The Association of Helicobacter pylori Infection and Virulence Factors in Gastric Cancer in Thi-Qar, Iraq
Download
Abstract
Background: The significance of Helicobacter pylori (H. pylori) infection in developing gastric cancer has been increasingly recognized. However, a noticeable gap exists in the literature regarding the prevalence of key virulence factors, particularly in Iraq. Therefore, Our study aimed to investigate the prevalence of H. pylori infection and the presence of virulence factors, namely the CagA and VacA genes, in gastric cancer patients.
Methods: A case-control study was conducted on 45 gastric cancer patients (38 males and 7 females) from September 2020 to February 2021. During routine endoscopic procedures, gastric biopsy specimens were collected from the tumour and adjacent non-tumour tissues. The presence of H. pylori was detected using quantitative real-time PCR, and the virulence factors CagA and vacA were identified using specific primers.
Results: H. pylori infection was detected in a significant 85% (38/45) of the patients, with a higher prevalence in males 89.5% (34/38) compared to females 10.5% (4/38) (P=0.021) (Chi-square test). No significant age-related differences were observed. The CagA gene was present in a substantial 58.8% (22/38) of H. pylori-positive patients, predominantly in tumor tissues at 81.81% (18/22), while the vacA gene was found in only one patient. These results underscore the importance of our research in understanding the prevalence of H. pylori infection and the presence of virulence factors in gastric cancer patients.
Conclusion: The high prevalence of H. pylori infection and the significant presence of the CagA virulence factor in tumour tissues underscore the bacterium’s role and risk factors in gastric carcinogenesis in Thi-Qar, Iraq.
Introduction
The etiology of most cancers stems from genetic instability, encompassing chromosomal and molecular genetic aberrations [1]. Stomach cancer, ranking as the fourth most common malignancy and the third leading cause of cancer-related deaths worldwide across both genders, poses a significant public health challenge. Late-stage diagnoses and dismal prognoses are common due to delayed patient identification [2, 3]. Notably, stomach cancer stands as the tenth least prevalent cancer type in Iraq, characterized by its rarity [4].
Helicobacter pylori (H. pylori) was identified as a class I carcinogen in 1994 by the World Health Organization (WHO) [5]. Over half of the adult population in the globe is infected with H. pylori [6]. H. pylori infection has long been recognized as a significant risk factor for the development of various gastrointestinal diseases, including peptic ulcers and gastric cancer [7]. Among the diverse array of virulence factors exhibited by H. pylori, the cytotoxicity-associated gene A (CagA) and vacuolating cytotoxin gene (vacA) stand out as pivotal contributors to its pathogenicity [8, 9]. Despite the global prevalence of H. pylori infection, its association with gastric cancer remains a pressing concern, particularly in regions such as Iraq [10]. The presence of CagA is strongly associated with more severe clinical outcomes, including atrophic gastritis, intestinal metaplasia, and gastric adenocarcinoma. Studies indicate that CagA-positive H. pylori strains significantly increase the risk of developing gastric cancer compared to CagA-negative strains [11, 12], while VacA contributes to creating a conducive environment for cancer development [13].
According to the Global Cancer Observatory (IARC 2024), gastric cancer remains a considerable public health issue in Iraq. The age-standardized incidence and mortality rates of cancer among the Iraqi population in 2018, as displayed in the Global Cancer Observatory [14], are 105.5 and 64.7, respectively (). The latest Iraqi Cancer Registry (ICR) has illustrated that the total number of new cancer cases during 2018 was 31,502, with an incidence rate of 82.6/100,000 population; 43% occurred in males and 57% in females [15]. Thi-Qar, situated in southern Iraq, presents a unique context for studying the molecular aspects of H. pylori infection and its correlation with gastric cancer [16]. Despite the significant burden of stomach cancer in the region, there remains a notable absence of research investigating the presence and impact of H. pylori virulence factors, such as CagA and vacA, in affected individuals. Understanding the molecular mechanisms underlying H. pylori-associated gastric carcinogenesis is essential for devising effective prevention and treatment strategies tailored to the specificities of the local population [17].
Considering this knowledge gap, the present study aims to elucidate the presence and significance of various virulence factors of H. pylori extracted from stomach cancer patients in Thi-Qar, Iraq. By employing robust detection methods and analyzing H. pylori isolates, we seek to contribute valuable insights into the pathogenesis of gastric cancer in this region. Ultimately, this research endeavours to pave the way for targeted interventions aimed at mitigating the impact of H. pylori infection on gastric cancer incidence and improving the management of affected individuals in Thi-Qar, Iraq, and similar settings worldwide.
Materials and Methods
Sample Collection
This case-control study collected forty-four samples of gastric cancer patients referred to oncology institutions in Thi-Qar, Iraq, from September 2020 to February 2021. Inclusion criteria were patients diagnosed with gastric cancer who were referred to oncology institutions in Thi-Qar, Iraq, between September 2020 and February 2021, patients who underwent routine endoscopic procedures, during which gastric biopsy specimens could be collected, individuals who provided informed consent to participate in the study, and patients with sufficient biopsy specimens obtained from both tumor and adjacent non-tumor tissue for comparative analysis. Also, patients who did not provide informed consent for participation in the study, individuals with insufficient or inadequate biopsy specimens that could not be used for DNA extraction and molecular analysis, patients who had previously undergone treatment for H. pylori infection, such as antibiotics or proton pump inhibitors, within the last 4 weeks before sample collection, as this could affect the bacterial load and detection were excluded from the study.
Sampling Protocol and Specimen Preparation
The gastric biopsy specimens were collected aseptically during routine endoscopic procedures from both the tumor and adjacent non-tumor tissue of gastric cancer patients. Immediately after collection, the samples were placed in sterile containers and transported to the laboratory under cold conditions to ensure sample integrity.
Specimen Processing
Once in the laboratory, each biopsy sample was cut into small pieces and homogenized to ensure uniform distribution of bacterial content. The homogenized samples were then suspended in 1500 mL of PBS buffer (pH 7.0) to create a uniform suspension for subsequent culturing and molecular analysis.
Differential Cultures and Isolation of H. pylori
A portion of each prepared sample (100 mL) was cultured on blood agar and a selective medium specifically designed for H. pylori isolation (Himedia Laboratories, Kennett Square, PA, USA). The selective medium contained antibiotics to inhibit the growth of non-H.pylori bacteria, allowing for the isolation of H. pylori colonies. The plates were incubated at 37°C under microaerophilic conditions (5% O2, 10% CO2, 85% N2) for 3-7 days.
Confirmatory Phenotypic Tests and Morphology
Colonies suspected of being H. pylori based on their morphological characteristics (small, translucent, and greyish colonies) were further confirmed using phenotypic tests, including oxidase, catalase, and urease tests, all characteristic of H. pylori. Only positive samples for these tests were considered for further molecular analysis.
Molecular Confirmation of H. pylori and Virulence Factors
According to the manufacturer’s instructions, genomic DNA was extracted from gastric tissue samples using Maxwell RCS Tissue DNA kit (Promega, WI, USA). The extracted DNA was stored at -20°C until further analysis. The concentration of total DNA was determined using fluorometric quantification with the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Vilnius, Lithuania). Quantitative RT-PCR was performed to detect the presence of H. pylori DNA, utilizing primers specific for the housekeeping gene (18S ribosomal RNA) as an internal control to assess DNA integrity. The ATCC’s (700392D-5) H. pylori strain 26695 genomic DNA was used to generate the standard curve and as a positive control. Specific primers previously described were used to identify H. pylori [18]. CagA and VacA virulence factors were detected using specific primers targeting their respective gene sequences (Table 1).
| Primers | Sequence of the primers (5 to 3) | Size of the product by base pairs |
| Cag.A | Forward: TGCTAAATTAGACAATTGACGA | 132bp |
| Reverse: AATAATCAACAAACATCACGCCT | ||
| Vac.A | Forward: GCGGTATCAATCTGTCCAATCA | 678bp |
| Reverse: TGATATTCCCGGTTAGTTTTCCA |
Five pmol of primers and ten μl of the master mix (FastStart Essential DNA Green Master; Roche, Mannheim, Germany) were used in the reactions. The LightCycler 96 Instrument was used to perform the PCR. After preparing the PCR mixture, the thermocycler program was set to 35 cycles. The time of the primary denaturation stage was 5 minutes at 95 cº, the denaturation stage45 sec at 94 Cº, the annealing stage 35 sec at 51 Cº, and the extension stage 45 sec at 72 cº. The amplified fragments were electrophoresed on 1% agarose gel and then visualized by DNA-safe stain (Cinaclone, Iran).
Statistical analysis
The Microsoft Excel program computed clinical and pathological feature rates (Table 1). Data on associations were analyzed employing R and SPSS version 20 (IBM, Inc., Armonk, NY, USA). Inter-agreement between methods was assessed using the Cohen kappa index. Statistical significance was determined at an alpha level of p < 0.05 with a confidence level of 95%.
Results
Forty-five gastric cancer patients, comprising 38 males and 7 females, participated in this study (Table 2).
| Variable | Result | Total | P-value | ||
| Positive | Negative | ||||
| Gender | Female | 4 | 3 | 7 | 0.021⁎ |
| Male | 34 | 4 | 38 | ||
| <10 | 2 | - | 2 | 0.879 | |
| 20-Nov | 4 | 1 | 5 | ||
| Age group | 21-30 | 8 | 1 | 9 | |
| 31-40 | 7 | 1 | 8 | ||
| 41-50 | 11 | 3 | 14 | ||
| >50 | 6 | 1 | 7 |
The majority of the participants were between 41 and 50 years old (n=14). H. pylori infections were identified using a Real-time PCR method with specific primers, revealing that 38/45 (85%) of the sample tested positive for H. pylori. The remaining 7/45 (15%) tested negative. Among the 38 positive cases, 4/38 (10.5%) were females, and 34/38 (89.5%) were males, indicating a significant gender difference (P=0.021). However, no significant difference was observed when comparing age groups (P>0.05). The distribution of H. pylori infection across different age groups did not show a significant association (P = 0.879). Infection was present across all age categories, with the highest number of positive cases found in the 41-50 age group (11 positives out of 14 total). CagA gene-positive subjects were detected in 22/45 (48.8%) of H. pylori-positive patients, and they were found significantly more in tumour tissue [18/22 (81.81%)] than in non-tumour tissue [4/22 (18.19%)] (P=0.03). The vacA gene was detected in the tumour tissue of only one H. pylori-positive patient.
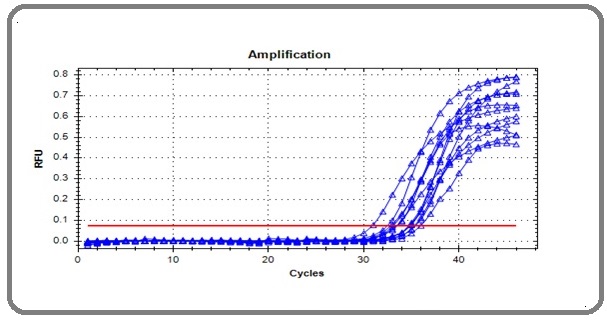
The amplification of the CagA gene appeared as a curve represented by the real-time cycler (Figure 1), each representing the amplification of a single sample.
Figure 1. The Cytotoxic Related Gene (CagA) Amplification Curve in Real-Time PCR Amplification Plot.
Discussion
In the present study, we investigated the prevalence of H. pylori infection and the presence of virulence factors, specifically the CagA and VacA genes, in gastric cancer patients. Our findings shed light on the association between H. pylori infection and gastric carcinogenesis and the distribution of virulence factors in tumour and non-tumour tissues.
The high prevalence of H. pylori infection observed in our study population (85%) aligns with previous reports highlighting the significance of H. pylori as a major risk factor for gastric cancer development [19, 20]. A study by Kumar et al. (2020) involving 371,813 veterans diagnosed with H. pylori infection found significantly higher risks of gastric cancer among racial and ethnic minorities [19]. Additionally, a study by Shirani et al. reported that Japan had the highest pooled prevalence of gastric cancer among H. pylori-positive patients, with a prevalence of 90.90% (95% CI: 83.61–95.14) [20].
The predominance of H. pylori infection in males compared to females is consistent with the established epidemiological pattern, which may be attributed to differences in lifestyle factors, dietary habits, and hormonal influences [21]. In the study by Kawai et al. (2022), the mean adjusted cumulative incidence risk for gastric cancer in the H. pylori-infected population was 17.0% for males and 7.7% for females, while the incidence risk in the uninfected population was 1.0% for males and 0.5% for females [21]. The significantly higher proportion of H. pylori-positive males underscores the importance of gender-specific considerations in H. pylori-associated gastric cancer risk assessment and management.
Furthermore, our study identified the presence of the CagA gene in approximately 58.8% of H. pylori-positive patients, with a significantly higher prevalence observed in tumour tissue compared to non-tumour tissue. These findings corroborate previous studies implicating the CagA protein as a major virulence factor associated with gastric cancer pathogenesis [22, 23]. The preferential localization of the CagA gene in tumour tissue underscores its potential role in promoting tumour progression and aggressiveness.
Blaser et al. study from North America has indicated that infection with cagA-positive H. pylori strains elevates the risk of atrophic gastritis and gastric cancer [24]. Conversely, this correlation has not been consistently observed in multiple studies involving Asian populations [25]. European studies have demonstrated a significant association between the vacA m1 genotype and epithelial damage, characterized by neutrophilic and lymphocytic infiltrates, atrophic gastritis, and intestinal metaplasia. Severe gastric epithelial damage has also been linked to vacA s1/m1 mosaicism [26].
Interestingly, we observed a relatively lower prevalence of the VacA gene in our study population, with only one H. pylori-positive patient exhibiting positivity in tumour tissue. The limited detection of the VacA gene may suggest its variable distribution among H. pylori strains or its differential expression during gastric carcinogenesis [24]. Further investigation is warranted to elucidate the specific role of the VacA gene in gastric cancer development and progression.
The differential distribution of virulence factors between tumour and non-tumour tissues underscores the complex interplay between H. pylori infection and gastric carcinogenesis. The preferential localization of virulence factors in tumour tissue highlights their potential contribution to tumour initiation, progression, and metastasis. Future studies exploring the mechanical interactions between H. pylori virulence factors and host factors within the tumour microenvironment may provide valuable insights into the molecular pathways underlying H. pylori-induced gastric carcinogenesis [27].
Although our study yielded interesting insights, it is important to acknowledge several limitations. Firstly, the limited sample size of 45 patients with stomach cancer could limit the generality of our findings to larger populations. More significant, multicenter studies with a broader range of demographic participants must confirm our findings and make more reliable conclusions on the virulence factors and prevalence of H. pylori infection in gastric cancer. Second, real-time PCR was the main method used in our investigation to identify H. pylori infection and virulence factors. Despite being extremely sensitive and specific, PCR-based techniques might not be able to detect all H. pylori strains and virulence factors that are present in the stomach mucosa. utilizing extra molecular methods, like multiplex PCR tests that target several virulence factors or whole-genome sequencing
In conclusion, these findings highlight a significant presence of the CagA virulence factor among H. pylori- positive gastric cancer patients in Thi-Qar, Iraq, with a notable gender disparity in infection rates. However, the VacA gene was rarely detected, suggesting a lesser role in the pathogenicity within this population. These findings underscore the importance of H. pylori eradication strategies and the potential utility of virulence factor-targeted therapies in gastric cancer management.
Acknowledgements
The authors would like to thank all who supported this survey.
Ethical approval
The research received ethical approval (DSM/HO- 15314) for scientific research from the ethics committees of the Ministry of Higher Education and Scientific Research (MOHESR) in Iraq.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors of this manuscript did not receive any funding to perform the present research
References
- Chromosome Instability; Implications in Cancer Development, Progression, and Clinical Outcomes Vishwakarma R, McManus KJ . Cancers.2020;12(4). CrossRef
- Epidemiology of gastric cancer: global trends, risk factors and prevention Rawla P, Barsouk A. Przeglad Gastroenterologiczny.2019;14(1). CrossRef
- Epidemiology of stomach cancer Ilic M, Ilic I. World Journal of Gastroenterology.2022;28(12). CrossRef
- Incidence Rates, Pattern and Time Trends of Registered Cancer in Iraq (1991-2008) Population and Hospital Based Registry Hamid Y, Al-Alawachi S. OALib.2014;01. CrossRef
- Helicobacter pylori in gastric carcinogenesis Ahn HJ , Lee DS . World Journal of Gastrointestinal Oncology.2015;7(12). CrossRef
- Epidemiology of Helicobacter pylori infection Burucoa C, Axon A. Helicobacter.2017;22 Suppl 1. CrossRef
- Helicobacter pylori infection Malfertheiner P, Camargo MC , El-Omar E, Liou J, Peek R, Schulz C, Smith SI , Suerbaum S. Nature Reviews. Disease Primers.2023;9(1). CrossRef
- Polysaccharides as Bacterial Antiadhesive Agents and "Smart" Constituents for Improved Drug Delivery Systems Against Helicobacter pylori Infection Menchicchi Bi, Hensel A, Goycoolea FM . Current Pharmaceutical Design.2015;21(33). CrossRef
- How to manage Helicobacter pylori infection beyond antibiotics: The bioengineering quest Fonseca DR , Chitas R, Parreira P, Martins MCL . Applied Materials Today.2024;37. CrossRef
- Association of Helicobacter Pylori Infection and Gastric Cancer AL-Faisal A., Lafta S. Iraqi journal of Biotechnology.2017;16. CrossRef
- Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer Park JY , Forman D, Waskito LA , Yamaoka Y, Crabtree JE . Toxins.2018;10(4). CrossRef
- Helicobacter pylori-induced DNA double-stranded break in the development of gastric cancer Murata-Kamiya N, Hatakeyama M. Cancer Science.2022;113(6). CrossRef
- Inflammation and Gastric Cancer Jaroenlapnopparat A, Bhatia K, Coban S. Diseases (Basel, Switzerland).2022;10(3). CrossRef
- Cancer Care at Times of Crisis and War: The Syrian Example Sahloul E, Salem R, Alrez W, Alkarim T, Sukari A, Maziak W, Atassi MB . Journal of Global Oncology.2017;3(4). CrossRef
- Cancer care in times of conflict: cross border care in Pakistan of patients from Afghanistan Yusuf MA , Hussain SF , Sultan F, Badar F, Sullivan R. Ecancermedicalscience.2020;14. CrossRef
- A study of Helicobacter pylori-associated gastritis patterns in Iraq and their association with strain virulence Hussein NR , Napaki SM , Atherton JC . Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association.2009;15(2). CrossRef
- Molecular mechanisms underlying the action of carcinogens in gastric cancer with a glimpse into targeted therapy Patrad E, Khalighfard S, Amiriani T, Khori V, Alizadeh AM . Cellular Oncology (Dordrecht, Netherlands).2022;45(6). CrossRef
- Helicobacter pylori Genotyping from American Indigenous Groups Shows Novel Amerindian vacA and cagA Alleles and Asian, African and European Admixture Camorlinga-Ponce M, Perez-Perez G, Gonzalez-Valencia G, Mendoza I, Peñaloza-Espinosa R, Ramos I, Kersulyte D, et al . PLoS ONE.2011;6(11). CrossRef
- Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study Kumar S, Metz DC , Ellenberg S, Kaplan DE , Goldberg DS . Gastroenterology.2020;158(3). CrossRef
- The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis Shirani M, Pakzad R, Haddadi MH , Akrami S, Asadi A, Kazemian H, Moradi M, et al . BMC infectious diseases.2023;23(1). CrossRef
- Lifetime incidence risk for gastric cancer in the Helicobacter pylori-infected and uninfected population in Japan: A Monte Carlo simulation study Kawai S, Wang C, Lin Y, Sasakabe T, Okuda M, Kikuchi S. International Journal of Cancer.2022;150(1). CrossRef
- Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis Hatakeyama M. Cell Host & Microbe.2014;15(3). CrossRef
- Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island Viala J, Chaput C, Boneca IG , Cardona A, Girardin SE , Moran AP , Athman R, et al . Nature Immunology.2004;5(11). CrossRef
- Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A.. Cancer Research.1995;55(10).
- Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan Maeda S., Ogura K., Yoshida H., Kanai F., Ikenoue T., Kato N., Shiratori Y., Omata M.. Gut.1998;42(3). CrossRef
- Helicobacter pylori genotypes may determine gastric histopathology Nogueira C., Figueiredo C., Carneiro F., Gomes A. T., Barreira R., Figueira P., Salgado C., et al . The American Journal of Pathology.2001;158(2). CrossRef
- Helicobacter pylori in health and disease Cover TL , Blaser MJ . Gastroenterology.2009;136(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details