Hepatoprotective Effects of Silybum Marianum Extraction Against Acetamiprid-Induced Stress Oxidative in Male Rats: Potential Anticancer Application
Download
Abstract
Objective: Silybum marianum, commonly known as milk thistle, has been extensively studied for its hepatoprotective effects. While most documented data focus on its benefits for liver disorders, recent research has highlighted its protective effects on other organs such as the kidneys. Studies have shown that Silybum marianum exhibits antioxidant, lipid-lowering, antihypertensive, antidiabetic, antiatherosclerotic, anti-obesity, and hepatoprotective properties.
Materials and Methods: In the present study we investigated the protective effect of aqueous extraction of Silybum marianum against acetamiprid-induced (N’-cyano-N-methyl-acetamidine phosphorothioate) stress oxidative on liver and kidney function by evaluating the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and urea in male rats. Furthermore, an anatomical study was conducted on the liver and kidney to enhance the research.
Results: The results showed the effective impacts of aqueous extraction of Silybum marianum on AST, ALT, creatinine, and urea serum levels. There was also a protective effect in the group of rats fed with both acetamiprid and extraction of Silybum marianum compared to the group fed with only acetamiprid.
Conclusion: Our results revealed that Marianum extract can reduce hepatotoxic and nephrotoxic effects caused by acetamiprid and has anti-hepatocyte cancer potential.
Introduction
Liver cancer, particularly hepatocellular carcinoma (HCC), is a significant global health concern due to its high mortality rates [1]. HCC accounts for over 90% of liver cancer cases and is a leading cause of cancer-related deaths worldwide [2]. Enzymes formation by liver cells play a critical role in a broad extent of reactions cellular metabolic. Indicators of liver damage or stress oxidative include increase liver enzymes levels. Liver enzyme levels, mostly ALT and AST this a result of hepatitis, cirrhosis, non-alcoholic fatty and liver disease [3]. The ALT (alanine aminotransferase) and AST (aspartate aminotransferase) are two liver enzymes. ALT is primarily localized in the cellular cytoplasm of the liver, while AST is present in both the cytosol and mitochondria [3]. Elevated ALT levels are closely associated with liver fat accumulation, making it a key marker for nonalcoholic fatty liver disease (NAFLD) [4]. It is also confirmed that elevated ALT is a key risk factor for HCC development in chronic hepatitis B patients [5]. Generally elevated levels of ALT and AST indicates liver damage [6]. Hepatocellular carcinoma presents a significant challenge due to limited curative treatment options. While orthotropic liver transplantation or surgical resection are curative, they are only suitable for a subset of patients [1]. Studies have shown that active ingredients such as flavonoids, saponins, acids, phenols, and alkaloids, can have hepatoprotective benefits, making them attractive for treating liver injury [7]. Additionally, herbal derived medicines like silibinin have demonstrated the ability to inhibit cell proliferation and promote apoptosis in HCC cells [8]. It has been extensively studied for its various pharmacological properties, including anti-hepatotoxic, anticancer, antioxidant, and anti-inflammatory effects [9, 10]. Additionally, silibinin has been found to decrease serum AST and ALT levels and hepatic necrosis induced by chemical liver damage [11]. Silibinin, also known as silybin, is a flavonolignan extracted from milk thistle seeds and fruits [12]. One of the daisy family asteraceae is the Silybum marianum [13]. It is found in the Mediterranean region and is also grown all over the world [14]. Milk thistle’s active ingredients include a group of flavonoids known as silymarin (silibinin); it is the main component of interest [15]. The resultant silymarin extract has several potential applications, from nutritional supplements and herbal medicines to pharmaceuticals. Commonly used to promote liver health and as a natural therapy for a variety of liver diseases, silymarin has gained notoriety for its possible hepatoprotective (liver-protecting) effects [15]. In terms of therapeutic value, milk thistle is most well-known for the assistance it may provide for the liver. As was noted before, silymarin is the aggregate name for the active chemicals in milk thistle. Herbal medicines and nutritional supplements containing silymarin are often utilized to treat liver-related disorders due to the compound’s presumed antioxidant and anti-inflammatory qualities [16, 17].
Creatinine, glomerular filtration rate, urea and other are some of the indicators mostly clinical tested in a medicine of blood and urine analyses used for determination kidney damage [18, 19]. Research on the effects of milk thistle, or Silybum marianum, on urea and creatinine levels has been conducted. Serum creatinine variations after angiography were not different between the silymarin and placebo groups, although the silymarin group had a decreased incidence of contrast-induced nephropathy (CIN) [10, 20]. According to another study, there was no statistically significant difference in serum urea levels between groups which received silymarin and groups which received milk thistle extract [21].
The effects of acetamiprid (N-(6-chloro-3- pyridylmethyl)-N’-cyano-N-methylacetamidine) on the liver and kidneys are not addressed in the search results. However, there is some information that ingesting or encountering acetamiprid might be harmful. It has also been proven to be quite stable in the environment and accumulate in living creatures while being very resistant to biological destruction. The Environmental Protection Agency (EPA) regulates the chemical because of its use as a pesticide. The Environmental Protection Agency (EPA) establishes limits for allowable levels of pesticides and other chemicals in food and agricultural raw materials. The toxicity and effects of a chemical may change based on the dosage, the route of exposure, and other variables [22-24].
This study aimed to evaluate the hepatoprotective effect of Silybum marianum aqueous extract against stress oxidative induced via the acetamiprid in male rats.
Materials and Methods
Preparation of Extraction
In this study we applied the aqueous extraction of Silybum marianum that purified and characterized in our previous studies. Briefly, following the collection and pulverization of plant components, the subsequent extraction procedure included immersion in the customary solvent, water. This was followed by filtering and drying operations, resulting in the acquisition of the extract in a powdered state.
Experiment animals
Healthy male Wistar rats (30) with an average mass of (120±10) g were obtained from the animal house in the College of Science, University of Al-Qadisiyah, where the study was conducted. The animals were housed in a temperature-controlled room at 21-28°C. The animals were randomly divided into five groups as illustrated in Figure 1.
Figure 1. The Study Design of Various Groups. G1) control group, G2) received only extraction of Syllibium marianum, G3) received only acetamiprid, G4) firstly received extraction of Silybum marianum then received acetamiprid, G5) firstly received acetamiprid then received extraction of Silybum marianum.
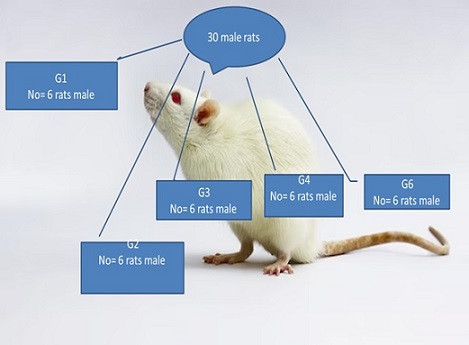
1- G1 group as control group
2- G2 group which were dosed with aqueous extraction of Syllibium marianum (100 mg/kg) for three weeks
3- G3 group which were dosed with acetamiprid (30 mg/kg) for three weeks.
4- G4 group which were dosed with aqueous extraction of Syllibium marianum (100 mg/kg) for three weeks and then acetamiprid (30 mg/kg) for another three weeks.
5- G5 group which were dosed with acetamiprid (30 mg/kg) for three weeks and then aqueous extraction of Syllibium marianum (100 mg/kg) for another three weeks
Biochemical assays
The levels of liver enzymes (AST and ALT) in the serum samples were measured (IU/L) using commercial Randox kit by automated analyzer device (HITACHI 911). Creatinine in alkaline solution reacts with picric acid to form a colored complex. The amount of the complex formed is directly proportional to the creatinine concentration. Randox test kit was used for this estimation. Using fresh double-distilled water (ddH2O), performed a new Gain Calibration in cuvette mode. Select CREA in the Run Test screen and carry out with water blank as instructed in the test manual. Urea is hydrolyzed in the presence of water and urease to produce ammonia and carbon dioxide. The ammonia produced in the first reaction combines with α-oxoglutarate and NADH in the presence of glutamate dehydrogenase. Using fresh ddH2O, performed a new Gain Calibration in flow cell mode. (Randox test kit was also used for this estimation).
Sections of the liver and kidney were examined histologically
Following a 24-hour fixation in 10% formalin saline and dehydration in ethyl alcohol (30, 50, 70, 90%, and 100%), the tissues were rinsed in running tap water. Paraffin (melting point 58–60°C) was used after specimens were washed in xylene for 24 hours. Tissue blocks were formed from paraffin wax and sectioned at 4°C using a slide sludge microtome. Hematoxylin and eosin (H&E) stain [25] was used to examine deparaffinized tissue sections under an electric light microscope for histopathological analysis.
Results and Discussion
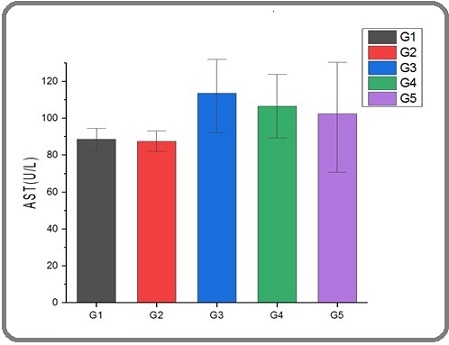
The liver assumes a pivotal role in the process of detoxification inside the human body [26]. The process involves the filtration of blood originating from the digestive organs, facilitating the transportation of essential nutrients, pharmaceutical compounds, and harmful chemicals [27]. Upon reaching the liver, these chemicals undergo a series of physiological processes, including processing, storage, alteration, detoxification, and subsequent re-entry into the bloodstream or elimination via the gastrointestinal tract [28]. Figures 2 and 3 shows a significant increase in ALT and AST enzyme activity in G3 group compared to G1, G2, G4, and G5 groups, the reason for these increases are attributed to effect of the acetamiprid.
Figure 2. Level of ALT in Serum Male Rats of G1, G2, G3, G4, and G5 Groups.
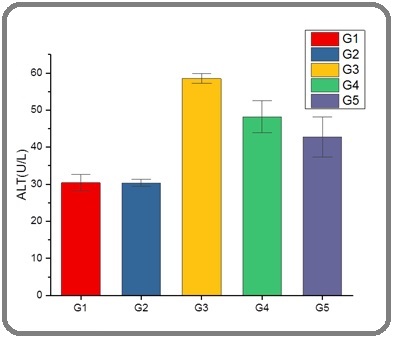
Figure 3. Level of AST in Serum Male Rats of G1, G2, G3, G4, and G5 Groups .
The chemical molecule known as acetamiprid is a subgroup of organophosphate insecticides [29]. The chemical in this compound has the potential to elicit detrimental effects on hepatic enzymes. Organophosphate pesticides have been identified as agents that impede the functionality of acetylcholinesterase, an enzymatic catalyst responsible for the degradation of the neurotransmitter acetylcholine [30]. This inhibition may lead to the buildup of acetylcholine in the synaptic cleft, producing overstimulation of the nervous system and leading to symptoms such as muscular twitching, weakness, and respiratory failure [31]. The liver assumes a pivotal role in the processes of metabolism and detoxification of organophosphate pesticides. The hepatic metabolism of these chemicals entails the involvement of the cytochrome P450 system, a crucial enzymatic pathway responsible for the oxidative biotransformation and detoxification of xenobiotics and a significant decreasing in (G3 and G4) groups this could be due to activate compounds in Silybum Marianum extraction. Its usage in aqueous medium has been proven to have good results due to preventive and therapeutic properties regarding liver disorders. The extract of Silybum marianum, known as silymarin, has been investigated to regulate enzymes that are implicated in the progression of cellular injury fibrosis, and cirrhosis [25]. The administration of an ethanolic extract derived from silymarin seeds has shown a significant reduction in liver enzymes within the rat population. For more than two millennia, silymarin has mostly been used in the management of liver and gall bladder problems, including jaundice, hepatitis, and cirrhosis.
Silymarin has antifibrotic, immunomodulatory, anti-inflammatory, and antioxidant characteristics [32]. The substance functions as an agent that scavenges free radicals and regulates enzymes which are implicated in the progression of cellular harm, fibrosis, and cirrhosis [33-36].
Figures 4 and 5 show a significant increase in Urea and creatinine levels in the G3 group compared to the G1, G2, G4, and G5 groups.
Figure 4. Serum Level of Urea in Male Rats of G1, G2, G3, G4 and G5 Groups .
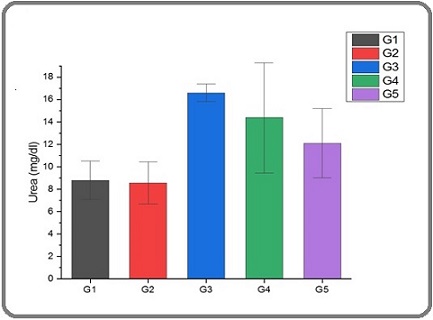
Figure 5. The Serum Level of Creatinine in Male Rats of G1, G2, G3, G4, and G5 Groups.
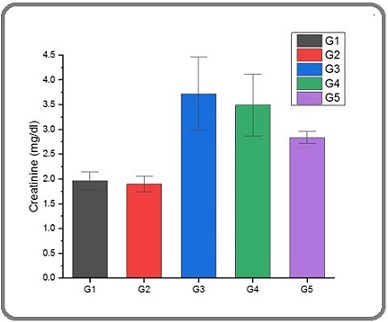
The reason for these increases is attributed to the effect of acetamiprid. Investigations have shown that the sub-chronic oral administration of acetamiprid has the potential to induce hepatotoxicity and nephrotoxicity in male rats [37]. In addition, the administration of acetamiprid resulted in oxidative harm and impacted the liver, as shown by the presence of injury indicators such as cholesterol levels as well as the enzymes alanine aminotransferase and aspartate aminotransferase [38]. Additionally, a reduction in plasma levels of urea, uric acid, and creatinine was seen, perhaps indicating liver damage. Based on histological tests, it was shown that the hepatotoxicity of acetamiprid was more pronounced compared to its nephrotoxicity [37]. There is a significant decrease in G2 and G5 groups compared to G3 group this could be due to ability of Silybum marianum aqueous extraction. The current study recommended that the aqueous extraction of Silybum marianum has the potential to play as therapeutic and prevention in protecting the kidneys and liver in mice from damages of free radicals. This role may be related to its ability to reduced oxidative stress, liver damage, kidney damage, and inflammation [39].
A study found that Silybum marianum and Taraxacum officinale extracts applied together had a protective effect against oxidative kidney injuries induced by carbon tetrachloride in rats [39]. More research suggested that giving milk thistle extract from Silybum marianum might help protect against kidney damage caused by cisplatin in animal models.
The review study examined the potential of plant-based therapeutics in the context of chronic kidney disease (CKD). It talked about the results of studying how Silybum marianum affects inflammation, oxidative stress, and kidney damage in rat models, showing big drops in these things. The use of Silybum marianum as a therapeutic intervention for hepatoprotection has been well established. However, a comprehensive review paper posits that this botanical treatment may potentially provide additional advantages beyond its traditionally recognized liver-protective properties [39-42].
Figures 6 represents the cross section of the liver tissue of mice. Cross section of the liver in the control G1 group shows normal liver histology (Figure 6.A).
Figure 6. A Cross Section of the Liver Tissue in Untreated Mice and Mice Treated with Extraction of Silybum Marianum and Acetamiprid.
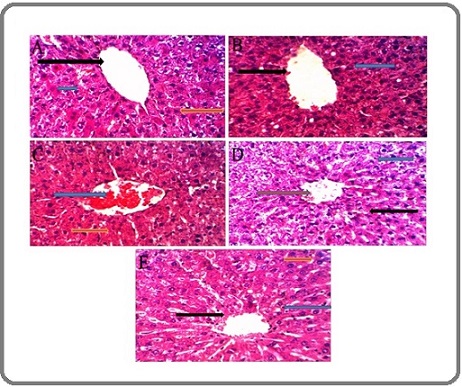
The G2 group was treated with Silybum marianum extract only. Result was same as control group (normal liver tissue) with an arrow (black) indicating a central vein, and the arrow (blue) indicates hepatocytes arranged in radial bands (Figure 6.B). Figure 6.C represents a cross-section of the liver tissue of G3 group, feed with acetamiprid only. Blue arrow indicates blood congestion in the central vein, and the yellow arrow indicates simple necrosis. A simple histological change occurs in livers tissue of G4 group. The blue arrow indicates a slight expansion of the hepatic sinusoids and slight congestion. The yellow arrow indicates the presence of a central vein, and the black arrow indicates the hepatocytes arranged in the form of radial bands. Finally, in liver tissue of the G5 group (Figure 6.E) which were feed with acetamiprid and then with aqueous extraction of Silybum marianum, the central vein (black arrow), indicates hepatocytes arranged normally (yellow arrow), the hepatic sinusoids (blue arrow) are evident.
Figure 7 represents the cross section of the kidney tissue of mice. In figure 7.A which represent G1 group (a normal kidney tissue), the bowman’s capsule (blue arrow), glomerulus (the black arrow), and the proximal urinary tubules (yellow arrow) are evident.
Figure 7. A Cross Section of the Kidney Tissue in Untreated Mice and Mice Treated with Extraction of Silybum Marianum and Acetamiprid.
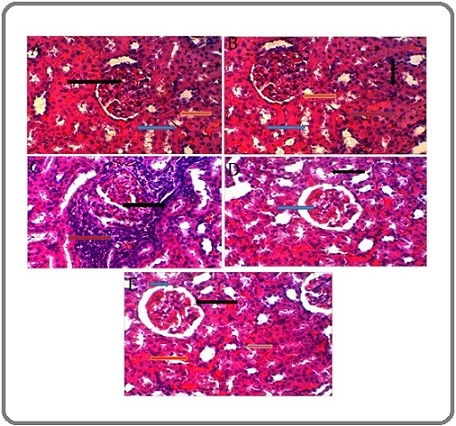
Figure 7.B shows the proximal urinary tubules (black arrow); Bowman’s capsule (yellow arrow); glomerulus (red arrow); and the distal urinary tubules (blue arrow) of kidney tissue of G2 group. Figure 7.C represents a cross section of the kidney tissue of G3 group. The red arrow indicates hemorrhage, and the black arrow indicates inflammatory cell infiltration. In G4 group (Figure 7.D) the occurrence of some simple pathological changes is notable the blue arrow indicates a slight expansion of bowman’s capsule, and the black arrow indicates a slight necrosis in the cells lining the urinary tubules. Finally, Figure 7.E represents a cross-section of kidney tissue in G5 group. The black arrow indicates the glomerulus, the blue arrow indicates bowman’s capsule, the red arrow indicates the distal urinary tubules, and the yellow arrow indicates the proximal urinary tubules.
In conclusion, we focused on investigating the effect of Silybum marianum aqueous extract on liver and kidney function in rats treated with acetamiprid and its potential to prevent liver and kidney damage. The results showed that the aqueous extract of Silybum marianum significantly affects the serum levels of AST, ALT, creatinine, and urea. Notably, mice administered together with acetamiprid and Silybum marianum extract showed a protective effect compared to mice that received only the phosphorothioate combination. This shows that Silybum marianum extract can reduce hepatotoxic and nephrotoxic effects caused by acetamiprid and has anti-hepatocyte cancer potential. More research is needed to fully understand the renal function of this plant extract on hepatocellular carcinoma.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Hepatocellular carcinoma: a review Balogh J, Victor D, Asham EH , Burroughs SG , Boktour A, Saharia A, Li X, Ghobrial RM , Monsour HP . Journal of Hepatocellular Carcinoma.2016;3. CrossRef
- Challenges in liver cancer and possible treatment approaches Anwanwan D, Singh SK , Singh S, Saikam V, Singh R. Biochimica Et Biophysica Acta. Reviews on Cancer.2020;1873(1). CrossRef
- Liver enzyme alteration: a guide for clinicians Giannini EG , Testa R, Savarino V. Canadian Medical Association journal.2005;172(3). CrossRef
- Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease Schindhelm RK , Diamant M, Dekker JM , Tushuizen ME , Teerlink T, Heine RJ . Diabetes/Metabolism Research and Reviews.2006;22(6). CrossRef
- A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis El-Serag HB , Kanwal F, Davila JA , Kramer J, Richardson P. Gastroenterology.2014;146(5). CrossRef
- Liver damage in severely obese patients: a clinical-biochemical-morphologic study on 1,000 liver biopsies Papadia FS , Marinari GM , Camerini G, Murelli F, Carlini F, Stabilini C, Scopinaro N. Obesity Surgery.2004;14(7). CrossRef
- Molecular mechanism and research progress on pharmacology of traditional Chinese medicine in liver injury Zhang HY , Wang HL , Zhong GY , Zhu JX . Pharmaceutical Biology.2018;56(1). CrossRef
- Silibinin: a potential old drug for cancer therapy Zhu X, Ding Y, Wu Y, Qian L, Zou H, He Q. Expert Review of Clinical Pharmacology.2016;9(10). CrossRef
- Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2 Hsieh Yi, Chu S, Yang S, Chen P, Liu Y, Lu K. Carcinogenesis.2007;28(5). CrossRef
- Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2 Chu S, Chiou H, Chen P, Yang S, Hsieh Y. Molecular Carcinogenesis.2004;40(3). CrossRef
- Comparison of the Hepatoprotective Effects of Four Endemic Cirsium Species Extracts from Taiwan on CCl₄-Induced Acute Liver Damage in C57BL/6 Mice Zhao Z, Chang J, Lin L, Tsai F, Chang H, Wu C. International Journal of Molecular Sciences.2018;19(5). CrossRef
- Influence of silybin on biophysical properties of phospholipid bilayers Wesołowska O, Łania-Pietrzak B, Kuzdzał M, Stanczak K, Mosiadz D, Dobryszycki P, Ozyhar A, et al . Acta Pharmacologica Sinica.2007;28(2). CrossRef
- The Plants of the Asteraceae Family as Agents in the Protection of Human Health Rolnik A, Olas B. International Journal of Molecular Sciences.2021;22(6). CrossRef
- Cultivation of milk thistle ( Silybum marianum L. Gaertn.), a medicinal weed Karkanis A, Bilalis D, Efthimiadou A. Industrial Crops and Products - IND CROPS PRODUCTS.2011;34. CrossRef
- Clinical applications of Silybum marianum in oncology Greenlee H, Abascal K, Yarnell E, Ladas E. Integrative Cancer Therapies.2007;6(2). CrossRef
- The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines Nathan M. Annals of Internal Medicine.1999;130(5). CrossRef
- Milk thistle (Silybum marianum). Nonvitamin and nonmineral nutritional supplements: Elsevier Devi KP . 2019;:321-325.
- Mayo Clinic gastroenterology and hepatology board review: Oxford University Press Hauser SC , Sweetser SR , Leise MD , Ravi K , Bruining DH . 2024.
- Hepatorenal syndrome Ginès P, Solà E, Angeli P, Wong F, Nadim MK , Kamath PS . Nature Reviews. Disease Primers.2018;4(1). CrossRef
- Silymarin for the Prevention of Contrast-Induced Nephropathy: A Placebo-Controlled Clinical Trial Sedighifard Z, Roghani F, Bidram P, Harandi SA , Molavi S. International Journal of Preventive Medicine.2016;7. CrossRef
- Silymarin in non alcoholic fatty liver disease Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. World Journal of Hepatology.2013;5(3). CrossRef
- Toxicity of the acetamiprid insecticide for mammals: a review Phogat A, Singh J, Kumar V, Malik V. Environmental Chemistry Letters.2022;20. CrossRef
- Influence of Acetamiprid on Soil Enzymatic Activities and Respiration Yao X, Jin S, Lü Z, Yuan H. European Journal of Soil Biology - EUR J SOIL BIOL.2006;42. CrossRef
- Apta-nanosensors for detection and quantitative determination of acetamiprid - A pesticide residue in food and environment Verdian A. Talanta.2018;176. CrossRef
- Progressive histologic injury in kidneys from heart and liver transplant recipients receiving cyclosporine Falkenhain M. E., Cosio F. G., Sedmak D. D.. Transplantation.1996;62(3). CrossRef
- Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation Guerville M, Boudry G. American Journal of Physiology. Gastrointestinal and Liver Physiology.2016;311(1). CrossRef
- Ameliorative effects of soy 11S protein on liver damage and hyperlipidemia in alcohol-fed rats Park KJ , Kim HY , Chang BJ , Lee HH . Biological & Pharmaceutical Bulletin.2004;27(10). CrossRef
- Biologie anatomie physiologie: Elsevier Health Sciences Menche N. 2023.
- Enzymes involved in the bioremediation of pesticides. Industrial applications of microbial enzymes: CRC Press Ahmad S, Bhatt P, Ahmad HW , Cui D, Guo J, Zhong G, et al . 2022;:133-168.
- Acetylcholinesterase Inhibition in Rats and Humans Following Acute Fenitrothion Exposure Predicted by Physiologically Based Kinetic Modeling-Facilitated Quantitative In Vitro to In Vivo Extrapolation Chen J, Zhao S, Wesseling S, Kramer NL , Rietjens IMCM , Bouwmeester H. Environmental Science & Technology.2023;57(49). CrossRef
- A Rare Presentation of Severe Organophosphate Poisoning: A Case Report and Review of Literature Ibrahim AE , Ghantarchyan H, Le T, Bhagat A, Maknouni B, Arabian S. Cureus.2022;14(11). CrossRef
- Silymarin Attenuates ELMO-1 and KIM-1 Expression and Oxidative Stress in the Kidney of Rats with Type 2 Diabetes Goli F, Karimi J, Khodadadi I, Tayebinia H, Kheiripour N, Hashemnia M, Rahimi R. Indian journal of clinical biochemistry: IJCB.2019;34(2). CrossRef
- Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review Gillessen A, Schmidt HH . Advances in Therapy.2020;37(4). CrossRef
- "Silymarin", a promising pharmacological agent for treatment of diseases Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. Iranian Journal of Basic Medical Sciences.2011;14(4).
- Milk Thistle (Silybum Marianum): A Valuable Medicinal Plant With Several Therapeutic Purposes Šimora V, Ďúranová H, Bilcikova J, Habán M. Journal of microbiology, biotechnology and food sciences.2020;9. CrossRef
- Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer Fallah M, Davoodvandi A, Nikmanzar S, Aghili S, Mirazimi SMA , Aschner M, Rashidian A, et al . Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2021;142. CrossRef
- Toxic effects of subchronic oral acetamiprid exposure in rats Karaca BU , Arican YE , Boran T, Binay S, Okyar A, Kaptan E, Özhan G. Toxicology and Industrial Health.2019;35(11-12). CrossRef
- Reactivity of hydroxyl radicals with neonicotinoid insecticides: mechanism and changes in toxicity Dell'arciprete ML , Santos-Juanes L, Sanz AA , Vicente R, Amat , Furlong JP , Mártire DO , Gonzalez MC . Photochemical & Photobiological Sciences: Official Journal of the European Photochemistry Association and the European Society for Photobiology.2009;8(7). CrossRef
- Protective effect of Silybum marianum and Taraxacum officinale extracts against oxidative kidney injuries induced by carbon tetrachloride in rats Karakuş A, Değer Y, Yıldırım S. Renal Failure.2017;39(1). CrossRef
- Cisplatin nephrotoxicity and protection by milk thistle extract in rats Karimi G, Ramezani M, Tahoonian Z. Evidence-Based Complementary and Alternative Medicine: eCAM.2005;2(3). CrossRef
- Silybum marianum: Beyond Hepatoprotection Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M. Journal of Evidence-Based Complementary & Alternative Medicine.2015;20(4). CrossRef
- Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats El-Khishin IA , El-Fakharany YMM , Abdel Hamid OI . Toxicology Reports.2015;2. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details