Molecular Epidemiology of Human Papilloma Virus (HPV) in Women’s Cervical Samples from Tehran, Iran
Download
Abstract
Background: Cervical cancer (CC) is the second-leading cause of cancer death among women. In this cancer, more than any other type of malignancy, the effects of prevention, early diagnosis, and timely treatment on reducing the death rate are evident. Hence the aim of this study was to Investigating the prevalence of human papilloma virus different genotypes in women’s cervical tissue samples.
Methods: In this cross-sectional study, 1045 samples obtained from referred patients were selected for periodic cervical examinations. Then, DNA extraction from cytology liquid brush was done in accordance with the standard method. The presence of HPV was determined by PCR. By using pyrosequencing method and then BLAST online search, HPV genotypes were identified.
Results: of 1045 patients, 159 (15%) had HPV infection in which 50.3% were LSIL by frequent HPV types 6, 18, 16, 11; 19.4% were HSIL by frequent HPV types 16, 18, and 30.1% were ASCUS by frequent HPV types 6, 11, respectively. The age group, 30–40 years had the highest prevalence of HPV infection (p-value = 0.6). 21.4% people diagnosed by mix genotype HPV infection. significant value identified by the pathological patterns and multiple types infections (p-value=0.007), HPV-16 infection and younger age (p-value=0.02).
Conclusion: our study showed 15% HPV infection identified in studied samples and the most HPV prevalent types were 6, 18, 11, 16, respectively. HPV-16 is common in younger ages. HPV vaccination and regular screening programs are major prevention measures.
Introduction
Cervical cancer (CC) stands as the second leading cause of mortality among women worldwide [1] and the third most prominent cause of death in Iran [2]. Human papillomavirus (HPV) serves as the primary etiological factor behind CC [3]. Various other risk factors also contribute to its development, including smoking, multiple pregnancies, oral contraceptive usage, inadequate dietary intake of vitamins A and E, and a family history of the disease. A notable correlation has been established between HPV infections and the onset of cervical cancer, with approximately 90% of anal cancer cases linked to HPV. HPV, responsible for an annual death toll of 250,000 individuals, encompasses over 200 genotypes, categorized as oncogenic or high-risk, with genotypes 58, 56, 52, 51, 45, 39, 33, 35, 31, 18, 16, and 59, probable high-risk types 26, 53, 66, and low HPVs 6, 11, 40, 43, 44, 54, 70 [4]. Notably, genotype 16, followed by genotypes 31, 16, 18, and 45, have been identified as the most prevalent HPV genotypes in over 70% of cervical cancer samples globally [5]. To date, researchers have identified a total of 100 types of human papillomaviruses (HPV), approximately one-third of which target the epithelial cells within the genital region. Human papillomaviruses that give rise to complications in the genital region can be categorized into two groups. The first group comprises low-risk papillomaviruses, such as HPV-11 and HPV-6, which are responsible for genital warts. The second group encompasses high-risk papillomaviruses, including HPV-18 and HPV-16, which are known to be causative agents of cervical cancer [6].
Human papillomaviruses have the capability to infect the skin and mucous epithelial tissue not only in the genital region but also on the hands and feet, thereby leading to various afflictions like skin warts, genital warts, laryngeal warts, and intra-epithelial neoplasia within the genital area, which frequently progress to malignancies. Notably, HPV-18 has been extensively isolated from cervical cancer cases and is accountable for approximately two-thirds of cervical cancer diagnoses in most countries across the globe. A consistent correlation has been established between infection by HPV-16, HPV-18, and HPV-31 and the presence of moderate to severe cervical dysplasia, and to a somewhat lesser extent, the development of invasive cancers affecting the cervix, penis, and anus. Furthermore, HPV DNA has been detected in up to 100% of cervical cancer specimens. Conversely, it has been identified in less than 10% of women exhibiting healthy uterine tissue [7, 8].
No specific treatment exists for HPV infection. Instead, destructive interventions, such as cryotherapy, trichloroacetic acid applications, lasers, and surgical procedures, are employed in the management of premalignant lesions [9]. The HPV vaccine prevents infection with certain types of HPV that are associated with the development of cervical and anogenital cancers and genital warts. So far, two types of HPV vaccines have been developed, Gardasil and Cervarix, and both of these vaccines are able to defend against HPV types 16 and 18. Gardasil alone protects against four types of HPV, including 11, 6, 16, and 18, and Cervarix alone protects against two types of HPV-16 and -18 [10].
The most effective strategy for mitigating the incidence and advancement of cervical cancer centers on the implementation of comprehensive screening programs. These programs encompass the utilization of genetic markers, rigorously validated molecular diagnostic methods distinguished by their superior sensitivity and specificity, and the deployment of vaccines designed to target the predominant HPV genotypes within a specific geographical area [10].
Aforementioned, cervical cancer, primarily attributed to human papillomavirus (HPV) infection, remains a significant global health concern, with varying HPV genotypes contributing to diverse disease outcomes.
While numerous studies have explored HPV prevalence, understanding the distribution of specific HPV genotypes within women’s cervical tissue samples remains a critical knowledge gap. Current HPV genotyping techniques, including pyrosequencing, offer a precise means to identify individual genotypes and assess their prevalence. However, a comprehensive investigation into the prevalence of different HPV genotypes in women’s cervical tissue samples is essential for informing targeted prevention and intervention strategies. This study seeks to address this gap by employing nested PCR and pyrosequencing to elucidate the prevalence of HPV genotypes in cervical tissue useful for HPV prevention and management efforts in Iranian population.
Materials and Methods
Sample collection
In order to conduct this study, cytology brush samples were obtained from 1045 patients who had recently referred for HPV screening by gynecologist. The hospitals affiliated to the Iran University of Medical Sciences, Tehran, Iran, laboratory databases from 2015 to 2022 was searched to identify patients’ data.
Eligible patients had HPV PCR test request, and had available and adequate sample for analysis. Patients samples which had not requested data or the sample was not available, or cases where did not meet the experimental requirements, were excluded from the study.
DNA extraction
To extract the virus genome, 200 ul liquid-based brush of cytology was obtained from all samples. The viral DNA was extracted from the genomic material using a Favoragene tissue genome DNA extraction kit (Favoragene Co., Taiwan) regarding to the manufacturer protocol. The isolated DNA was frozen at -20 °C for use in subsequent experiments.
HPV nested-PCR
In the initial reaction, universal primers MY09 and MY11 were employed to detect HPV infection using the PCR method targeting the human papillomavirus L1 gene. The target fragment in this reaction had a length of 451 bp, visualized on a 1.5% agarose gel. In HPV-negative samples, a second reaction was performed using the primers GP5+ and GP6+, which amplified a 150 bp fragment of the L1 ORF region of the HPV genome. This conserved region in the HPV genome allowed for the isolation of most types of the virus. Heating protocol obtained from previous studies [4, 11]. The positive control was obtained from previous studies conducted by the group on this virus [4, 11], with its sequence confirmed and submitted to the GeneBank and It was included in each PCR round as an experiment positive control. A negative control was also used by extracting DNA from HPV- negative samples obtained in this study. To validate the accuracy of DNA extraction, we employed PCR primers, specifically designed for the beta globin gene as an internal control to amplify a 110 bp fragment [11].
Pyrosequencing
To identify the HPV type of each sample, we performed pyrosequencing on the immobilized single-stranded templates that had annealed to the GP5+ sequencing primer. We aimed to obtain sequence data from 20 to 25 bases which are enough among the most common HPV types to enable typing. We used BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) to analyze the sequence data and determine the HPV genotype.
Statistical analysis
We performed stats with SPSS 16. We characterized the specimens by age, gender, and signs. We expressed continuous variables as median and IQR and categorical variables as number and percentage. We employed the chi-square and Wilcoxon-Mann-Whitney tests to examine categorical and continuous variables. We considered p < 0.05 as significant.
Results
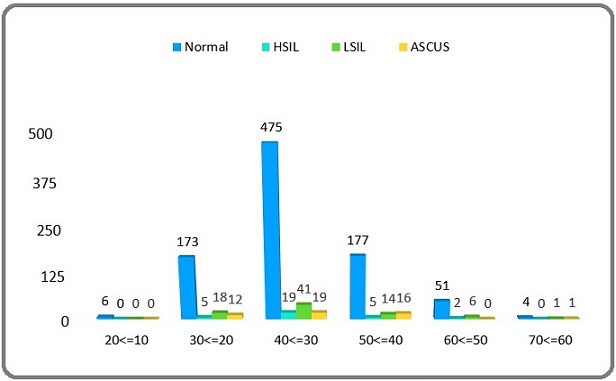
Of a total 1045 women cases, age was categorized into 6 groups (Figure 1). The most of cases (554/1045) were into the age group of 30–40 years, and followed by the age group of 40–50 years (212/1045). Cytology lesions based on pathology result was analyzed in each age category and shown in Figure 1.
Figure 1. Age Stratified Distribution of HPV Infection among the Cases. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ASCUS, Atypical Squamous Cells of Undetermined Significance.
HPV prevalence
By the nested PCR results, HPV-specific DNA was detected in the collected samples. Out of the 1045 cervical tissue samples obtained from participants, 159 (15%) were diagnosed as HPV positive, while 886 were negative. Overall, the study showed a 15% prevalence rate of HPV infection. Based on cytology analysis of different lesions, revealed that out of the 159 HPV positive samples, 80 cases (50.3%) were identified as LSIL, 31 cases (19.4%) as HSIL, and 48 cases (30.1%) as ASCUS. The age group 30–40 years showed highest amounts of HSIL, LISI, and ASCUS lesions.
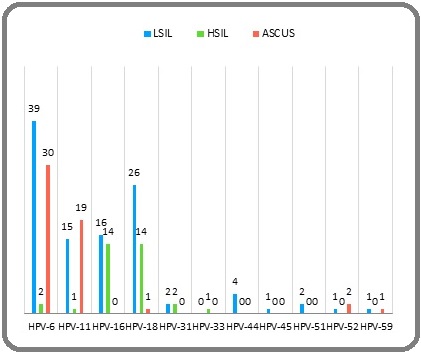
HPV genotyping based on pyrosequencing method and further BLAST search showed the most common HPV types were types 6, 18, 11, 16, respectively. By combining cytology and HPV genotyping results, in the LSIL samples the most common HPV types were 6, 18, 16, 11; in the ASCUS samples there were HPV types 6, 11; and in the HSIL cases there were HPV types 16, 18, respectively (Figure 2).
Figure 2. Prevalence of HPV genotypes, in positive HPV samples based on cytology lesions. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ASCUS, Atypical Squamous Cells of Undetermined Significance.
Based on the age group, 30–40 years had the highest prevalence of HPV. The statistical analysis showed that there was no clear link between age and pathological features (p = 0.6). The average age in HPV positive cases was 36.1± 8.2 years, and 34 (21.4%) people diagnosed by mix infection (Figure 3).
Figure 3. Age Stratified Distribution of HPV Infection among the Cases. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ASCUS, Atypical Squamous Cells of Undetermined Significance.
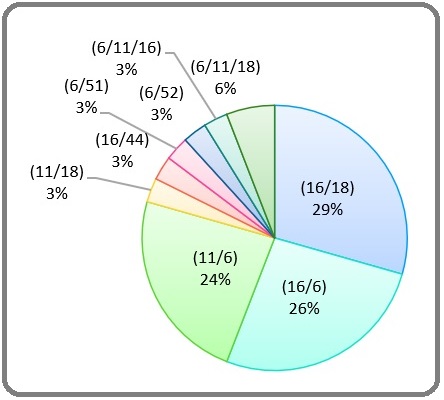
Statistically, a noteworthy distinction is evident among various pathological patterns and the occurrence of simultaneous infections with multiple types of infections (p=0.007). Predominantly, the mix genotype infected cases exhibited LSIL pathology. Furthermore, statistically significant findings indicate a lower prevalence of HPV-6 and HPV-18 among patients who have contracted simultaneous infections (p=0.0001). Conversely, there is no statistically significant association observed between age and the occurrence of simultaneous infections with different types (p=0.09). However, a statistical correlation is discerned between HPV-16 infection and age (p=0.02), with a higher prevalence noted among younger individuals. Importantly, the analysis reveals no statistically significant relationships between age and pathological features (p=0.6), as well as for all other unmentioned variables (p>0.05). It is noteworthy that the Chi-square and U-Mann Whitney tests were employed in all the aforementioned statistical analyses.
Discussion
The findings of this study provide valuable insights into the prevalence of various genotypes of human papillomavirus (HPV) in cervical tissue samples from women, as determined through pyrosequencing. A total of 1045 patients referred for periodic cervical examinations were included in this study, with 159 (15%) of them testing positive for HPV infection, while 886 exhibited no evidence of infection. This yielded an overall HPV infection rate of 15% within this study population, indicating a relatively low prevalence of HPV. The 15% prevalence rate for HPV infection observed in this study is lower than that reported in several other Iranian studies, ranging from 39% to 87% [12-17]. Comparing the prevalence of HPV in this study to other studies conducted in Iran, Kayhan et al. found a prevalence of 73% [12], Al-Lawati et al. reported an HPV prevalence rate of 82% [13], and Onsory et al. reported a prevalence of 79% [14]. These differences in prevalence rates may be attributed to variations in the methodologies employed across the different studies. The current study utilized cervical tissue samples, while some previous studies relied on cervical smears or swabs. Tissue samples may have lower viral loads compared to direct cervical samples.
In the present study, 20% of the examined HSIL cases were found to be associated with HPV, which is often a precursor to cervical cancer. Additionally, 50% of the LSIL cases examined were linked to HPV, as were 30% of the ASCUS cases. Discrepancies in these prevalence rates may be attributed to variations in the methodologies employed across the different studies. Regarding pathological lesions, our HSIL HPV prevalence aligns with Esmaeili et al.’s 39-66% range [16] but is lower than Allameh et al.’s 82% [15]. For LSIL, our 50% rate is lower than Esmaeili et al.’s 83%. These variations could reflect geographic differences in genotype distribution across Iran. Additionally, the use of tissue samples in our study compared to direct cervical samples may have impacted HPV detection sensitivity in precancerous lesions.
Regarding HPV genotypes, our finding that types 6, 18, 11, and 16 are the most common matches previous reports of type 16 dominance in Iran [17-19]. Safaei et al. identified HPV type 16 as the most common type in Shiraz [17], while Mortazavi et al. associated it with types 16, 18, and 33 [18]. Mobini et al. also found type 16 prevalent in their study [19]. In some studies, types 6 and 11 are more common than other genotypes in the low-risk group [20, 21].
In this study, according to the age group, 30 to 40 years had the highest prevalence of HPV. In another study, the age group of 21 to 40 years had the highest prevalence of HPV [22]. Liao et al. reported that the age group of 40 to 49 years had the highest prevalence [23]. Sabet et al. reported that the age group of 26 to 35 years had the highest prevalence of HPV [5]. This difference in age distribution between the results of the present study and other studies indicates a significant limitation in the present study, which is due to the limited number of the studied population.
Findings indicate a lower prevalence of HPV-6 and HPV-18 among patients who have contracted simultaneous infections (p=0.0001). One study reported that co-infections were more prevalent with types 11 and 18 [24]. The results of another study showed that types 16, 18, 6, and 11 have a high tendency to be involved in co-infection [25].
Moreover, no statistically significant relationship exists between the age of simultaneous infection with different types (p=0.09). However, in a study, co- infections in 35% of women’s cases, which was higher in people under 31 years of age, indicating that co-infections and the prevalence of HPV decrease with age [5]. Another study showed that women aged 35 to 49 years had a lower risk of HPV co-infections [26].
However, there is a statistically significant relationship between HPV-16 infection and age (p=0.02). HPV-16 is more common in young adults. Markowitz et al. reported that the prevalence of HPV 16 and 18 is related to increasing age [27]. Also, Lu et al. reported that HPV 16 reaches its highest level between the ages of 35 and 44 [28].
This study had no significant relationship between age and pathological features (p=0.06). Baandrup et al. reported that some lesions associated with HPV 16 occur at a younger age than lesions associated with other high-risk HPV infections [29].
Our use of tissue samples versus cervical cytology specimens in other studies may have impacted overall HPV detection sensitivity. However, applying nested PCR and pyrosequencing genotyping likely conferred good analytical sensitivity and specificity.
In summary, while some variance in prevalence was observed compared to other Iranian studies, possibly related to sample type differences, we confirm the ubiquity of high-risk HPV-16 in cervical tissues. The study’s limitations include the lack of follow-up clinical data correlating HPV status with disease progression. Additionally, the reliance on pathology referrals may bias the patient cohort versus population-based screening. Moreover, there is a lack of information on the prevalence of multiple genotypes in one patient and the potential impact of HPV genotypes on disease progression. Additionally, the study needed to directly compare the types of samples and methods used in other studies conducted in Iran.
In conclusion, this study contributes to the body of knowledge regarding HPV prevalence and genotype distribution in cervical tissue samples. The observed high prevalence of HPV infection, particularly with high-risk genotypes, underscores the importance of continued vigilance and comprehensive cervical cancer prevention measures, including vaccination and regular screening programs. Future research may delve deeper into genotype-specific risks and outcomes, enabling more targeted interventions for the protection of women’s cervical health.
The results showed that out of 1045 samples collected from the cervical tissue of patients with cervical cancer, 159 (15%) were positive for HPV infection and 886 were negative. Among the different genotypes found in these samples, frequent types were 6, 18, 11, 16, respectively. The age group of 30 to 40 years had the highest prevalence of HPV. HPV-16 is common in younger ages. Further studies by greater sample sizes recommend to better understanding.
Ethical Approval
Ethics was approved in Iran University of Medical Sciences, Tehran, Iran, Ethical Committee (ethics code: IR.IUMS.REC.1402.955).
Funding
Iran University of Medical Sciences, Tehran, Iran (grant code: 1402-4-30-28055).
Conflict of interest
None to declare.
Authors Contribution
Study conception and design: M.H.K.N.; data collection: M.A., N.E., S.Y., M.S.; analysis and interpretation of results: M.F., G.H., M.A., N.E.; draft manuscript preparation: N.S.K., M.E., M.H.K.N. All authors reviewed the results and approved the final version of the manuscript.
References
- Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, Arbyn M, et al . The Lancet. Global Health.2023;11(2). CrossRef
- Human Papillomaviruses-Related Cancers: An Update on the Presence and Prevention Strategies in the Middle East and North African Regions Fernandes Q, Allouch S, Gupta I, Elmakaty I, Elzawawi KE , Amarah A, Al-Thawadi H, Al-Farsi H, Vranic S, Al Moustafa A. Pathogens (Basel, Switzerland).2022;11(11). CrossRef
- Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation Kombe Kombe AJ , Li B, Zahid A, Mengist HM , Bounda G, Zhou Y, Jin T. Frontiers in Public Health.2020;8. CrossRef
- Detection of Human Papilloma Virus (HPV) Infection in Colorectal Cancer and Viral Genome Integration Status Karbalaie Niya MH , Tameshkel F, Motamed N, Miri S, Mortazavi H, Ajdarkosh H, Zamani F, Keyvani H. Shiraz E-Medical Journal.2022;23. CrossRef
- Prevalence, genotypes and phylogenetic analysis of human papillomaviruses (HPV) in northeast Iran Sabet F, Mosavat A, Ahmadi Ghezeldasht S, Basharkhah S, Shamsian SAA , Abbasnia S, Shamsian K, Rezaee SA . International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases.2021;103. CrossRef
- Malignancy Associated with Low-Risk HPV6 and HPV11: A Systematic Review and Implications for Cancer Prevention Silva LL , Teles AM , Santos JMO , Souza de Andrade M, Medeiros R, Faustino-Rocha AI , Oliveira PA , et al . Cancers.2023;15(16). CrossRef
- Trends in Cervical Cancer Incidence in Iran According to National Cancer Registry Vafaeinezhad Z, Kazemi Z, Mirmoeini M, Piroti H, Sadeghian E, Mohammad Ali-Vajari M, Fattah N, Roza M, Jafari M. Journal of Mazandaran University of Medical Sciences.2018;28(161).
- Cervical cancer in Iran: integrative insights of epidemiological analysis Momenimovahed Z, Salehiniya H. BioMedicine.;8(3). CrossRef
- Investigating feasibility of 2021 WHO protocol for cervical cancer screening in underscreened populations: PREvention and SCReening Innovation Project Toward Elimination of Cervical Cancer (PRESCRIP-TEC) Sultanov M, Zeeuw J, Koot J, Schans J, Beltman JJ , Fouw M, Majdan M, et al . BMC public health.2022;22(1). CrossRef
- Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices Petrosky E, Bocchini JA , Hariri S, Chesson H, Curtis CR , Saraiya M, Unger ER , Markowitz LE . MMWR. Morbidity and mortality weekly report.2015;64(11).
- Human Papillomavirus Investigation in Head and Neck Squamous Cell Carcinoma: Initial Report from the Low Risk HPV Types Associations Karbalaie Niya MH , Safarnezhad Tameshkel F, Panahi M, Bokharaei Salim F, Monavari SHR , Keyvani H. Asian Pacific journal of cancer prevention: APJCP.2017;18(9). CrossRef
- The prevalence of human papilloma virus(HPV)in malignant cervical lesion, using multiplex PCR Keyhani E, Kohannia N, Izadimood N, Keyhkhaee MR , Najmabadi H. Tehran-Univ-Med-J.2006;64(3):905-101.
- Prevalence of human papilloma virus in Oman: Genotypes 82 and 68 are dominating Al-Lawati Z, Khamis FA , Al-Hamdani A, Al-Kalbani M, Ramadhan FA , Al-Rawahi TR , Al-Kobaisi MF . International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases.2020;93. CrossRef
- Epidemiology Of Human Papilloma Virus (Hpv) Type 16 And 18 In The Patients With Cervical Cancer In Tehran Elahe J, Reza AA , Khadijeh O. 2016;8(425).
- Reviewing the Prevalence of Human Papillomavirus (HPV) in Married Women Aged 18-60 Years with Normal Pap Smear and Referring to Gynecology Clinics in Hospitals Affiliated to Isfahan University of Medical Sciences, Iran Allameh T, Moghim S, Farahbod F. Journal of Isfahan Medical School.2011;29(163).
- HPV typing in women with cervical precancerous and cancerous lesions in northwestern Iran Esmaeili M, Bonyadi M, Dastranj A, Alizadeh M, Melli MS , Shobeiri MJ . Gynecologic and Obstetric Investigation.2008;66(1). CrossRef
- Prevalence of high-risk human papillomavirus types 16 and 18 in healthy women with cytologically negative pap smear in Iran Safaei A, Khanlari M, Momtahen M, Monabati A, Robati M, Amooei S, Valibeigi B, Azarpira N. Indian Journal of Pathology & Microbiology.2010;53(4). CrossRef
- The Prevalence of Human Papillomavirus in Cervical Cancer in Iran Mortazavi SH , Zali MR , Raoufi M, Nadji M, Kowsarian P, Nowroozi A. Asian Pacific journal of cancer prevention: APJCP.2002;3(1).
- Identification of human papillomavirus type 16 among thinprep samples from 11 Provinces of Iran Mobini Kesheh M, Kafashi A, Bagheri G, Shahkarami MK , Mohamadi M, Naji SA . Iranian Journal of Obstetrics, Gynecology and Infertility.2013;16:22-8.
- Distribution of human papillomavirus types in anogenital warts of men Chan PKS , Luk ACS , Luk TNM , Lee K, Cheung JLK , Ho K, Lo K. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology.2009;44(2). CrossRef
- Human papillomavirus infection: etiopathogenesis, molecular biology and clinical manifestations Leto MDGP , Santos Júnior GFD , Porro AM , Tomimori J. Anais Brasileiros De Dermatologia.2011;86(2). CrossRef
- Distribution of human papillomavirus genotypes in suspected women cytological specimens from Tehran, Iran Tabibzadeh A, Panahi M, Bouzari B, Haghi Ashtiani MT , Zamani F, Teimoori Arzati H, Karbalaie Niya MH . Iranian Journal of Microbiology.2022;14(1). CrossRef
- Multi-Infection Patterns and Co-infection Preference of 27 Human Papillomavirus Types Among 137,943 Gynecological Outpatients Across China Liao G, Jiang X, She B, Tang H, Wang Z, Zhou H, Ma Y, et al . Frontiers in Oncology.2020;10. CrossRef
- Prevalence of human papillomavirus types 6, 11, 16, 18, 31, and 33 in a cohort of Greek women Panotopoulou E, Tserkezoglou A, Kouvousi M, Tsiaousi I, Chatzieleftheriou G, Daskalopoulou D, Magiakos G. Journal of Medical Virology.2007;79(12). CrossRef
- Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease Chaturvedi AK , Katki HA , Hildesheim A, Rodríguez AC , Quint W, Schiffman M, Van Doorn L, et al . The Journal of Infectious Diseases.2011;203(7). CrossRef
- Association of HIV status with infection by multiple HPV types Camargo M, Del Río-Ospina L, Soto-De León SC , Sánchez R, Pineda-Peña AC , Sussmann O, Patarroyo ME , Patarroyo MA . Tropical medicine & international health: TM & IH.2018;23(11). CrossRef
- Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004 Markowitz LE , Sternberg M, Dunne EF , McQuillan G, Unger ER . The Journal of Infectious Diseases.2009;200(7). CrossRef
- Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: results from the multinational HPV Infection in Men Study (HIM Study) Lu B, Viscidi RP , Lee J, Wu Y, Villa LL , Lazcano-Ponce , Silva RJC , et al . Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2011;20(5). CrossRef
- HPV16 is associated with younger age in women with cervical intraepithelial neoplasia grade 2 and 3 Baandrup L., Munk C., Andersen K. K., Junge J., Iftner T., Kjær S. K.. Gynecologic Oncology.2012;124(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details