki-67 and E-cadherin; our finding is consistent in this aspect.
TNBC patients are usually younger than the patients with other types of breast cancer. The age of the TNBC patients varies according to geographic location and population. An Italian study reported the median age of TNBC patients was 58.8 years [3]. Nurses’ Health study observed the mean age at onset of TNBC in Ki-67 index ≥14% and Ki-67 index ˂14% positive patients was 60 years and 58.9 years respectively in a study involving 2653 African Americans and White Americans nurses [18]. The median age of the Arab TNBC patients was 52 years [17] and the mean age of the Chinese patients was 52.8 years [20]. An Indian study reported the mean age of TNBC patients was 47 years [14]. Our TNBC patients were the youngest (mean age 43.79 years) than the patients participated in those studies. Studies revealed that the Bangladeshi breast cancer patients are relatively younger than the patients from other part of Asia. Amee et al. (2023) [11] found the mean age of Bangladeshi breast cancer patients was 45.12 years, Khan et al. (2021) [22] reported the mean age as 48.7 years, Chowdhury et al. (2020) [23] and Nishat et al. (2019) [24] observed the mean age as 44.66 and 44.64 years respectively. These findings indicate that the Bangladeshi females are more prone to develop TNBC in their early ages.
Our study has some limitation. We did not follow up the patients to evaluate the prognosis or response to therapy. Thus, though we cannot conclude on aggressiveness of TNBC in Bangladeshi population from our finding. Our research was a cross-sectional study, after selecting the patients from histological report; we found seven cancer related death of the listed TNBC patients shortly after diagnosis. This evidence of seven (8.75%) death in one and half a year supports the aggressiveness of TNBC in our population.
In conclusion, this study indicates that the TNBC has an early age at onset and Ki-67 expression is associated with lymph node metastasis pointing its importance in prognosis of the TNBC patients. Negative expression of E-cadherin suggests close monitoring and follow up might be needed in left sided TNBC. Therefore, the assessment of Ki-67 and E-cadherin routinely in TNBC tissue would be helpful for evaluating the prognosis of this cancer patient.
Acknowledgements
We would like to acknowledge the authority of BSMMU for funding support of this study (Research Grant for Residents, BSMMU). We convey our thanks to the Department of Anatomy, BSMMU and the National Institute of Cancer Research and Hospital (NICRH), Bangladesh for providing the infrastructure, and other research facilities. We also express our gratitude to the patients participated in our research.
Funding statement
The study was funded by the Research Grant for Residents, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Statement conflict of Interest
The authors declare no conflict of interest.
References
- Molecular classification and molecular forecasting of breast cancer: ready for clinical application? Brenton JD , Carey LA , Ahmed AA , Caldas C. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2005;23(29). CrossRef
- The prognostic relevance of p53 and Ki-67 to chemotherapy sensitivity and prognosis in triple-negative breast cancer Zhang G, Shi Z, Liu L, Yuan H, Pan Z, Li W, Tao Y, et al . Translational Cancer Research.2021;10(2). CrossRef
- Androgen Receptor (AR), E-Cadherin, and Ki-67 as Emerging Targets and Novel Prognostic Markers in Triple-Negative Breast Cancer (TNBC) Patients Ricciardi GRR , Adamo B, Ieni A, Licata L, Cardia R, Ferraro G, Franchina T, Tuccari G, Adamo V. PloS One.2015;10(6). CrossRef
- Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67 Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, et al . The American Journal of Pathology.1991;138(4).
- The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins Schlüter C, Duchrow M, Wohlenberg C, Becker MH , Key G, Flad HD , Gerdes J. The Journal of Cell Biology.1993;123(3). CrossRef
- Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group Nielsen TO , Leung SCY , Rimm DL , Dodson A, Acs B, Badve S, Denkert C, et al . Journal of the National Cancer Institute.2021;113(7). CrossRef
- The prognostic and predictive potential of Ki-67 in triple-negative breast cancer Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, Di G, et al . Scientific Reports.2020;10(1). CrossRef
- Regulation of cadherin-mediated adhesion in morphogenesis Gumbiner BM . Nature Reviews. Molecular Cell Biology.2005;6(8). CrossRef
- E-cadherin deregulation in breast cancer Corso G, Figueiredo J, De Angelis SP , Corso F, Girardi A, Pereira J, Seruca R, et al . Journal of Cellular and Molecular Medicine.2020;24(11). CrossRef
- The Global Cancer Observatory (2021) ‘Cancer Fact Sheet Bangladesh’, 745, pp. 1–2 .
- ‘Expression of BRCA1 mRNA in FFPE tissue breast cancer’ Amee SS , Nishat LRS , Arjuman F, , Yesmin ZA , Laboni UH TR , Akther Z. Cancer Journal of Bangladesh.2022;3(2):55-62.
- Metastatic behavior of breast cancer subtypes Kennecke H, Yerushalmi R, Woods R, Cheang MCU , Voduc D, Speers CH , Nielsen TO , Gelmon K. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(20). CrossRef
- Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013 Goldhirsch A, Winer EP , Coates AS , Gelber RD , Piccart-Gebhart M, Thürlimann B, Senn HJ . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2013;24(9). CrossRef
- Expression of E cadherin and Ki 67: Emerging Prognostic Markers in Triple-Negative Breast Cancer Shetty J, Rao C. Indian Journal of Surgical Oncology.2019;10(2). CrossRef
- Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on breast cancer excision whole sections: an international multicentre collaboration Leung SCY , Nielsen TO , Zabaglo LA , Arun I, Badve SS , Bane AL , Bartlett JMS , et al . Histopathology.2019;75(2). CrossRef
- Ki67 as a predictor of poor prognosis in patients with triple-negative breast cancer Li H, Han X, Liu Y, Liu G, Dong G. Oncology Letters.2015;9(1). CrossRef
- KI-67 LI Expression in Triple-Negative Breast Cancer Patients and Its Significance Arafah MA , Ouban A, Ameer OZ , Quek KJ . Breast Cancer: Basic and Clinical Research.2021;15. CrossRef
- Assessment of Ki67 expression for breast cancer subtype classification and prognosis in the Nurses’ Health Study Healey MA , Hirko KA , Beck AH , Collins LC , Schnitt SJ , Eliassen AH , Holmes MD , Tamimi RM , Hazra A. Breast Cancer Research and Treatment.2017;166(2). CrossRef
- Prognostic Value of E-Cadherin and β-Catenin in Triple-Negative Breast Cancer Shen T, Zhang K, Siegal GP , Wei S. American Journal of Clinical Pathology.2016;146(5). CrossRef
- E-cadherin expression phenotypes associated with molecular subtypes in invasive non-lobular breast cancer: evidence from a retrospective study and meta-analysis Liu J, Feng C, Deng M, Ge D, Liu D, Mi J, Feng X. World Journal of Surgical Oncology.2017;15(1). CrossRef
- Significance of E-cadherin expression in triple-negative breast cancer Kashiwagi S, Yashiro M, Takashima T, Nomura S, Noda S, Kawajiri H, Ishikawa T, Wakasa K, Hirakawa K. British Journal of Cancer.2010;103(2). CrossRef
- P15-2 Risk factors and barriers of early breast cancer diagnosis and treatment outcome in Bangladesh Khan A, Akhtar P, Ali M, Khatun N, Alam M, Rahman M, Hossen N, et al . Annals of Oncology.2021;32. CrossRef
- Mutation in Exon2 of BRCA1 Gene in Adult Bengali Bangladeshi Female Patients with Breast Cancer: An Experience from Two Tertiary-Care Hospitals Chowdhury SS , Khatun M, Khan TH , Laila AB . Asian Pacific Journal of Cancer Prevention : APJCP.2020;21(8). CrossRef
- Identification of Mutation in Exon11 of BRCA1 Gene in Bangladeshi Patients with Breast Cancer Nishat L, Yesmin ZA , Arjuman F, Rahman SHZ , Banu LA . Asian Pacific Journal of Cancer Prevention : APJCP.2019;20(11). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details