Unusual Clinical Presentation of Clear Cell Sarcoma Masquerading as Cutaneous Lymphoma, in a Young Male
Download
Abstract
Background: Clear cell sarcoma of soft tissue is an exceedingly rare tumor that originates from the neural crest cells. These tumors are histologically characterized by the presence of clear cells that contain accumulated glycogen. Clear cell sarcomas share histological and immunohistochemical features with malignant melanoma. They commonly arise from tendinous sheaths and aponeuroses, with the lower limbs, particularly around the ankles, being the most common site. Tumors arising in the upper extremities are rare, and very few primary clear cell sarcoma cases have been described in the chest wall and scapular soft tissues. They usually present as a solitary mass.
Materials and Methods: Here we are reporting a case of clear cell sarcoma in a young boy, presenting with multifocal lesions involving the trunk, both upper and lower extremities, lymph nodes, bone marrow, and many internal viscera.
Results: The case described an unusual presentation of clear cell sarcoma with multifocal involvement, which is rare compared to the typical presentation as a solitary mass.
Conclusion: The reported case demonstrates a very unusual presentation of clear cell sarcoma involving multiple sites, highlighting the importance of considering a wide differential diagnosis when encountering multifocal soft tissue lesions.
Introduction
Clear cell sarcoma (CCS) was first described by Dr. Franz Enzinger in 1965. It is a rare type of soft tissue tumor that constitutes approximately 1% of all sarcomas [1]. CCS primarily occurs in deeper soft tissues near tendons and aponeuroses of distal extremities and is often misdiagnosed. It is thought to arise from neural crest cells within the soft tissue. It is a distinct entity characterized by the presence of the EWS-ATF1 fusion transcript [2]. Although CCS predominantly affects adolescents and young adults with a similar incidence between males and females, its true frequency remains unknown [3].
We report a case of CCS in an adolescent male, which was clinically misdiagnosed as cutaneous lymphoma.
Case Report
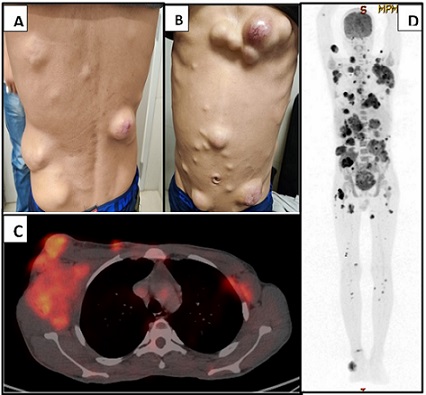
A 20-year-old-male presented to our outpatient department with complaints of mild fever since 5 months and multiple swellings all over the body in both upper and lower limbs, chest, and back along with pain abdomen. The swellings were gradually progressing in size, with mild pain and itching. On examination, he had cervical and bilateral axillary lymphadenopathy (right>left), and multiple mobile swellings all over the skin, the largest measuring approximately 3 cm (Figure-1A, B). There was no history of weight loss.
Figure 1. A, B, 20-year-old Male Patient Presented with Multiple Subcutaneous Swellings of Varying Size All over the body. C, CECT scan shows hypermetabolic Multiple Intermuscular, Intramuscular, and Cutaneous Deposits. D, PET scan shows bulky right axillary adenopathy with discrete supra and infra diaphragmatic adenopathy along with multiple intermuscular, intramuscular, and cutaneous deposits, multiple abdominal and pelvic deposits, marrow lesions, right middle lobar mass and bilateral suprarenal masses.
An outside biopsy from one of the lesions was reported as high-grade large cell Non-Hodgkin Lymphoma involving the deep dermis. Based on this presentation, the clinical diagnosis was deemed as Lymphoma, favoring cutaneous T cell lymphoma.
On PET CT scan, he showed multiple FDG avid lymphadenopathy in right cervical level II, left cervical level I/IV nodes, bilateral axillary nodes (SUV max 6.45), mediastinal nodes (bilateral upper paratracheal, prevascular, right pulmonary ligament, left epiphrenic), abdominal nodes, right obturator node, and right inguinal node. Hypermetabolic multiple intermuscular, intramuscular, cutaneous, abdominal and pelvic deposits were identified. FDG avid foci were also noted in the left femur, lung, and bilateral suprarenal region (Figure-1 C, D).
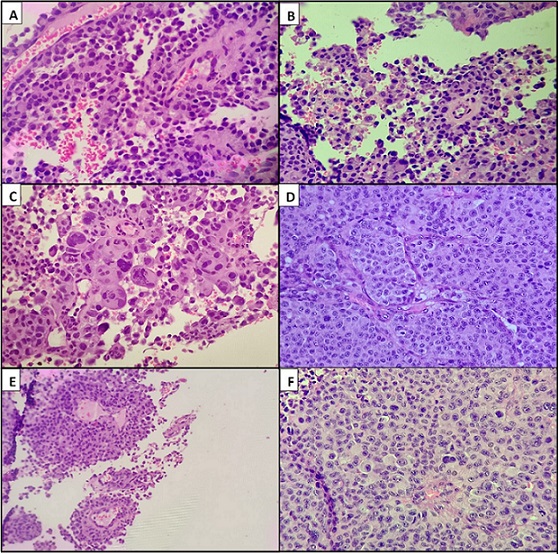
The previous biopsy was reviewed in-house. It revealed multiple fragmented tissue pieces infiltrated by diffuse sheets and nests of malignant cells having plasmacytoid morphology. Individual cells had eccentrically placed nuclei, vesicular chromatin, conspicuous to prominent nucleoli and a moderate amount of eosinophilic cytoplasm. Focal areas of necrosis were also noted. Few places showed perivascular arrangement of tumor cells resembling papillae. Good number of tumor giant cells and cells having bizarre nuclei were also noted (Figure 2).
Figure 2. [400x H and E]. A, Perivascular Arrangement of Tumor Cells with Prominent Fibrovascular Core. B, Tumor cells having plasmacytoid morphology. C, Numerous bizzare tumor cell nuclei along with tumor giant cells. D, Cells arranged in cohesive clusters resembling poorly differentiated carcinoma. E, Papillary pattern of arrangement of tumor cells. F, Sheets of tumor cells with occasional tumor giant cells resembling wreath-like cells of ALCL.
The main differentials based on the morphology of the tumor cells included cutaneous lymphoma especially Anaplastic large-cell lymphoma, Plasmacytoma and malignant melanoma.
Other differentials considered were metastatic poorly differentiated carcinoma, Langerhan’s cell histiocytosis.
On first panel of immunohistochemistry, atypical cells were focally positive for CD138, while negative for PanCK, CD30 and CD45, thus ruling out carcinoma and lymphoma. On further evaluation tumor cells were diffusely positive for HMB-45, S-100, Melan-A, and SOX-10 while focal positivity was noted for CD56 and CD99. Atypical cells were negative for EMA and CD1a. Ki-67 proliferative index was approximately 65% (Figure 3 and 4).
Figure 3. [400x H and E]. A, On Immunohistochemistry Tumor Cells Show Membranous Positivity for CD99. B, Tumor cells are strongly positive for HMB-45. C, CD138 is focally positive. D, Tumor cells are positive for CD56. E, Diffuse strong MelanA positivity is demonstrated in the tumor cells. F, S100 is positive. G, Tumor cells show diffuse strong nuclear positivity for SOX10. H, The Ki67 index is high (50-60%).
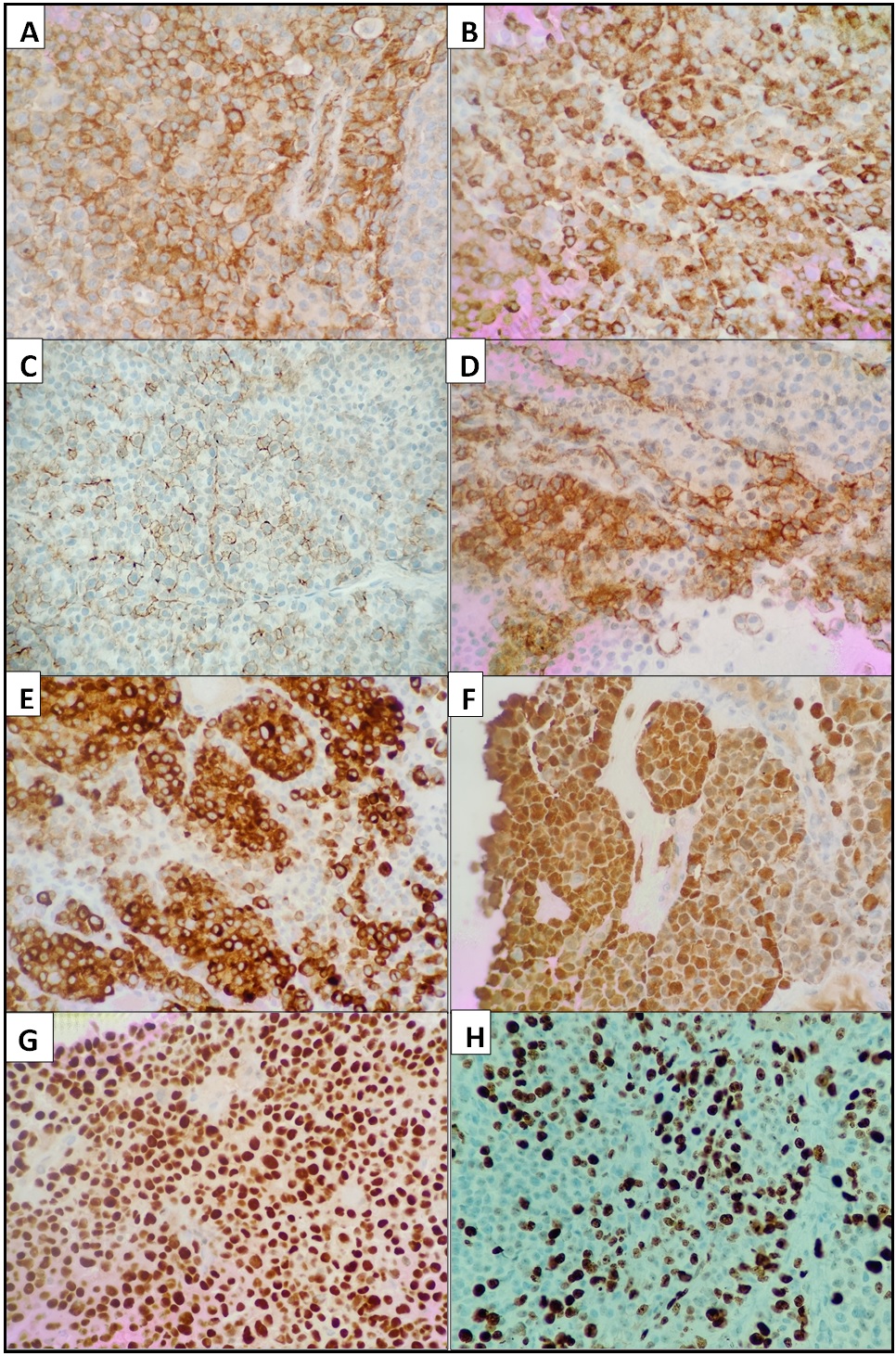
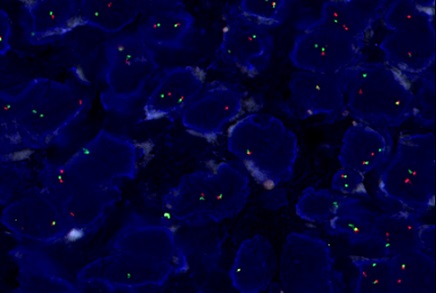
Figure 4. FISH Break Apart Probe Showing Split Signal in Most Nuclei (EWSR1 rearrangement positive).
Since the multiple subcutaneous lesions were not associated with the overlying skin, we rendered the diagnosis of clear cell sarcoma of the soft tissue.
The patient was advised to undergo EWSR1 rearrangement studies, which was done outside, came out to be positive, confirming the diagnosis of clear cell sarcoma.
Discussion
The present case of CCS presented as multiple subcutaneous nodular lesions, which on examination was misdiagnosed as Lymphoma. Other studies have shown CCS to commonly present as solitary subcutaneous masses [4]. Multifocal presentation of CCS is very rare.
In CCS, the lower limbs are most commonly affected, with the foot and ankle being the primary sites, followed by the knee, thigh, hand, forearm, elbow, and shoulder in descending order [5].
These tumors have a high tendency for local recurrence, regional lymph node involvement, and distant metastasis. Lymph nodes and lungs are the most common sites of metastasis, while skin, bones, liver, heart, and brain are less frequently affected [6].
Despite exhibiting some immunoreactivity for melanoma markers like HMB45 and S100, CCS can be distinguished from malignant melanoma (MM) through molecular biology testing such as RT-PCR and FISH, as well as characteristic chromosomal abnormalities and the genetic translocation t (12;22) (q13;q12). Clinically, CCS can also be differentiated by its extension within the cutaneous layer and susceptibility to lymph node and lung metastasis [4,7].
Histologically, CCS is characterized by uniform polygonal to fusiform eosinophilic or clear cells with central round nuclei and prominent basophilic nucleoli [8]. Approximately 60-70% of CCS patients develop metastases within 18 months to 6 years, and the 5-year survival rate is estimated to be around 63% [9]. According to a retrospective review and analysis of 31 cases, patients with lung or lymph node involvement have a 0% 10-year survival rate [10].
Prognosis is influenced by tumor size, with larger tumors and the presence of tumor necrosis under microscopic examination indicating a poorer outcome. Other factors such as tumor extent, gender, stage, mitotic index, and surgical margins have also been proposed as prognostic indicators [11].
The first line treatment protocol for CCS is considered to be wide local excision although there are high chances of local recurrence [12]. Amputation is done only when vascular or neural involvement is noted, as both procedures have shown similar prognostic and survival rates [12]. CCS shows limited response to chemotherapy or radiation therapy and thus these treatment options are reserved for cases with metastatic disease [11, 12].
References
- Clear-cell sarcoma of tendons and aponeuroses. an analysis of 21 cases Enzinger FM . Cancer.1965;18:1163-1174. CrossRef
- Clear cell sarcoma of tendons and aponeuroses: a review Dim DC , Cooley LD , Miranda RN . Archives of Pathology & Laboratory Medicine.2007;131(1). CrossRef
- Clear-cell sarcoma of tendons and aponeuroses: a clinicopathologic study. Presentation of six additional cases with review of the literature Pavlidis NA , Fisher C, Wiltshaw E. Cancer.1984;54(7). CrossRef
- Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases Hisaoka M, Ishida T, Kuo T, Matsuyama A, Imamura T, Nishida K, Kuroda H, et al . The American Journal of Surgical Pathology.2008;32(3). CrossRef
- Malignant melanoma of soft parts. A reassessment of clear cell sarcoma Chung EB , Enzinger FM . The American Journal of Surgical Pathology.1983;7(5). CrossRef
- Clear cell sarcoma of the scapula. A case report and review of the literature Kazakos CJ , Galanis VG , Giatromanolaki A, Verettas DJ , Sivridis E. World Journal of Surgical Oncology.2006;4. CrossRef
- Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Wang W, Mayordomo E, Zhang W, Hernandez VS , Tuvin D, Garcia L, Lev DC , Lazar AJ , López-Terrada D. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2009;22(9). CrossRef
- Cutaneous clear cell sarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 12 cases emphasizing its distinction from dermal melanoma Hantschke M, Mentzel T, Rütten A, Palmedo G, Calonje E, Lazar AJ , Kutzner H. The American Journal of Surgical Pathology.2010;34(2). CrossRef
- Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases Hocar O., Le Cesne A., Berissi S., Terrier P., Bonvalot S., Vanel D., Auperin A., Le Pechoux C., Bui B., Coindre J. M., Robert C.. Dermatology Research and Practice.2012;2012. CrossRef
- Biology and management of clear cell sarcoma: state of the art and future perspectives Cornillie J, Cann T, Wozniak A, Hompes D, Schöffski P. Expert Review of Anticancer Therapy.2016;16(8). CrossRef
- Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, Tsuchiya H, Beppu Y, Morioka H, Yabe H. Cancer.2007;109(1). CrossRef
- Clear cell sarcoma of soft tissues Malchau SS , Hayden J, Hornicek F, Mankin HJ . Journal of Surgical Oncology.2007;95(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details