The Relationship between Tumor Budding and Stromal Maturity with Tumor Characteristic and Survival in Patients Gastric Cancer
Download
Abstract
Background: Considering the prognostic role of stromal maturity and tumor budding in various cancer types, along with the importance of gastric cancer as the fifth most prevalent cancer globally and the second leading cause of cancer-related mortality, this study aimed to investigate the impact of stromal maturity and tumor budding on patients with gastric cancer.
Methods: In this retrospective cohort study, 91 patients diagnosed with gastric cancer and who underwent total gastrectomy in educational hospitals of Babol between 2016 and 2021 were evaluated. Slides corresponding to the deepest invasive tumor edge were reviewed by two independent pathologists to assess tumor budding and stromal maturity. Immunohistochemical staining with AE1/AE3 marker was used to correlate the results of tumor budding evaluation. Other pathological information was collected from patients’ records, and patient survival data were obtained through telephone follow-up. Data analysis was performed with SPSS V.22, utilizing T-test, Anova, Chi-square, Fisher’s exact test, Kaplan-Meier and Cox regression method. P-value <0.05 was considered as significant level.
Results: The average survival of patients without tumor budding was 64.64 months, while the average survival of the low tumor budding group (less than 5 tumor buds in 10 microscopic fields at ×400 magnification) was 58.38 months, and the high tumor budding group (less than 5 tumor buds) had an average survival of 28.52 months (p=0.002). The average survival of patients with and without stromal maturity was 41.43 and 61.36 months, respectively (p=0.501).
Conclusion: Tumor budding is an independent prognostic factor in gastric adenocarcinoma, but its predictive value is limited to the intestinal type of adenocarcinoma
Introduction
Stomach cancer ranks fifth globally in terms of both incidence and mortality in 2022 [1]. The TNM classification, based on the depth of tumor invasion, the number of involved lymph nodes, and the presence or absence of distant metastasis, is the most common method for determining tumor prognosis in cancer patients [2]. However, a significant number of patients still succumb to gastric cancer in the early stages of the disease [3]. Determining the histological pattern may be useful in improving the accuracy of the TNM staging and identifying high-risk patients who would benefit from aggressive treatment [4, 5].
Tumor budding and stromal maturity are two characteristics that can aid in determining the level of cancer invasion. Tumor budding refers to the presence of single cells or clusters of 2 to 4 cells at the invasive front of the tumor [3]. High tumor budding is recognized as a poor prognostic marker in colorectal cancer [6] and has also been associated with poor prognosis in other types of cancer, such as breast cancer [7], squamous cell carcinoma of the head and neck [8], and pancreatic adenocarcinoma [9].
Furthermore, stromal tissue and fibroblasts play a significant role in tumor growth [10, 11]. In gastric cancer, tumors with desmoplastic stroma types have prognostic value [12, 13]. Immature stroma characterized by myxoid stroma or the presence of thick collagen fibers and eosinophilic hyalinization, is associated with poor prognosis. On the other hand, mature desmoplastic stroma, characterized by thin collagen fibers in the stroma, indicates a good prognosis [14, 15].
Tumor budding associated with poor prognosis in gastric cancer [3] [16] but there was limited focus on stromal maturity and due to importance of gastric cancer as a global health concern [17], the aim of this study was to investigate the prognostic effect of tumor budding and stromal maturity in patients referred to teaching hospitals in Babol city between 2016 and 2021.
Materials and Methods
Study design
The present study is a retrospective cohort study in which 90 patients with gastric adenocarcinoma, who underwent total gastrectomy in educational hospitals in Babol city during the years 2016-2021, were included, and their H&E slides were entered into the study from the pathology department archives of the hospitals. Patients with a previous history of gastric cancer or other malignancies, patients who received radiotherapy and chemotherapy prior to surgery, patients with incomplete recorded information in their medical records, patients with an unknown date of death, and patients who died due to causes other than gastric adenocarcinoma were excluded from the study. In addition, after reviewing the H&E slides, cases with histological subgroups of signet ring cell carcinoma and poorly differentiated tumors were excluded from the study.
Data collection
Patient survival data were collected through telephone follow-up since the time of surgery. After reviewing all stained sections with the H&E method related to the gastrectomy specimens, the histological subgroups of gastric adenocarcinoma were determined based on the criteria of WHO, Goski classification, and Lauren classification. Samples of intestinal-type adenocarcinoma with good and moderate differentiation were separated, and slides corresponding to the deepest invasive tumor area in terms of tumor budding and stromal maturity were examined by two experienced pathologists. The pathologists were blinded to the output and clinical data. Stromal maturity was determined by examining the deepest invasive edge of the tumor and the desmoplastic reaction at the invasive edge of the tumor. In tumors where the desmoplastic reaction was not assessable at the invasive edge, especially tumors invading the subserosal tissue, the entire tumor stroma was studied for more accuracy. The “hot spot” technique was used to study tumor budding, which is one of the most practical methods for evaluating tumor budding in colorectal cancers [6]. H&E slides related to the deepest invasive edge were initially examined with low magnification to find the area with the highest number of tumor buds. If it was not clear which area had the highest number of tumor buds or if the first area under examination had less than 15 tumor buds, several areas were examined, and the area with the highest number of tumor buds was analyzed at a magnification of *400. An average of 5 tumor buds per ten high-power fields (HPF) was considered as the cut-off [7]. High tumor budding was defined as 5 or more tumor buds per ten HPF, and low tumor budding was defined as fewer than 5 tumor buds per ten HPF. The observed results were compared with the immunohistochemistry- stained slides with the AE1/AE3 marker. Immature stroma is defined as the presence of thick bundles and scanty collagen with eosinophilic hyalinization; the Mochizuki grouping of stroma was performed based on the most immature portion of the tumor stroma. If there was no hyalinized collagen or if it was present in less than 5% of the tumor stroma and and there was desmoplastic area in front of the tumor, the stroma was considered mature, and tumors containing colloid-like collagens in 5% or more of the tumor stroma or having myxoid stroma were considered immature stroma.
Statistical analysis
The data were analyzed using SPSS V.22 software. T-test, Anova, Chi-square and Fisher’s exact test were used to compare between groups, and Kaplan-Meier and Cox regression methods were used for survival analysis.. A P-value less than 0.05 was considered significant.
Results
Patient Characteristics
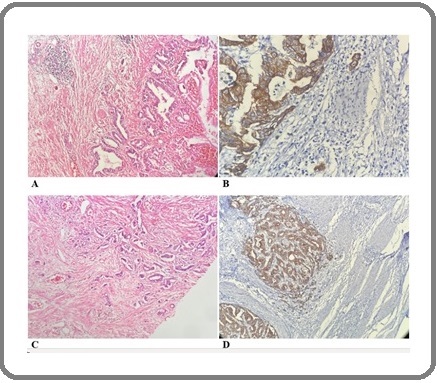
In this study, 90 patients with gastric cancer who underwent gastrectomy and had not received neoadjuvant chemotherapy were evaluated. The mean age of the individuals was 67.31 years (SD=11.59). The minimum age reported was 33, and the maximum age was 86. Of this number, 67 individuals (73.6%) were male, and 24 individuals (26.4%) were female. Thirty-two individuals (35.2%) had tumors with moderate differentiation, and 59 individuals (64.8%) had tumors with good differentiation. The highest proportion of T stage was T3, with 22 individuals (24.2%), and the lowest proportion was T4, with 1 case (1.1%). Seventeen individuals (18.7%) had no tumor budding, 22 individuals (24.2%) had low tumor budding, and 52 individuals (57.1%) had high tumor budding (Figure 1).
Figure 1. Low Tumor Budding in GastricAdenocarcinoma with H and E Staining (A) and Immunohistochemistry for AE1/AE3 (B), and High Tumor Budding in Gastric Adenocarcinoma with H and E Staining (C) and Immunohistochemistry (D).
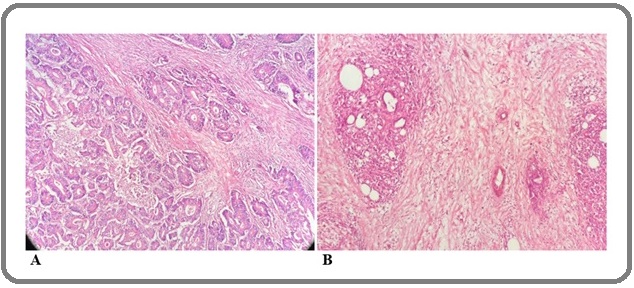
Fifty-four individuals (59.3%) had stromal maturity (Figure 2).
Figure 2. Mature (A) and Immature (B) Stroma in Gastric Adenocarcinoma.
The mean (standard deviation) age in mature patients was 65.96 (11.06) years, and in immature patients, it was 69.27 (12.21) years (p = 0.183). Patients without tumor budding had a mean (standard deviation) age of 65.12 (13.6) years, those with low tumor budding had a mean age of 65.55 (12.83) years, and those with high tumor budding had a mean age of 68.77 (10.53) years, (p = 0.283).
Associations Patient
As shown in Table 1, gender and stage variables did not show a significant association with tumor budding and stromal maturity. While stromal maturity did not have a significant association with tumor differentiation (p = 0.19), tumor differentiation showed a significant association with tumor budding, where patients with moderate differentiation had a higher percentage of high tumor budding compared to patients with good differentiation (p = 0.048).
| Sex | Differentiation | Stage | |||||
| Female | Male | Well | Moderate | I/II | III/IV | ||
| Tumor budding | Negative | 3 (12.5) | 14 (20.9) | 2 (6.2) | 1 (25.4) | 13 (24.5) | |
| Low | 5 (20.8) | 17 (25.4) | 7 (21.9) | 15 (25.4) | 14 (26.4) | 8 (21.1) | |
| High | 16 (66.7) | 36 (53.7) | 23 (71.7) | 29 (49.2) | 26 (49.1) | 26 (68.4) | |
| Total | 24 (100) | 67 (100) | 32 (100) | 59 (100) | 53 (100) | 38 (100) | |
| p-value | 0.513 | 0.048 | 0.133 | ||||
| Stroma | Mature | 14 (58.3) | 40 (59.7) | 16 (50) | 38 (64.4) | 32 (60.4) | 22 (57.9) |
| Immature | 10 (41.7) | 27 (40.3) | 16 (50) | 21 (35.6) | 21 (39.6) | 16 (42.1) | |
| Total | 24 (100) | 67 (100) | 32 (100) | 59 (100) | 53 (100) | 38 (100) | |
| p-value | 1 | 0.19 | 0.832 |
Survival Analysis
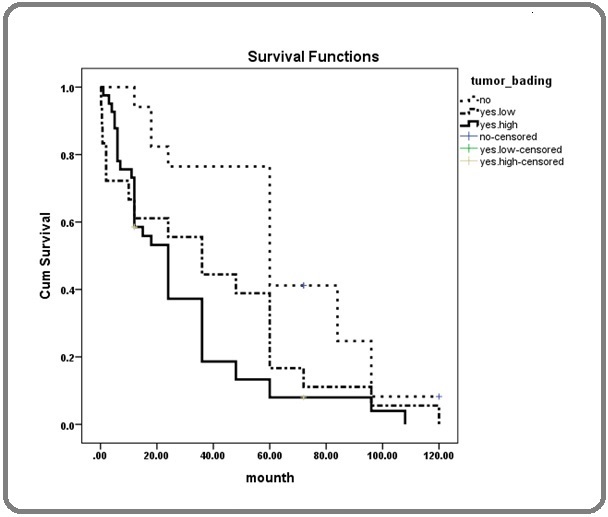
The overall survival of the study patients was 39.52 months with a 95% confidence interval (31.8- 47.23). Individuals without tumor budding had a mean survival of 64.64 months, individuals with low tumor budding had a mean survival of 38.58 months, and individuals with high tumor budding had a mean survival of 28.52 months (Figure 3), showing a statistically significant difference (p = 0.002).
Figure 3. Patient Survival Using the Kaplan-Meier Method Based on Tumor Budding Levels.
However, there was no significant difference in survival among different stromal maturity levels (Table 2) (Figure 4).
| Mean Survival | CI 95% | p-value | ||
| Lower | Upper | |||
| Tumor budding | ||||
| Negative | 64.641 | 49.699 | 80.184 | 0.002 |
| Low | 38.587 | 22.334 | 55.38 | |
| High | 28.523 | 20.025 | 37.022 | |
| Total | 39.52 | 31.808 | 47.231 | |
| Stroma | ||||
| Mature | 41.439 | 31.368 | 51.511 | 0.501 |
| Immature | 36.615 | 24.701 | 48.529 | |
| Total | 39.52 | 31.808 | 47.231 |
Figure 4. Patient Survival Based on Stromal Maturity Levels.
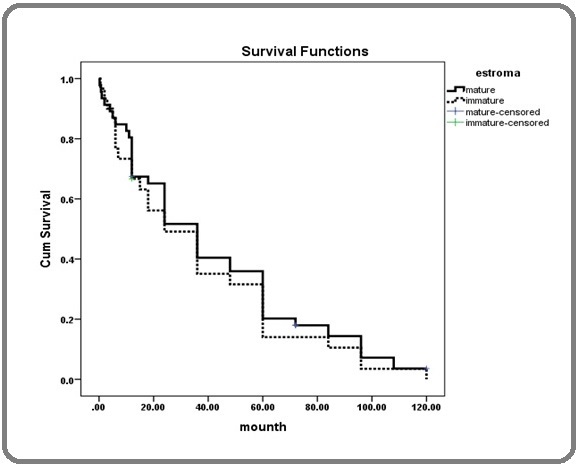
Table 3 and Cox regression analysis also demonstrated a significant association between tumor budding status and patient survival, with a hazard ratio of 2.681 (p = 0.002).
| Hazard Ratio | CI 95% | P-value | |||
| Lower | Lower | ||||
| Stroma (Immature/ Mature) | 1.078 | 0.666 | 1.743 | 0.761 | |
| Tumor budding | Negative | - | - | - | - |
| Low | 1.894 | 0.935 | 3.838 | 0.076 | |
| High | 2.681 | 1.418 | 5.068 | 0.002 |
Discussion
In present study, average survival in patients with high tumor budding was significantly lower than patients with low and negative tumor budding. As well as, the survival effect size for higher tumor budding compared to without tumor budding was high. These findings are consistent with the study conducted by Kemi et al. in 2019, where approximately 583 gastric cancer patients undergoing surgery at the University Hospital of Oulu were examined. The patients were divided into low and high-budding groups, and tumor budding was evaluated in relation to 5-year survival and overall survival. In intestinal-type adenocarcinoma, the high-budding group had significantly lower 5-year survival compared to the low-budding group. There was no difference in 5-year survival between the budding groups in diffuse-type adenocarcinoma [3]. These results are also in line with the study by Che et al. in 2017, which focused on evaluating tumor budding and single-cell invasion in gastric adenocarcinoma. Patients with high-budding tumor had lower overall survival compared to patients with low-budding tumor. According to the Lauren classification, patients with intestinal-type adenocarcinoma had better outcomes than those with diffuse-type adenocarcinoma. Based on the results of this study, tumor budding and single-cell invasion in gastric adenocarcinoma are associated with unfavorable prognosis [18]. In another study by Yavuz, et al in Turkey in 130 patients with gastric adenocarcinoma show that Intratumoral budding was a significant risk factor associated with poor prognosis in gastric cancer. The multivariate Cox regression analysis confirms that Intratumoral budding is an independent prognostic variable. Additionally, univariate analysis highlights Intratumoral budding as a strong parameter for predicting survival , and it is also found to be an independent prognostic factor [19]. In comparison to the results of the present study and other studies regarding the prognostic role of tumor budding in gastric cancer, it is evident that tumor budding plays a crucial role in predicting survival outcomes in patients with gastric cancer.
In our study, survival was examined in relation to stromal maturity at the invasive tumor edge, and the results showed that individuals with mature stroma had high average survival compared to immature stroma. These results are consistent with the study conducted by Kemi et al. in 2019, which evaluated patients with gastric adenocarcinoma undergoing surgical treatment [3], but did not show a statistically significant difference. This lack of significance is likely due to the duration of the present study, as our patients were enrolled over a period of 5 years, while the study by Kemi et al. included patients from 1983 to 2016, resulting in a larger sample size for them. Similar to these result, in another study, Cheng, et al. investigated predictive indicators role of tumor stroma maturity, in 695 esophagogastric junction adenocarcinoma patients. High stromal levels or immature stroma were associated with worse prognoses and high stromal levels in preoperative biopsies correlated with poor neoadjuvant therapy response [20].
In present study, relative frequency of high tumor budding and stromal immaturity in higher stage patients were higher than lower stage. But there was direct association between tumor budding and staging in gastric cancer patients in Kim, et al, study [18]. It’s possible that the study had a small sample size or lacked statistical power to detect significant associations between tumor budding and stage. Additionally poorly differentiated intestinal type had been excluded in present study.
One of the strengths of this study is the concordance of the findings from the examination of H and E-stained slides with those from AE1/AE3-immunohistochemistry- stained slides, which increases the accuracy of the method [21]. Additionally, patients with neoadjuvant chemotherapy were excluded. Limitations of the study include the limited number of gastric resection samples and the limited follow-up period for assessing patient survival.
Based on the findings from the present study, we recommend investigating the underlying molecular mechanisms that drive tumor budding in gastric cancer. Understanding the cellular processes involved could offer valuable insights into potential therapeutic targets and prognostic markers.
In conclusion, the present study’s findings regarding the prognosis of the disease based on tumor budding and stromal maturity indicate that individuals without tumor budding had a significantly higher average survival. However, the evaluation of stromal maturity showed that patients with mature stroma had a higher average survival compared to those with immature stroma, although this difference was not statistically significant and may needs large sample size study. So, it appears that tumor budding plays a crucial role in predicting survival outcomes in patients with gastric cancer.
Acknowledgments
Statement of Transparency and Principals:
Conflict of interest
The authors declare no competing interests.
Author contribution
NN, AH, NN, MR and GK performed study concept and design. RAN analyzed the data. NN, GK and RAN drafted the manuscript. NN, AH, NN, MR, GK and RAN reviewed the manuscript and approved final.
Ethics approval and consent to participate
This research approved by Research Ethics Committees of Babol University of Medical Science with approval ID: IR.MUBABOL.REC.1399.474.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- Survival rates in T1 and T2 gastric cancer: A Western report Ikoma N, Blum M, Chiang Y, Estrella JS , Roy-Chowdhuri S, Fournier K, Mansfield P, Ajani JA , Badgwell BD . Journal of Surgical Oncology.2016;114(5). CrossRef
- Tumor Budding and Prognosis in Gastric Adenocarcinoma Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ , Kauppila JH . The American Journal of Surgical Pathology.2019;43(2). CrossRef
- Time trends in the occurrence of major GI cancers in Iran Yazdizadeh B, Jarrahi AM , Mortazavi H, Mohagheghi MA , Tahmasebi S, Nahvijo A. Asian Pacific journal of cancer prevention: APJCP.2005;6(2).
- Determining the Effective Factors in the Incidence of Gastric Cancer by Using Data Mining Approach Mahmoodi SA , Mirzaie K, Mahmoodi SM . Payavard Salamat.2017;11(3).
- Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016 Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, e al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2017;30(9). CrossRef
- The prognostic value of tumor budding in invasive breast cancer Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z. Pathology, Research and Practice.2013;209(5). CrossRef
- Tumour budding in head and neck squamous cell carcinoma - a systematic review Almangush A, Salo T, Hagström J, Leivo I. Histopathology.2014;65(5). CrossRef
- Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma O'Connor K, Li-Chang HH , Kalloger SE , Peixoto RD , Webber DL , Owen DA , Driman DK , et al . The American Journal of Surgical Pathology.2015;39(4). CrossRef
- Desmoplastic Pattern at the Tumor Front Defines Poor-prognosis Subtypes of Colorectal Cancer Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, et al . The American Journal of Surgical Pathology.2017;41(11). CrossRef
- The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer Bremnes RM , Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund L. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2011;6(1). CrossRef
- Disentangling the prognostic heterogeneity of stage III colorectal cancer through histologic stromal categorization Ueno H, Sekine S, Oshiro T, Kanemitsu Y, Hamaguchi T, Shida D, Takashima A, et al . Surgery.2018;163(4). CrossRef
- Prognostic Significance of Tumour Budding and Desmoplastic Reaction in Intestinal-Type Gastric Adenocarcinoma Pun C, Luu S, Swallow C, Kirsch R, Conner JR . International Journal of Surgical Pathology.2023;31(6). CrossRef
- Histological assessment of stromal maturity as a prognostic factor in surgically treated gastric adenocarcinoma Kemi NA , Eskuri M, Pohjanen V, Karttunen TJ , Kauppila JH . Histopathology.2019;75(6). CrossRef
- Desmoplastic Reaction, Immune Cell Response, and Prognosis in Colorectal Cancer Akimoto N, Väyrynen JP , Zhao M, Ugai T, Fujiyoshi K, Borowsky J, Zhong R, et al . Frontiers in Immunology.2022;13. CrossRef
- Prognostic and pathological impact of tumor budding in gastric cancer: A systematic review and meta-analysis Guo Y, Zhang Z, Zhao G, Zhao E. World Journal of Gastrointestinal Oncology.2019;11(10). CrossRef
- Tumour-stroma ratio and prognosis in gastric adenocarcinoma Kemi N, Eskuri M, Herva A, Leppänen J, Huhta H, Helminen O, Saarnio J, Karttunen TJ , Kauppila JH . British Journal of Cancer.2018;119(4). CrossRef
- Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma Che K, Zhao Y, Qu X, Pang Z, Ni Y, Zhang T, Du J, Shen H. OncoTargets and Therapy.2017;10. CrossRef
- Prognostic significance of tumor budding, desmoplastic reaction, and lymphocytic infiltration in patients with gastric adenocarcinoma Yavuz A, Simsek K, Alpsoy A, Altunay B, Gedik EO , Unal B, Bassorgun CI , Tatli AM , Elpek GO . World Journal of Gastrointestinal Pathophysiology.2024;15(1). CrossRef
- Tumor stroma ratio, tumor stroma maturity, tumor-infiltrating immune cells in relation to prognosis, and neoadjuvant therapy response in esophagogastric junction adenocarcinoma Cheng N, Wang B, Xu J, Xue L, Ying J. Virchows Archiv: An International Journal of Pathology.2024. CrossRef
- Digital image analysis of pan-cytokeratin stained tumor slides for evaluation of tumor budding in pT1/pT2 colorectal cancer: Results of a feasibility study Jepsen RK , Klarskov LL , Lippert MF , Novotny GW , Hansen TP , Christensen IJ , Høgdall E, Riis LB . Pathology, Research and Practice.2018;214(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details