Acute Toxicities and Treatment tolerance of Neoadjuvant Chemoradiation in Rectal Cancer: A Tertiary Cancer Centre Analysis
Download
Abstract
Background: To assess acute toxicities and treatment tolerance in patients with carcinoma rectum undergoing neoadjuvant chemoradiotherapy (NACTRT) at a tertiary care center in North-East India.
Materials and Methods: A total of 50 patients with histologically proven rectal adenocarcinoma, recruited from July 2022 to May 2023, were included in the study. Patients were planned for either NACTRT (n=44) or total neoadjuvant treatment (TNT) (n=6) followed by surgical assessment. The treatment protocol included a total dose of 50.4 Gy delivered in 28 fractions with concurrent oral Capecitabine at a dose of 825 mg/m² twice daily. Acute toxicities were assessed using RTOG grading criteria. Toxicity assessments were performed weekly through clinical examination and laboratory tests, with additional evaluations at the end of radiotherapy and 4 weeks after completing NACTRT.
Results: All patients completed the prescribed dose of external beam radiotherapy (EBRT) (50.4 Gy in 28 fractions) over a period of 6 weeks. Toxicity assessment revealed Grade 1 dermatitis in 14 patients (56%), Grade 2 dermatitis in 14 patients (54%), and Grade 3 dermatitis in 2 patients (7.6%). For lower gastrointestinal toxicities, Grade 1 diarrhea occurred in 4 patients (50%), Grade 2 diarrhea in 3 patients (37.5%), and Grade 3 diarrhea in 1 patient (7.6%). Grade 1 genitourinary toxicity was observed in 4 patients (8%). Hematological toxicities were reported in 40 patients, with Grade 1 anemia in 10 (38.4%), Grade 2 anemia in 14 (54%), and Grade 3 anemia in 2 (7.6%). Grade 1 and Grade 2 leucopenia were observed in 4 (57%) and 3 (43%) patients, respectively. Liver function test abnormalities were noted in 7 patients (15%), with dose modifications required for 5 patients (10%). Treatment was temporarily put on hold for 3 patients (6%) for 3 days due to Grade 3 toxicities.
Conclusion: NACTRT in rectal cancer patients was associated with acceptable levels of toxicity and treatment tolerance. To further reduce radiation-related toxicities, modern external beam radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT), should be evaluated.
Introduction
Neoadjuvant chemoradiation (NACTRT) followed by surgery and adjuvant chemotherapy is considered to be a standard treatment practice for clinically T3-T4 or node positive rectal cancers in many tertiary cancer centres [1, 2]. Current evidence also suggests the advent of Total Neoadjuvant Therapy (TNT) as a treatment option for locally advanced rectal cancer (LARC).
The addition of preoperative radiation along with 5-fluoro-uracil based chemotherapy has shown to improve the local control rates in LARC in many phase III studies [3, 4]. The toxicities of NACTRT were also found to be significantly less than adjuvant chemoradiation (CTRT) [5]. Even though radiation has lesser toxicities in the preoperative setting compared to the post operative period, the side effects can still be a limiting factor in the overall treatment tolerance or compliance of the patients. The combined effect of concurrent chemotherapy with radiation can further exacerbate the toxicities, resulting in increasing the overall treatment time of NACTRT and also delay the definitive surgery as well as adjuvant chemotherapy. Although techniques like Intensity Modulated Radiotherapy (IMRT) can reduce radiation induced toxicities compared to Three-Dimensional Conformal Radiotherapy (3DCRT) [6, 7], not all cancer centres employ IMRT as 3DCRT also has acceptable tolerance.
A large proportion of LARC is seen in our country, with colorectal cancers constituting almost 5% of all cancers in India [8]. The main aim of our study is to prospectively analyse the acute toxicities and treatment tolerance of NACTRT in LARC at a high-volume tertiary cancer centre in North Eastern region of India.
Materials and Methods
A total of 50 patients of histologically proven locally advanced Rectal Adenocarcinoma from July 2022 to May 2023 were recruited in the study. All patients were planned for either NACTRT (n=44) or Total Neoadjuvant Treatment (TNT) (n=6) followed by assessment for surgery and adjuvant chemotherapy. Informed consent was taken for all patients before starting the treatment. Patients were immobilised using pelvic thermoplastic mould. CT simulation was done for all patients in prone position with arms above head and a marker at anal verge. Proper bowel preparation with stimulant laxatives like bisacodyl was advised for all patients prior to the CT simulation. Bladder protocol was followed for all patients during the CT scan. The CT images were taken of the lower abdomen and pelvic region with 3mm slice thickness. The images were then transferred to Monaco and Eclipse TPS for radiation planning. External beam radiotherapy (EBRT) planning was done using 3DCRT technique using one posterior beam and two lateral beams (Figure 1) to a total dose of 50.4 Gray (Gy) in 28 fractions over five and a half weeks at 1.8 Gy per fraction.
Figure 1. Three Field EBRT Planning Showing Dose Colour Wash of 50.4Gy in Axial, Sagittal and Coronal Sections (left to right).
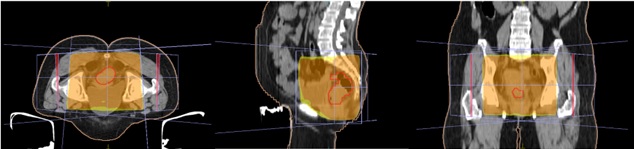
The given field ensured the coverage of the entire rectum, mesorectum and pelvic lymph nodal regions. Concurrent oral chemotherapy with Tab. Capecitabine at a dose of 825 mg/m2 twice daily after food was prescribed along with radiation. A baseline clinical examination was done prior to starting radiation. Weekly review of patients for toxicity assessment and any required dose modifications of Capecitabine was done. Hematological investigations like complete blood count, kidney function tests, serum electrolytes and liver function tests were advised weekly during the review along with clinical examination. Radiation and capecitabine induced acute toxicities were assessed using RTOG (Radiotherapy and Oncology Group) [9] toxicity criteria. Patients were also assessed at the end of NACTRT and at 4 weeks post completion of NACTRT. Response assessment imaging with CECT/MRI whole abdomen was done at 4-6 weeks post completion of NACTRT. The response evaluation was done using RECIST 1.1 [10] Criteria.
Data collection and Analysis
The data of the patient characteristics, disease related specifications and toxicities during each review of all patients were collected and stored in Microsoft Excel. Descriptive statistics like percentages, mean and median were calculated.
Results
This was a prospective study with a sample size of 50 patients recruited from July 2022 till May 2023. The patient characteristics have been detailed in Table 1.
| Characteristic | Total (n=50) |
| Age (years) | |
| Range | 16 – 80 |
| Median | 48.5 |
| Gender | |
| Male | 31 |
| Female | 19 |
| Location of tumor | |
| Upper Rectum | 5 |
| Mid Rectum | 29 |
| Lower Rectum | 12 |
| Entire Rectum | 4 |
| HPE | |
| Well differentiated Adenocarcinoma | 25 |
| Moderately Differentiated Adenocarcinoma | 14 |
| Poorly Differentiated Adenocarcinoma | 1 |
| Mucinous Adenocarcinoma | 5 |
| Intramucosal Adenocarcinoma | 3 |
| Signet Ring Cell Adenocarcinoma | 2 |
| T (Tumor) stage | |
| T1 | - |
| T2 | 4 |
| T3 | 38 |
| T4 | 8 |
| N (Node) stage | |
| N0 | 16 |
| N1 | 23 |
| N2 | 11 |
| N3 | - |
| AJCC 8 th Edition Staging | |
| Stage I | - |
| Stage II | 6 |
| Stage III | 36 |
| Stage IV | 8 |
| Radiation dose | 50.4 Gy |
| RT Technique | 3D-CRT |
| Concurrent chemo with Tab. Capecitabine @ 825 mg/m 2 BD (Total Dose per day) | |
| 1500 mg | 11 |
| 2000 mg | 25 |
| 2500 mg | 14 |
HPE – Histopathological Examination, AJCC - American Joint Committee on Cancer, Gy – Gray, 3D-CRT – Three-Dimensional Conformal Radiotherapy, BD – Twice daily
The median age of the patients was 48.5 years with ages ranging from 16 to 80 years. The most frequent site of primary tumor was mid rectum (n=33), and most common histopathology was Well Differentiated Adenocarcinoma (n=25). Majority of the patients had clinical staging of AJCC stage III disease. NACTRT followed by surgery followed by adjuvant chemotherapy was planned 44 patients (78%) patients and TNT followed by surgery for 6 patients (12%).
All patients could complete the prescribed dose of EBRT (50.4Gy/28#) over a period of 6 weeks. Treatment was put on hold for patients (6%) for 3 days due to grade 3 toxicities.
The acute toxicities recorded are summarized in Table 2.
| Type of Toxicity | Total (n=50) |
| Dermatitis | |
| Grade I | 16 – 80 |
| Grade II | 48.5 |
| Grade III | |
| Diarrhea | |
| Grade I | 31 |
| Grade II | 19 |
| Grade III | |
| Genitourinary | |
| Grade I | |
| Anemia | |
| Grade I | 25 |
| Grade II | 14 |
| Grade III | 1 |
| Leucopenia | - |
| Grade I | 4 |
| Grade II | 38 |
| Response Assessment (RECIST 1.1) | Total (n=36) |
| Complete Response (CR) | 3 |
| Partial Response (PR) | 22 |
| Stable Disease (SD) | 8 |
| Progressive Disease (PD) | 3 |
RECIST – Response Evaluation Criteria in Solid Tumors
The radiation induced toxicities that were assessed included skin toxicity (Figure 2), lower gastrointestinal tract toxicity and genitourinary toxicity.
Figure 2. Grade II Dermatitis.

Grade I dermatitis was seen in 22 patients (44%), Grade II in 25 patients (50%), Grade III in 3 patients (6%). In lower gastrointestinal toxicities, Grade I diarrhea occurred for 4 patients (8%), Grade II diarrhea in 3 patients (6%) and Grade III diarrhea in 1 patient (2%). None of the patients had Grade IV dermatitis or Grade IV diarrhea. There was only Grade I genitourinary toxicity, which was observed in 4 patients (8%). After completion of NACTRT, patients were again assessed after 4 weeks. Majority of the toxicities were resolved, with 30 patients (60%) having grade 1 dermatitis, and 3 patients (6%) having grade 1 diarrhea at 4 weeks post NACTRT.
Hematological toxicities were seen in 80% of the patients (n=40). Grade I, Grade II, Grade III Anemia were seen in 10 (20%), 14 (28%), 2 (4%) patients respectively.
Grade 1 and Grade 2 leucopenia were seen in 4 (8%) and 3 (6%) patients respectively. None of the patients had any significant neutropenia or thrombocytopenia. Deranged LFT was seen for 7 patients (14%), out of whom dose modifications were required for 5 patients (10%). All hematological toxicities were resolved at 4 weeks post completion of NACTRT.
Response assessment CECT/ MRI pelvis and abdomen was done in 36 patients (72%) at four to six weeks interval after completion of NACTRT (Table 2). Response evaluation was done using RECIST 1.1 criteria which showed that 3 patients (6%) had complete response, 22 patients (44%) had partial response, 8 patients (16%) had stable disease and 3 patients (6%) had progressive disease.
Discussion
Colorectal cancers are the third most common cancer worldwide and second most common cause of cancer death, constituting ten percent of the newly diagnosed cancer cases [11]. The highest incidence and mortality rates are seen in the Asian continent compared to the rest of the world. Although Total Mesorectal Excision (TME) is the primary treatment modality of rectal cancers, the addition of NACTRT has shown improved locoregional control and sphincter preservation rates compared to surgery alone [1]. The addition of systemic chemotherapy addresses the micrometastases prevalent in these cancers, thereby reducing the distant failure as well as improving the overall survival [4, 12].
The entire course of treatment easily takes up to 9 to 12 months, assuming minimal treatment breaks and good compliance from patients. The protracted treatment of rectal cancers is challenging for the patients as multiple visits to the hospital as well as admissions are required. Our study aimed to assess the first step of this long treatment pathway, as any delays or increased toxicities in NACTRT would determine the further prolonging of the remaining management.
We have observed that our patients had minimal radiation induced toxicities (Table 2) that were less than what were seen in the existing literature [4]. Dermatitis is expected to occur in all patients as there are presence of skin folds and mucosal interface in the gluteal region. The use of 15 MV photons for the radiation planning resulted in lesser grade III dermatitis (6%) because of increased skin sparing effect. The lower G.I toxicities were also less frequent in these patients, with predominantly grade I diarrhea that could be due to irradiation of some portion of the large bowel. The prone position of the patient has resulted in lesser instances of diarrhea as the bowel bag falls anteriorly. The patients were treated with three field EBRT that included one posterior beam and two lateral beams. This could explain why only few patients had grade I genitourinary toxicity since there was no anterior component of EBRT that could irradiate bladder and external genitalia. Overall, the radiation induced toxicities did not lead to any significant treatment breaks as only 1 patient with grade III dermatitis required a gap of 3 days. All the toxicities were managed conservatively with supportive treatment, and none of the patients required in-patient admission.
The hematological toxicities due to concurrent Tab. Capecitabine were majorly limited to Grade I and Grade II toxicities. Patients with anemia received hematinics and blood transfusions as required. None of the patients developed hand-foot syndrome commonly seen in patients receiving capecitabine [13]. Weekly review of the blood parameters also ensured prompt dose modification if required in case of any intolerability of capecitabine. We observed that all chemotherapy related toxicities resolved after 4 weeks of completion of NACTRT. Overall, our patients had good tolerability of both radiation as well as concurrent oral chemotherapy.
Modern radiation techniques such as IMRT also play an important role in reducing the acute toxicities faced by the patients. A Korean meta-analysis by Chan et al showed that overall lower G.I toxicities was less in IMRT technique compared to 3DCRT techniques [14]. On the other hand, a few studies have shown no difference in patient reported toxicities between these two planning techniques [15]. Our study has used only 3DCRT planning for all patients and we have seen minimal grade 2 and grade 3 G.I toxicities. Although use of IMRT has been advocated by many researchers [14, 16], we have found similar toxicities with 3DCRT as seen with IMRT in the literature. This could be due to the fact of shielding of bowel bag with multi-leaf collimators (MLCs) and also due to the prone position of the patients. As there was no head on comparison of 3DCRT versus IMRT in our study, we do recommend a prospective study comparing these two modalities for a definite conclusion.
After completion of NACTRT, the response assessment scans that were advised at 4 to 6 weeks interval showed partial response in many patients. Various studies have shown the importance of complete resolution of the disease after neoadjuvant treatment that might also affect the overall survival [17]. In this study, only 6% of the patients had a complete response to NACTRT. However, not all patients had a response assessment imaging done during the analysis. As the primary aim of our study was acute toxicities, we have observed the patients only till the 4 to 6 weeks interval post radiation.
The main drawbacks of our study are the small sample size and the short follow up period of the patients. The information regarding subsequent surgery and adjuvant chemotherapy is not available for further review. The patients need to be followed up further for assessing the overall compliance, timely completion, and long-term toxicities of the entire course of management. We recommend conducting prospective studies with a larger number of patients assessing both acute and chronic radiation induced toxicities. Future research analysing the complete treatment along with the radiological as well as pathological responses will help us understand the current ramifications of the management of locally advanced rectal cancers.
In conclusion, NACTRT in rectal cancer patients was associated with acceptable toxicities, minimal treatment breaks and good tolerance in our study. Further follow up of patients is required to assess the successful completion of the remaining treatment with surgery and chemotherapy. The assessment of late toxicities of radiation would require a long term follow up of these patients. Modern EBRT techniques like IMRT should be evaluated in an effort to further reduce the radiation related toxicities. The authors suggest a larger sample size and comprehensive observation of the entire treatment course for a better understanding of the toxicity profile of rectal cancer patients.
Acknowledgements
NIL
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
The authors declare no conflict of interest.
References
- Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial Gijn W, Marijnen CAM , Nagtegaal ID , Kranenbarg EM , Putter H, Wiggers T, Rutten HJT , et al . The Lancet. Oncology.2011;12(6). CrossRef
- Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial Hong YS , Kim SY , Lee JS , Nam B, Kim K, Kim JE , Park YS , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2019;37(33). CrossRef
- Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study Bosset J, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun R, Bardet E, et al . The Lancet. Oncology.2014;15(2). CrossRef
- Preoperative versus postoperative chemoradiotherapy for rectal cancer Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, et al . The New England Journal of Medicine.2004;351(17). CrossRef
- Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer Braendengen M, Tveit KM , Berglund A, Birkemeyer E, Frykholm G, Påhlman L, WiigJN , Byström P, Bujko K, Glimelius B. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(22). CrossRef
- Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits Jabbour SK , Patel S, Herman JM , Wild A, Nagda SN , Altoos T, Tunceroglu A, et al . International Journal of Surgical Oncology.2012;2012. CrossRef
- Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer Samuelian JM , Callister MD , Ashman JB , Young-Fadok TM , Borad MJ , Gunderson LL . International Journal of Radiation Oncology, Biology, Physics.2012;82(5). CrossRef
- International Agency for Research on Cancer (2020).Population Fact Sheets: India [online]. Website https://gco.iarc.fr/today/data/factsheets/populations/356-india-factsheets.pdf [accessed 17/09/2023] .
- Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Cox JD , Stetz J, Pajak TF . International Journal of Radiation Oncology, Biology, Physics.1995;31(5). CrossRef
- New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eisenhauer EA , Therasse P, Bogaerts J, Schwartz LH , Sargent D, Ford R, Dancey J, et al . European Journal of Cancer (Oxford, England: 1990).2009;45(2). CrossRef
- International Agency for Research on Cancer (2020). Population Fact Sheets: India [online]. Website https://gco.iarc.fr/today/data/factsheets/populations/356-india-factsheets.pdf [accessed 17/09/2023] .
- Micrometastases and survival in stage II colorectal cancer Liefers GJ , Cleton-Jansen AM , Velde CJ , Hermans J, Krieken JH , Cornelisse CJ , Tollenaar RA . The New England Journal of Medicine.1998;339(4). CrossRef
- Comparison of 5-FU-based and Capecitabine-based Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: A Meta-analysis Zou X, Wang Q, Zhang J. Clinical Colorectal Cancer.2017;16(3). CrossRef
- Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta-analysis and pooled-analysis of acute toxicity Wee CW , Kang H, Wu H, Chie EK , Choi N, Park JM , Kim J, et al . Japanese Journal of Clinical Oncology.2018;48(5). CrossRef
- [Comparison of the application among intensity-modulated radiotherapy, 3D-conformal radiotherapy and conventional radiotherapy for locally advanced middle-low rectal cancer] Zhang C, Dong J, Shen T, Li Y, Yang Z, Cheng X, Luo H, et al . Zhonghua Wei Chang Wai Ke Za Zhi = Chinese Journal of Gastrointestinal Surgery.2018;21(12).
- Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer Ng SY , Colborn KL , Cambridge L, Hajj C, Yang TJ , Wu AJ , Goodman KA . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2016;121(2). CrossRef
- Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(15). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details