Expression of PD-L1 (DAKO 28-8) in Urothelial Carcinoma of Urinary Bladder
Download
Abstract
Background: Around 90% of bladder cancers are urothelial carcinoma. Recent advancements in treatment have shifted towards targeted therapies, particularly immunotherapies targeting PD-L1 (Programmed Cell Death Ligand 1). PD-L1, a ligand for PD-1 receptors, helps cancer cells evade the immune system. Various factors, including genetic alterations, lead to its upregulation in cancer cells. Immunotherapies aim to block this pathway, making cancer cells more susceptible to the immune response.
Materials and Methods: This study was conducted at the Department of Pathology, Pakistan Institute of Medical Sciences, Islamabad. Fifty-six cases of urothelial carcinoma were included. Immunohistochemistry was performed using the PD-L1 Dako 28-8 clone on prepared tissue sections. PD-L1 positivity was determined by brown membrane staining in tumor cells, and the expression was scored using the Tumor Proportion Score (TPS).
Results: The median patient age was 63 years (±7 years). The highest frequency (58.9%) of PD-L1 expression occurred in the age range of 56 to 65 years. However, there was no significant association between PD-L1 expression and age groups. Out of the 56 cases, 64.3% had a TPS of ≥5%. Among the 76.7% of high-grade cases, 72% had a TPS ≥5%, whereas 28% had a TPS <5%. Among the 23.2% of low-grade cases, 61.5% had a TPS <5%, and 38.5% had a TPS ≥5%.
Conclusion: Sixty-four percent of urothelial carcinoma cases showed PD-L1 expression. A significant association (p = 0.02) between PD-L1 expression and high-grade urothelial carcinoma was found using Pearson’s Chi-square test.
Introduction
Urinary bladder is a frequent site for development of urothelial malignancies. Bladder cancers are one of the most frequently encountered causes of mortality around the globe. According to global cancer statistics, they represent 3% of global cancer diagnoses. In the United States, it is the sixth most commonly reported malignancy [1]. Throughout the world, the male gender appears to be affected more as compared to female. In Pakistan, a meta-analysis done by collaborating different institutes for the Punjab Cancer Registry showed an age-standardized incidence rates of 5.2 and 1.4 per 100,000 in males and females respectively for bladder cancer [2].
About 90% of the malignant cancers of the bladder are urothelial carcinoma. A study conducted in Sindh Institute of Urology and Transplant Pakistan showed a high proportion of urothelial type i.e. 93.3% [3]. Another study conducted in Armed Forces Institute of Pathology (AFIP) Rawalpindi Pakistan revealed a 100% frequency of urothelial type [4].
Previously, surgical excision followed by chemotherapy was the available treatment modality for Urothelial carcinoma. With the advent of molecular pathology and precision medicine, immune therapy is now a breakthrough in oncology. One such immunotherapy has been targeted against PD-L1 for Urothelial carcinoma [5].
PD-L1 (Programmed cell death ligand 1) is encoded by the PDCDL1 gene and is found on chromosome 9 in humans at position p24.1. It is a ligand for the PD1 Receptors on cytotoxic T lymphocytes (CTLs). In normal cells, interaction of PD1 and CTLs plays a major role in immune tolerance and preventing the development of autoimmunity in the body [6]. The cancer cells have an upregulated PDL-1/ PD-1 mechanism by which they escape the immune checkpoints. This upregulation of the PDL-1 in cancer cells is by various mechanisms. Inducible expression is by extrinsic factors like IFN-Ɣ and EGF, whereas constitutive expression is by genetic alteration in the cancer cells e.g. RAS and EGFR mutation, ALK fusion and MYC amplification.
The rationale is to target PDL-1 and render the cancer cells more susceptible to body’s immune response [7]. Two theories have been proposed as to what role PDL-1 pathway plays in anticancer treatment; 1. Immune checkpoint inhibitors (ICIs) activate the cytotoxic T cells in the tumor bed, 2. They improve the systemic antitumor immunity by increasing the tumor antigen presentation by dendritic cells to the naïve T cells in the draining lymph nodes [8].
Although widely studied and established in carcinomas of breast, colon, lung and lymphomas, immunotherapy against PD-L1 in urothelial carcinoma is relatively a newer modality on the block. Currently, different ICIs are being used for specific antibody clones of PD-L1. It has also been shown that the clones Dako 22c3, Dako 28-8, Ventana 263 may be used interchangeably. For clone 28-8, Nivolumab is the FDA approved drug for locally advanced and metastatic Urothelial carcinoma in patients resistant to platinum-based chemotherapy [5].
This study focuses on the expression of PD-L1 in Urothelial carcinoma in our population. The stratification of patients according to PD-L1 status in Urothelial carcinoma may be helpful in optimizing individualized treatment, as patients showing PD-L1 expression may potentially benefit from target immunotherapy against PD-L1.
Objectives
1. To determine the frequency of PDL-1 Dako 28-8 expression by immunohistochemistry in urothelial carcinoma of urinary bladder
2. To analyze the association of PDL-1 Dako 28-8 expression with histological grade of urothelial carcinoma
Materials and Methods
This cross-sectional study was conducted in Department of Pathology Shaheed Zulfiqar Ali Bhutto Medical University Islamabad. Approval from Ethical Review Board (ERB) committee, Shaheed Zulfiqar Ali Bhutto Medical University (SZABMU) was taken.
All urinary bladder biopsies and TURBT specimens received in the Department of Pathology, Pakistan Institute of Medical Sciences between March 2023 and August 2023 were collected. Patients’ data and registration number with relevant details were recorded.
After fixation of the specimen in 10% formalin, gross examination was done according to American Joint Committee on Cancer (AJCC) protocols and submitted in tissue cassettes. Tissue was then processed in automated tissue processor, LIECA TP-1020, followed by cutting of 3-to-4-micron tissue sections. The sections were then mounted on glass slides and staining of the tissue with Hematoxylin and Eosin (H&E) in a tissue stainer Shandon Varistan 24-4 was done.
The slides were examined under Olympus CX 22 LED series microscope by post-graduate resident along with two consultants Histopathologist and diagnoses were recorded. Fifty-six cases of Urothelial carcinoma were taken.
All cases reported as Urothelial carcinoma were included in the study, including resection, biopsy and TURBT specimen. Metastatic, non-representative and autolyzed cases were excluded from the study.
Three-to-four-micron sections of the selected blocks were prepared. Immunohistochemistry was applied for PD-L1 using Dako 28-8 clone using LEICA bond III staining platform [9].
The slides stained for PDL-1 were examined under light microscope. PDL-1 positivity was interpreted as brown colored membrane staining with variable faint, moderate and strong intensity in the cell membrane of tumor cells. The scoring of PDL-1 was done according to recommended scoring system for Dako 28-8 clone as follows: [5]
Tumor proportion score (TPS)= no. of positive tumor cells/ total no. of tumor cells x 100
The cases were divided into two groups i.e.<5% and ≥5% based on the TPS. The cases showing TPS ≥5% were considered positive [5].
All data was assembled on a Master chart and data analysis was done by using SPSS version 26.
Qualitative variables such as gender, histological grade and PDL-1 expression were expressed as frequencies.
For age, mean and standard deviation were calculated. The age categorization was done according to two criteria. First, the patients were categorized into two groups i.e. less than or equal to 60 years and greater than 60 years. The association of PDL-1 expression with these two groups was determined. Second, the patients were distributed into four categories according to age range i.e. 46-55 years, 56-65 years, 66-75 years and 76-80 years. The age range containing the maximum number of PDL-1 positive cases was indicated.
Chi square test was applied for analyzing the association of PDL-1 expression with histological grade In addition, association of PDL-1 expression with age groups was also determined.
p value of ≤0.05 was considered significant.
Results
Of the 56 cases, 12 (21.4%) were females while 44 (78.6%) were males.
The median age of the patients was 63±7years.
The highest frequency i.e. 33/56 (58.9%) of PD-L1 expression was seen in the age range of 56-65 years. However, there was no significant correlation of PD-L1 expression with age groups (Table 1).
| TPS | Total | |||
| <5% | ≥5% | |||
| Age Range | 46-55 | 1 | 6 | 7 |
| 56-65 | 13 | 20 | 33 | |
| 66-75 | 5 | 8 | 13 | |
| 76-85 | 1 | 2 | 3 | |
| Total | 20 | 36 | 56 |
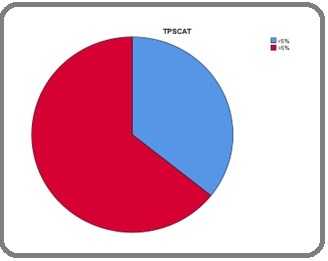
Of the total 56 cases, 36 (64.3%) showed a TPS ≥5% (Figure 1).
Figure 1. Frequency of PD-L1 Expression in Urothelial Carcinoma.
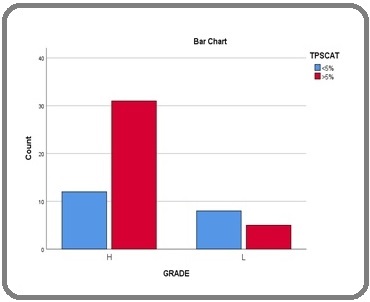
Forty-three 43/56 (76.7%) cases were of high grade while 13/56 (23.2%) were of low grade. Of the cases reported as high-grade Urothelial carcinoma, 31/43 cases (72%) showed a TPS of ≥5% and 12/43 (28%) showed a TPS of <5. Of the 13 low-grade cases, 8/13 (61.5%) showed a TPS of <5% and 5/13 (38.5%) showed a TPS of ≥5%. There was significant association of PD-L1 expression with high-grade Urothelial carcinoma, p value of 0.02 was calculated using Pearson’s Chi square test (Figure 2).
Figure 2. Association of PD-L1 Expression with Grade of Urothelial Carcinoma.
Discussion
In our population, more cases of urothelial carcinoma were found in males than females. This is comparable to the results shown by Doburch et al. from Europe [10]. The male to female ratio in our study was 3.6:1. Mubarak et al. from a study done in SIUT, Pakistan showed a male to female ratio of 5.3:1. The difference in our study may be due to variation in the accessibility of female patients to health care facilities in our part of the country [3].
The mean age of our patients was 63±7years, which is comparable to the results reported by Mubarak et al. and Ahmed et al. from different regions of Pakistan [3, 4]. There was no significant association of PDL-1 expression with age of the patient. This was similar to the results shown by Kumar et al. from India and Inman et al. from Canada [11, 12].
The frequency of PDL-1 expression in our population was 64.3%. To the best of our knowledge, our study is the first of any published data in Pakistan. In South Asian region, Kumar et al. from India have shown a positivity of 51% using the cut off value as ≥5% [12]. In a similar study conducted in the Middle East region, Nabhani et al. from Oman have shown a PD-L1 positivity of 45% [13]. The differences may be explained due to the use of different clones of PDL-1 in these studies.
Our study showed a significant association of PDL1 expression with high grade carcinoma. This is similar to the results shown by Kumar et al., Nabhani et al. and Inman et al [11-13].
The reporting of PDL1 is a challenging task, owing to its different clones, organ-specific cut off, different staining platforms, scoring criteria and clone-specific therapies. The scoring of PD-L1 in Urothelial carcinoma for specific clones are recommended by different scoring algorithms. The CPS scoring is used for the Dako 22c3 for which the approved drug is Pembrolizumab. For Dako 28-8, the recommended algorithm is the TPS, for which most studies have used a cut off value of ≥1% and others have used cut off ≥5%. The drug Nivolumab was approved by FDA in December 2014 for the 28-8 clone [5, 12]. The studies have shown an Objective Response Rate of up to 28.4% at cut off ≥5% for patients treated with Nivolumab in platinum refractory urothelial carcinoma. [14].
In conclusion, PDL-1 positivity was seen in 64.3% cases of urothelial carcinoma.
PDL-1 expression is significantly associated with high-grade urothelial carcinoma.
Limitations
The staining platform recommended by the clone manufacturer is Autolink 48, the one used in our study was LEICA Bond III. Although studies have shown this platform to be comparable, it may have affected the percentage of expression of PDL-1 [9].
PDL-1 being an expensive immunotherapy may be a constraint for the patients potentially eligible for therapy.
Recommendations
Further studies in South Asian population are suggested with patient follow up to determine the prognostic value of PDL-1 in urothelial carcinoma.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Epidemiology of bladder cancer. Prog Clin Biol Res. 2020;162 A:11–25. Saginala K, Barsouk D, Aluru JS , Rawla P, Barsouk SAP and A . .
- Cancer Epidemiology in Lahore, Pakistan - 2010-2015 Badar F, Mahmood S, Mahmood MT , Masood M, Tanvir I, Chughtai OR , Niazi S, Ahmad A. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP.2020;30(2). CrossRef
- Urinary Bladder Tumors in Southern Pakistan: A Histopathological Perspective Mubarak M, Kazi J, Hashmi A, Hussain M, Naqvi SA , Rizvi SAH . Middle East Journal of Cancer.2014;5(3).
- Clinicopathological spectrum of urothelial carcinoma of the urinary bladder - A study of 541 cases at AFIP Pakistan Ahmed R, Hashmi SN , Din H, Muhammad I. Pakistan Armed Forces Medical Journal.2015.
- PD-L1 assessment in urothelial carcinoma: a practical approach Eckstein M, Cimadamore A, Hartmann A, Lopez-Beltran A, Cheng L, Scarpelli M, Montironi R, Gevaert T. Annals of Translational Medicine.2019;7(22). CrossRef
- PD-L1 Kythreotou A, Siddique A, Mauri FA , Bower M, Pinato DJ. Journal of Clinical Pathology.2018;71(3). CrossRef
- Regulatory mechanisms of PD-L1 expression in cancer cells Shi Y. Cancer immunology, immunotherapy: CII.2018;67(10). CrossRef
- Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications Amin MB . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2009;22 Suppl 2. CrossRef
- Optimization and validation of PD-L1 immunohistochemistry staining protocols using the antibody clone 28-8 on different staining platforms Koppel C, Schwellenbach H, Zielinski D, Eckstein S, Martin-Ortega M, D'Arrigo C, Schildhaus H, Rüschoff J, Jasani B. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2018;31(11). CrossRef
- Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP , Resnick MJ , Shariat SF , Zlotta AR , Boorjian SA . European Urology.2016;69(2). CrossRef
- PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression Inman BA , Sebo TJ , Frigola X, Dong H, Bergstralh EJ , Frank I, Fradet Y, Lacombe L, Kwon ED . Cancer.2007;109(8). CrossRef
- Immunoexpression of PD-L1 and PD-1 and Its Clinicopathological Correlation in Urothelial Carcinomas Kumar U, Anthony ML , Sahai R, Mittal A, Durgapal P, Kishore S. Journal of Laboratory Physicians.2022;14(2). CrossRef
- Programmed Death-ligand 1 (PD-L1) Expression in Bladder Cancer and its Correlation with Tumor Grade, Stage, and Outcome Al Nabhani S, Al Harthy A, Al Riyami M, Al Sinawi S, Al Rashdi A, Al Husseni S, Kumar S. Oman Medical Journal.2022;37(6). CrossRef
- PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer Stenehjem DD , Tran D, Nkrumah MA , Gupta S. OncoTargets and Therapy.2018;11. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details