Overexpression of Mammaglobin-A in Primary Breast Tissue Tumor and High Concentration of mRNA Mammaglobin-A in Peripheral Blood as Risk Factors for Metastatic Breast Cancer
Download
Abstract
Objective: There is still limited evidence for the use of biomarkers in breast cancer. However, mammaglobin-A in tissue and blood has recently been found as a promising biomarker for detecting metastases. Therefore, this study will examine the overexpression of mammaglobin-A in primary breast tissue tumors and the elevated concentration of mammaglobin-A messenger RNA (mRNA0 in peripheral blood as potential risk factors for breast cancer metastasis.
Methods: The study was conducted at Sanglah General Hospital in Bali, Indonesia, from July 2017 to March 2018. It employed a hybrid research design, combining both cross-sectional (n = 70) and case-control (metastasis = 20 and non-metastasis = 20) approaches. Peripheral blood samples were collected using specialized L6 tubes and were analyzed for the expression of mammaglobin-A mRNA using quantitative real-time PCR. The diagnosis of breast cancer was established through open biopsy, considered the gold standard. Biopsy specimens underwent histopathological examination and standard immunohistochemistry (IHC) analysis to assess ER, PR, HER2, and Ki67 markers. The evaluation of mammaglobin-A protein expression was conducted via IHC at the Anatomical Pathology Laboratory of Prima Medika Hospital.
Result: The mean mammaglobin-A mRNA level in the metastatic group was 11.59±1.37, while in the non-metastatic group was 8.17±1.27-fold change relative to the housekeeping gene beta microglobulin. The mean mammaglobin-A mRNA levels in the two groups were significantly different (p<0.05). Overexpression of mammaglobin-A in cancer tissue was 7.36 times more likely for metastasis compared to non-metastatic (OR = 7.36; 95% CI = 1.34-40.55; p = 0.013). Additionally, mammaglobin-A mRNA concentration was found to be nine times higher for breast cancer metastasis compared to the non-metastatic group (OR = 9.00; 95% CI = 2.15-37.66; p = 0.002).
Conclusion: The mean mammaglobin-A level in the metastatic group significantly differs from that in the non-metastatic group. Overexpression of mammaglobin-A and a high concentration of Mammaglobin-A mRNA are influential risk factors for metastatic breast cancer.
Introduction
Breast cancer is a significant global health problem, with a high rate of morbidity and mortality. Globocan (2018) showed that there were 2.1 million new cases of breast cancer annually worldwide [1]. In Indonesia, data showed that the incidence rate of breast cancer was 42.1 per 100,000 women with a mortality rate of 17 per 100,000 women [2]. There was no exact data of breast cancer cases in Bali, but around 70% of patients were found in an advanced stage [3, 4].
The metastasis of breast cancer is initiated by the mesenchymal-epithelial transition of breast epithelial cells to a mutated type [5, 6]. The occurrence of metastasis might worsen the prognosis. Thus, recognizing the metastasis process early into the disease progression would be a valuable step in further managing breast cancer. However, the initial process, which is known as the micrometastasis process, is still difficult to be detected even by the existing marker [7]. Further efforts are needed to find other molecular prognostic factors for early detection of metastasis.
Mammaglobin-A, a breast-specific 93-amino acids, is overexpressed in most breast carcinomas [8]. Very little has been explored about its potential as a promising biomarker for breast cancer progression detection [9, 10]. Mitas et al., 2001 [11] reported that from 12 associated breast cancer genes, the most accurate diagnostic marker was mammaglobin-A (99.6%), followed by prolactin-inducible protein (PIP) (93.3%), cytokeratin 19 (CK19) (91.0%), mammaglobin B (mamB) (87.9%), mucin 1 (muc1) (81.5%) and carcinoembryonic antigen (CEA) (79.4.0%). Several studies have shown that the serum level of mammaglobin-A was sensitive enough to detect breast cancer circulating tumor cells (CTC) [12-14]. This clinical biomarker could detect breast cancer metastasis greater than 0.2 mm2 [15]. In addition, several studies have shown the relationship between mammaglobin-A expression and clinical prognostic parameters of breast carcinoma [16-18]. However, the prognostic role of mammaglobin-A in breast carcinoma is still debated.
Conflicting studies have arisen regarding the impact of mammaglobin-A expression on cancer behavior and prognosis [19-22]. While some studies have linked higher mammaglobin-A levels to better prognosis and clinicopathological parameters, others have reported the opposite trend [23, 24]. Notably, in metastatic breast cancer, positive mammaglobin-A expression has been associated with a high prevalence of bone metastasis [19]. These findings reinforce the potential of mammaglobin-A as a biomarker for breast cancer progression. However, there is a dearth of research in Indonesia examining the role of mammaglobin-A mRNA in peripheral blood or its expression in primary tumors concerning the occurrence of distant metastasis in breast cancer patients. Therefore, this study aims to address this gap and investigate whether higher levels of mammaglobin-A mRNA in peripheral blood or its expression in primary tumors are indicative of an increased risk of developing breast cancer metastasis.
Materials and Methods
Study Design
This study was done by combining a cross-sectional and case-control design. The cross-sectional study aims to determine the difference in the average level of Mammaglobin-A mRNA in peripheral blood between metastatic and non-metastatic breast cancer patients while also determining an optimal cutoff point (Figure 1).
Figure 1. Cross-sectional Study Design.
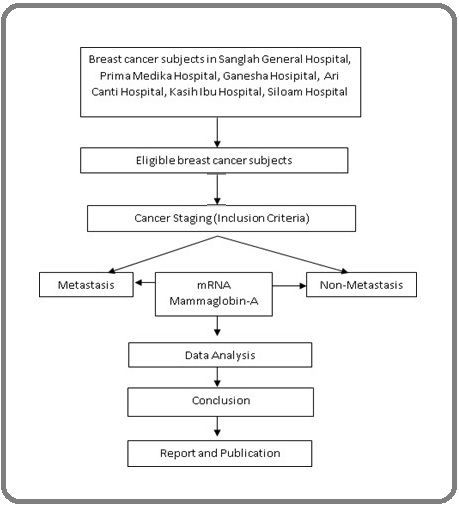
Then, after a cutoff point was obtained, a case-control study involving 40 patients selected from a cross-sectional sample consisting of 20 people as a case group (metastasis) and 20 people as a control group (nonmetastatic) was conducted.
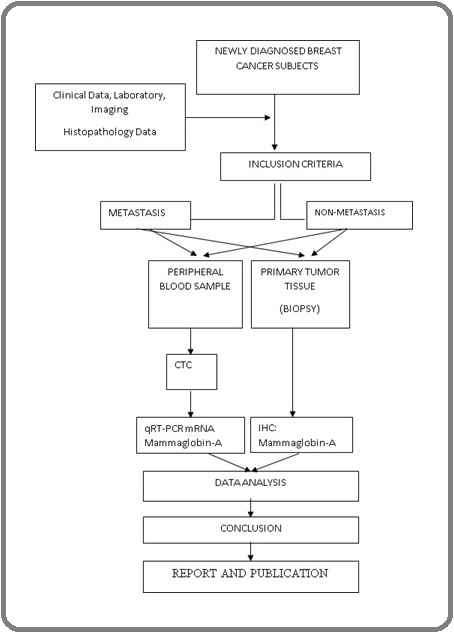
The results of the cross-sectional study were then applied to complement the case-control design, which was applied to discover the magnitude of the risk of metastasis among breast cancer patients based on Mammaglobin-A mRNA levels circulating in the peripheral blood and its expression in primary tumor tissue (Figure 2).
Figure 2. Case-control Study Design.
Location and Duration
This study was conducted in several hospitals in Indonesia, namely the Division of Surgical Oncology in Sanglah Hospital Denpasar, Prima Medika Hospital, Ganesha Hospital, Ari Canti Hospital, Kasih Ibu Hospital, and Siloam Hospitals. The study was conducted between July 2017 and March 2018.
Study Parameters
The primary parameters in this study included the concentration of mRNA mammaglobin-A in peripheral blood, the level of mammaglobin-A expression in breast cancer tissue, and the presence or absence of metastasis. The secondary parameters are demographic data, cancer staging, and the history of treatment management.
Study Procedure
The criteria for allocation of participants to the case and control group can be found in Figure 2. From all the eligible subjects who consented to the study, demographic data about age, occupation, risk factors for mammary carcinoma, menstrual status, and a history of using hormonal drugs were collected. The subjects were asked to do imaging tests such as chest radiographs, liver ultrasound, and laboratory examinations. All subjects were required to have 2.5 ml of blood drawn in a special L6 tube. Blood samples were sent to the microbiology laboratory of the Faculty of Medicine of Hasanuddin University for a quantitative real-time polymerase chain reaction (PCR) examination to identify Mammaglobin-A mRNA expression.
An open biopsy was performed as the gold standard in establishing a diagnosis of breast cancer. The biopsy sample was sent for histopathological examination and a standard immunohistochemistry (IHC) examination consisting of ER, PR, HER2, and Ki67. By IHC, the expression of Mammaglobin-A protein was then examined in the Anatomical Pathology Laboratory of Prima Medika Hospital.
Results
Demographic Analysis
From July 2017 to March 2018, there were 74 patients, aged 18-65 years, visiting the Division of Surgical Oncology in the locations of the study. Four patients were excluded because histopathology showed no tumors, metaplastic, ADH, and pleomorphic sarcomas. Of the 70 remaining subjects, the mean age was 49.49 ± 9.37 years old, mostly had more than two children (85.5%), 73% breastfed for more than two years, 50.7% premenopausal, 14.5% had a Karnofsky score of ≤ 70, and 11.6% had a history of breast cancer in the family. The mean diameter of the tumor was 7.7 ± 4.913 centimeters. Most of the tumors (37.7%) had high-grade histological grading, 75.4% had positive tumor-infiltrating lymphocytes (TIL), and 71% had negative lymphovascular invasion (LVI). About forty-six percent (46.4%) had no enlargement of axillary lymph nodes, 30.2% experienced distant metastasis (17.4% lungs and 1.4% to the liver, brain, breast, and contralateral and bone, respectively). This study showed no difference in age, menstrual status, histology type, and grade between metastatic and non-metastatic groups, while there was a significant difference in Tumor, Node, Metastasis (TNM) stage and IHC status metastatic and non-metastatic group (p<0.05) as seen in Table 1.
| Variable | Metastatic Group (n=23) N (%) | Non-Metastatic Group (n=47) N (%) | p |
| Age (mean ± SD) | 47.65±10.74 | 50.38±8.61 | 0.255 |
| Menstruation status | 0.323 | ||
| Pre-menopause | 13 (18.57) | 23 (32.86) | |
| Post-menopause | 10 (14.29) | 24 (34.29) | |
| Tumor stages | 0.001* | ||
| T1 | 1 (1.43) | 0 (0.00) | |
| T2 | 3 (4.29) | 25 (35.71) | |
| T3 | 0 (0.00) | 10 (14.29) | |
| T4 | 19 (27.14) | 12 (17.14) | |
| Lymph node stages | 0.001* | ||
| N0 | 4 (5.71) | 28 (40.00) | |
| N1 | 6 (8.57) | 15 (21.43) | |
| N2 | 6 (8.57) | 4 (5.71) | |
| N3 | 7 (10.00) | 0 (0.00) | |
| Type of histopathology | 0.546 | ||
| Invasive | 23 (32.86) | 44 (62.86) | |
| Noninvasive | 0 (0.00) | 3 (4.29) | |
| Grade | 0.405 | ||
| Low/Moderate | 12 (17.16) | 32 (45.76) | |
| High | 11 (15.71) | 15 (21.43) | |
| TIL | 0.728 | ||
| Negative | 5 (7.14) | 12 (17.14) | |
| Positive | 18 (25.71) | 35 (50.00) | |
| LVI | 0.171 | ||
| Negative | 14 (20.00) | 36 (51.43) | |
| Positive | 9 (12.86) | 11 (15.71) | |
| Breast cancer subtype | 0.008* | ||
| Luminal A | 0 (0.00) | 12 (17.14) | |
| Luminal B HER2-negative | 13 (18.57) | 16 (22.86) | |
| Luminal B HER2-positive | 2 (2.86) | 9 (12.86) | |
| HER2 Type | 3 (4.29) | 8 (11.43) | |
| TNBC | 5 (7.14) | 2 (2.86) |
statistically significant if p < 0.05; LVI, Lymphovascular Invasion; N, Nodes; M: Metastasis; SD, Standard Deviation; T, Tumor; TIL, Tumor-Infiltrating Lymphocytes; TNBC, Triple-Negative Breast Cancer
Mammaglobin-A in Primary Breast Tissue Tumor and Mammaglobin-A mRNA Concentration in Peripheral Blood
The mean mRNA level of mammaglobin-A in the metastatic group was 11.59 ± 1.37-fold change, while in the non-metastatic group, it was 8.17 ± 1.27-fold change relative to the housekeeping gene beta microglobulin. An independent t-test demonstrated a significant difference in the mean mRNA level of mammaglobin-A between the metastatic and non-metastatic groups (p<0.001) (Table 2).
| Subject groups | n | Mean difference | SD | t | p |
| Metastatic | 23 | 11.59 | 1.37 | 10.32 | 0.001* |
| Non-Metastatic | 47 | 8.17 | 1.27 |
*statistically significant if p < 0.05
In the second arm of the study, we found that high mammaglobin-A mRNA levels were a risk factor for breast cancer metastasis (OR 9.0; 95% CI 2.15-37.66; p=0.002) compared to low mammaglobin- A mRNA level within the peripheral blood (Table 3). This study also showed that the overexpression of mammaglobin-A is a risk factor for metastatic breast cancer by 7.36 times (OR = 7.36; 95% CI = 1.34-40.55; p = 0.013) compared to non-expression of mammaglobin-A in tumor tissues (Table 3).
| Variable | Group | OR | 95% CI | p | |
| Case (Metastatic) | Control (Nonmetastatic) | ||||
| mRNA Mammaglobin-A level | 9 | 2.15-37.66 | 0.002* | ||
| High | 15 | 5 | |||
| Low | 5 | 15 | |||
| Mammaglobin-A Expression | |||||
| Overexpression | 9 | 2 | 7.36 | 1.34-40.55 | 0.013 |
| Non-overexpression | 11 | 18 |
*statistically significant if p < 0.05
Discussion
Breast cancer is thought to progress gradually through several stages, starting with hyperplasia and advancing to intraductal carcinoma, followed by invasion and growth within the breast. In some cases, this progression may be accompanied by metastasis to lymph nodes and distant organs [25, 26]. Despite significant advancements in treatment, approximately 30% of early breast cancer patients experience distant metastasis within 5 years [27]. Understanding the role of biomarkers can help explain the association between anaplasia, tumor size, vascularity, prognosis, and the likelihood of local recurrence, as these biomarkers may be indicative of the presence of seed cells that have disseminated, even after complete tumor excision [28].
In accordance with this study, there was a significant relationship between the increase in biomarkers mammaglobin-A in tumor cells and peripheral blood and the occurrence of metastasis in breast cancer. This correlation highlights the potential significance of mammaglobin-A as a predictive indicator of metastatic breast cancer. Notably, the variables of age, menstrual status, histopathological type, LVI, TIL, and grade in this study are comparable, with no significant differences observed between metastatic and non-metastatic cases. This further emphasizes the potential relevance of mammaglobin-A as a key factor associated with metastatic progression in breast cancer, distinct from the parameters examined in this study.
Significantly higher mean mRNA mammaglobin-A levels were observed in the metastatic group (p < 0.05). In the metastatic group, CTCs in the blood also exhibited significantly different mean levels compared to the non-metastatic group. These findings suggest that CTCs in metastatic breast cancer may originate not only from the primary tumor but also from metastatic tumors and bone marrow. The differences in CTC levels between stage IV and stage I/II highlight the evolving nature of CTC dynamics and their potential as an indicator of disease progression. It is worth noting that while CTCs can also be found in ‘carcinoma in situ’, their levels and behavior differ in advanced stage IV cancer, where they often exhibit increased aggressiveness [28, 29]. This underscores the importance of monitoring CTCs in the context of breast cancer pathophysiology.
There are retention properties of CTC in microvascular tissue reported in metastasis of breast and cervical cancer. Therefore, detecting the presence of CTC during the progression of cancer becomes an important tool for estimating the pathophysiological processes of breast cancer [30, 31].
Our study’s findings align with previous research [32] demonstrated a close correspondence between reverse transcription polymerase chain reaction (RT-qPCR) in the target gene and mRNA expression with primary tumor IHC protein and metastasis. This observation is consistent with the broader body of literature, which generally reports a high concordance between primary tissue and metastatic lesions. However, it’s important to acknowledge that there may be slight variations in mutation status, proliferative markers, or gene expression between primary and metastatic sites [33]. In our study, the IHC expression of mammaglobin-A in primary tumors closely mirrors the levels of mammaglobin-A mRNA in peripheral blood, underscoring its potential as an indicator of breast cancer metastasis.
In the case-control study, it was found that the high level of mammaglobin-A mRNA was a risk factor for breast cancer metastasis by 9 times compared to low mammaglobin-A mRNA level. In addition, mammaglobin-A overexpression is a risk factor for breast cancer metastasis by 7.36 times compared to non- expression of mammaglobin-A. It can be explained that the expression of mammaglobin-A mRNA is breast tissue- specific; about 90% of primary breast cancers express mammaglobin-A [8].
Besides that, when compared to other genes that are overexpressed in breast cancer such as human epidermal growth factor receptor 2 (HER-2)/neuprotooncogene (neu) and MUC1, mammaglobin-A is expressed at higher levels in breast cancer cell lines, including primary tumors [8]. Furthermore, almost 91% of patients with metastatic breast cancer tested positive for mammaglobin-A mRNA in lymph nodes and/or blood [34]. The most important finding is that mammaglobin-A mRNA is not detected in lymph nodes and or healthy donor blood samples, which means independently it has the potential to be an ideal marker that has the ability to specifically detect breast cancer cells accurately when compared to markers used previously such as HER2 / neu, CEA, and CK19 [31]. Using RT-PCR and Northern Blotting, a study showed that the expression of Mammaglobin-A mRNA in various fetal and adult tissues is mainly limited in adult breast glands [35, 36, 8].
Mammaglobin-A expression is also found to be related to the proliferation and terminal differentiation of the breast gland [37, 38]. The results of this study were also supported by several studies that stated that overexpression of mammaglobin-A occurs in breast tumors [39, 8]. Mammaglobin protein expression is controlled by steroid hormones in the normal human endometrium with its peak expression usually found during the luteal phase [40]. Based on its specific expression in breast cancer, it can be assumed that there is potential in which mammaglobin-A can detect the presence of CTC and DTC, and also allow for the confirmation of tissue origin of breast metastasis [41]. Watson et al., 1999 [36] reported the presence of mammaglobin-A in blood samples of 9 out of 15 (60%) breast cancer patients, whereas Suchy et al., 2000 [42] reported the detection of mammaglobin-A protein expression in only 11/98 (11%) blood samples of breast cancer. However, it was reported that this study used a small amount of blood (150 microliters) for each reaction, which might pose as a limitation in their study. In addition, this study experienced a primary and probe error, so it is difficult to verify the sequence’s specificity. Several studies showed that in combination with CEA or cancer antigen 15-3 (CA15-3), there is an increase in mammaglobin-A’s diagnostic strength [43]. However, even as a single independent biomarker, mammaglobin-A mRNA was proven to have the potential to become a routine additional test as a marker for serum breast cancer. This study demonstrates that mammaglobin-A mRNA levels exhibit a significant association with metastatic progression in breast cancer. These findings support the potential utility of mammaglobin-A as a biomarker for predicting the risk of metastasis. Moreover, overexpression of mammaglobin-A in breast tumor tissues appears to be indicative of an increased risk of metastasis. Furthermore, the elevated level of mammaglobin-A mRNA in peripheral blood emerges as a robust risk factor for metastasis when compared to primary tumor tissues. This body of work provides evidence for the potential utility of mammaglobin-A as a biomarker in the diagnosis of primary breast cancer, particularly in the context of metastasis and prognosis. This biomarker identification can be done through peripheral blood and biopsy. Future research can further enhance our understanding of these biomarkers’ roles in metastasis diagnosis.
Acknowledgements
None
Funding Statement
None
Approval
This study has received approval from the Ethical Committee of Udayana University.
Conflict of Interest
None
Ethical Declaration
The Ethical Committee of Udayana University has approved this study. Informed consent was obtained from all the patients.
Authors Contribution
In this single-authored paper, XY made contributions across all aspects of the research process.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- Retrieved November 14, 2023, from https://www.kemkes.go.id/id/rilis-kesehatan/hari-kanker-sedunia-2019 Hari Kanker Sedunia 2019. (n.d.). . .
- Faktor-faktor yang berhubungan dengan keterlambatan pengobatan pada penderita kanker payudara di Rumah Sakit Umum Pusat (RSUP) Sanglah, Denpasar. Rossalia N, Manuaba I. 2016.
- High Ki-67 and Vascular Endothelial Growth Factor (VEGF) Protein Expression as Negative Predictive Factor for Combined Neoadjuvant Chemotherapy in Young Age Stage III Breast Cancer: Sudarsa IW , Manuaba I, Maliawan S, Sutirtayasa I. Bali Medical Journal.2016;5. CrossRef
- Epithelial-mesenchymal transitions in development and disease Thiery JP , Acloque H, Huang RYJ , Nieto MA . Cell.2009;139(5). CrossRef
- Tumor metastasis: molecular insights and evolving paradigms Valastyan S, Weinberg RA . Cell.2011;147(2). CrossRef
- Detection of micrometastasis in axillary lymph nodes of breast carcinoma patients and its association with clinical outcome Nair IR , Mathew AJ , Kottarathil VD . Indian Journal of Pathology & Microbiology.2018;61(3). CrossRef
- Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer Watson MA , Fleming TP . Cancer Research.1996;56(4).
- Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer Fritzsche FR , Thomas A, Winzer KJ , Beyer B, Dankof A, Bellach J, Dahl E, Dietel M, Kristiansen G. Histology and Histopathology.2007;22(11). CrossRef
- Expression of mammaglobin and gross cystic disease fluid protein-15 in breast carcinomas Luo M, Huang Y, Ni Y, Tsang JYS , Chan S, Shao M, Tse GM . Human Pathology.2013;44(7). CrossRef
- Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel Mitas M, Mikhitarian K, Walters C, Baron PL , Elliott BM , Brothers TE , Robison JG , Metcalf JS , Palesch YY , Zhang Z, Gillanders WE , Cole DJ . International Journal of Cancer.2001;93(2). CrossRef
- Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, Chlouverakis G, Stathopoulos E, Lianidou E, Georgoulias V, Mavroudis D. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2008;14(9). CrossRef
- Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR Ntoulia M, Stathopoulou A, Ignatiadis M, Malamos N, Mavroudis D, Georgoulias V, Lianidou ES . Clinical Biochemistry.2006;39(9). CrossRef
- Human mammaglobin mRNA is a reliable molecular marker for detecting occult breast cancer cells in peripheral blood Roncella S, Ferro P, Bacigalupo B, Pronzato P, Tognoni A, Falco E, Gianquinto D, et al . Journal of experimental & clinical cancer research: CR.2005;24(2).
- Efficacy of intraoperative GeneSearch Breast Lymph Node (BLN) Assay for breast cancer metastasis detection in sentinel lymph node in Chinese patients Liu Y, Xu F, Liao N, Li L, Zhang G, Zhuang H, Mei P, et al . Cancer Science.2010;101(8). CrossRef
- Detection of mammaglogin A in blood from breast cancer patients, before and after treatment, using a one-tube nested PCR protocol. Association with the absence of tumor estrogen receptors Ceballos MP , Zumoffen C, Massa E, Cipulli G, Funes CC , Gil AB , Morales C, Tozzini R, Ghersevich S. Clinical Biochemistry.2011;44(17-18). CrossRef
- Detection of circulating tumour cells in breast cancer patients using human mammaglobin RT-PCR: association with clinical prognostic factors Ferro P, Franceschini MC , Bacigalupo B, Dessanti P, Falco E, Fontana V, Gianquinto D, Pistillo MP , Fedeli F, Roncella S. Anticancer Research.2010;30(6).
- Circulating tumor cells (CTCs) detected by triple-marker EpCAM, CK19, and hMAM RT-PCR and their relation to clinical outcome in metastatic breast cancer patients Zhao S, Yang H, Zhang M, Zhang D, Liu Y, Liu Y, Song Y, Zhang X, Li H, Ma W, Zhang Q. Cell Biochemistry and Biophysics.2013;65(2). CrossRef
- Detection of micrometastatic cells in breast cancer by RT-pCR for the mammaglobin gene Bossolasco P, Ricci C, Farina G, Soligo D, Pedretti D, Scanni A, Deliliers GL . Cancer Detection and Prevention.2002;26(1). CrossRef
- Mammaglobin a in breast cancer: existence of multiple molecular forms O'Brien NA , O'Donovan N, Ryan B, Hill ADK , McDermott E, O'Higgins N, Duffy MJ . International Journal of Cancer.2005;114(4). CrossRef
- Peripheral blood mammaglobin gene expression for diagnosis and prediction of metastasis in breast cancer patients Radwan WM , Moussa HS , Essa ES , Kandil SH , Kamel AM . Asia-Pacific Journal of Clinical Oncology.2013;9(1). CrossRef
- Relationship between human mammaglobin mRNA expression in breast cancer tissue and clinico-pathologic features of the tumors Roncella S, Ferro P, Bacigalupo B, Dessanti P, Giannico A, Gorji N, Moroni M, et al . Journal of experimental & clinical cancer research: CR.2006;25(1).
- Prognostic role of plasma mammaglobin A expression in breast carcinoma patients: a meta-analysis Hu Y, Liu P, Wu D, Jiang Y. OncoTargets and Therapy.2018;11. CrossRef
- Detection of epithelial messenger RNA in the plasma of breast cancer patients is associated with poor prognosis tumor characteristics Silva JM , Dominguez G, Silva J, Garcia JM , Sanchez A, Rodriguez O, Provencio M, España P, Bonilla F. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2001;7(9).
- Combination of cytology, fluorescence in situ hybridization for aneuploidy, and reverse-transcriptase polymerase chain reaction for human mammaglobin/mammaglobin B expression improves diagnosis of malignant effusions Fiegl M, Haun M, Massoner A, Krugmann J, Müller-Holzner E, Hack R, Hilbe W, Marth C, Duba H, Gastl G, Grünewald K. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2004;22(3). CrossRef
- Breast cancer metastasis: markers and models Weigelt B, Peterse JL , Veer LJ . Nature Reviews. Cancer.2005;5(8). CrossRef
- New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes Buonomo OC , Caredda E, Portarena I, Vanni G, Orlandi A, Bagni C, Petrella G, Palombi L, Orsaria P. PloS One.2017;12(9). CrossRef
- Tumor self-seeding by circulating cancer cells Kim M, Oskarsson T, Acharyya S, Nguyen DX , Zhang XH , Norton L, Massagué J. Cell.2009;139(7). CrossRef
- Circulating tumor cells in patients with carcinoma in situ of the cervix uteri Song J, Nettles JB . American Journal of Obstetrics and Gynecology.1969;104(5). CrossRef
- Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer Peeters DJE , Brouwer A, Van den Eynden GG , Rutten A, Onstenk W, Sieuwerts AM , Van Laere SJ , et al . Cancer Letters.2015;356(2 Pt B). CrossRef
- The detection of circulating tumor cells expressing E6/E7 HR-HPV oncogenes in peripheral blood in cervical cancer patients after radical hysterectomy Weismann P, Weismanova E, Masak L, Mlada K, Keder D, Ferancikova Z, Vizvaryova M, et al . Neoplasma.2009;56(3). CrossRef
- Tumor biomarker conversion between primary and metastatic breast cancer: mRNA assessment and its concordance with immunohistochemistry Stefanovic S, Wirtz R, Deutsch TM , Hartkopf A, Sinn P, Varga Z, Sobottka B, et al . Oncotarget.2017;8(31). CrossRef
- Targeted biomarker profiling of matched primary and metastatic estrogen receptor positive breast cancers Schleifman EB , Desai R, Spoerke JM , Xiao Y, Wong C, Abbas I, O'Brien C, et al . PloS One.2014;9(2). CrossRef
- Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via a nested reverse transcriptase polymerase chain reaction assay for mammaglobin mRNA Zach O, Kasparu H, Krieger O, Hehenwarter W, Girschikofsky M, Lutz D. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1999;17(7). CrossRef
- Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer Fleming TP , Watson MA . Annals of the New York Academy of Sciences.2000;923. CrossRef
- Mammaglobin expression in primary, metastatic, and occult breast cancer Watson MA , Dintzis S, Darrow CM , Voss LE , DiPersio J, Jensen R, Fleming TP . Cancer Research.1999;59(13).
- Structure and transcriptional regulation of the human mammaglobin gene, a breast cancer associated member of the uteroglobin gene family localized to chromosome 11q13 Watson MA , Darrow C, Zimonjic DB , Popescu NC , Fleming TP . Oncogene.1998;16(6). CrossRef
- Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies Zafrakas M, Petschke B, Donner A, Fritzsche F, Kristiansen G, Knüchel R, Dahl E. BMC cancer.2006;6. CrossRef
- Discovery of differentially expressed genes in human breast cancer using subtracted cDNA libraries and cDNA microarrays Jiang Y, Harlocker SL , Molesh DA , Dillon DC , Stolk JA , Houghton RL , Repasky EA , Badaro R, Reed SG , Xu J. Oncogene.2002;21(14). CrossRef
- Mammaglobin 1 promotes breast cancer malignancy and confers sensitivity to anticancer drugs Picot N, Guerrette R, Beauregard A, Jean S, Michaud P, Harquail J, Benzina S, Robichaud GA . Molecular Carcinogenesis.2016;55(7). CrossRef
- Disseminated and circulating tumour cells and their role in breast cancer Čabiňaková M, Tesařová P. Folia Biologica.2012;58(3).
- Detection of mammaglobin expressing cells in blood of breast cancer patients Suchy B, Austrup F, Driesel G, Eder C, Kusiak I, Uciechowski P, Grill HJ , Giesing M. Cancer Letters.2000;158(2). CrossRef
- Mammaglobin gene expression: a superior marker of breast cancer cells in peripheral blood in comparison to epidermal-growth-factor receptor and cytokeratin-19 Grünewald K, Haun M, Urbanek M, Fiegl M, Müller-Holzner E, Gunsilius E, Dünser M, Marth C, Gastl G. Laboratory Investigation; a Journal of Technical Methods and Pathology.2000;80(7). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details