ALDH1A1 as a Stem Cell Marker and its Correlation with the Clinico-pathological Parameters in Invasive Mammary Carcinoma
Download
Abstract
Background: Breast cancer is a major public health concern due to its high incidence worldwide. Globally, it is considered the most common cancer to be diagnosed and one of the main causes for cancer deaths in women. Breast cancer stem cells (BCSCs) are the leading cause of adverse clinical outcomes and resistance to therapeutic agents because of their great capacity for self-renewal and differentiation. Aldehyde dehydrogenase1A1 (ALDH1A1) belongs to the aldehyde dehydrogenase (ALDH) superfamily of enzymes. It is one of the most important markers for cancer stem cells (CSCs). Its expression level in tumor cells is higher than in normal tissues. Recently, it has been widely investigated as a potential prognostic factor and therapeutic target.
Objectives: The purpose of this study was to assess the prognostic value of ALDH1A1 expression in invasive mammary carcinoma by correlating its expression to various clinicopathological characteristics and molecular subtypes and highlight the relationship between ALDH1A1 expression and tumor-infiltrating lymphocytes (TILs).
Methods: Seventy-two samples of invasive mammary carcinoma were retrieved from the archive of the Pathology Laboratory, Sohag University Hospital; of which 70 were of ductal origin and only 2 were of lobular origin, which were excluded from statistical analysis and discussed separately. Immunohistochemical (IHC) expression of ALDH1A1 was evaluated using an anti-human ALDH1A1 antibody. To elucidate the prognostic value of ALDH1A1 in breast cancer, its expression was statistically correlated with the available clinicopathological data.
Results: This study revealed positive ALDH1A1 expression in 54.3% of mammary invasive ductal carcinoma (IDC) specimens. ALDH1A1 up-regulation was significantly positively correlated with poor prognostic indicators, including larger tumor size (p= 0.007), high grade (p= 0.001), advanced stage (p< 0.001), poor Nottingham Prognostic Index (NPI) (p= 0.01), lymphovascular invasion (LVI) (p= 0.03), lymph node metastasis (LNM) (p=0.04), and a triple negative phenotype (p< 0.001). Moreover, we observed that tumors with positive ALDH1A1 expression exhibited higher levels of TILs with a statistically significant correlation (p= 0.001).
Conclusion: The current study revealed that ALDH1A1 up-regulation in invasive breast carcinoma is linked to aggressive behavior and different unfavorable prognostic indicators. It could be useful as a promising potential prognostic biomarker, serving as a prospective target for anti-cancer therapy.
Introduction
Breast cancer is a serious public health problem. According to Global Burden of Cancer (GLOBOCAN) statistics for 2022, it accounts for approximately 11.6% of all cancers and 6.9% of all deaths. It represents about 30% of all female cancers worldwide, and it is a leading cause of mortality in females globally, ranking the most frequent for incidence and mortality in most countries [1]. Breast cancer is the most prevalent cancer among Egyptian females; it ranks first in incidence and second in deaths, accounting for about 35% of all cancer cases [2].
Breast cancer represents a heterogeneous group of neoplasms with varying histopathology, behavior, prognosis, and response to treatment. Despite diagnostic and therapeutic advances in breast cancer, the clinical outcome for patients with breast cancer is still unsatisfactory due to relapse, metastasis, or resistance to chemotherapy [3]. Certain molecular subtypes, triple negative breast cancer (TNBC) and human epidermal growth factor receptor 2 positive (HER2+), have been linked to a more immunogenic tumor microenvironment, with a higher levels of immune cells like TILs [4], and associated with the activation of several signaling pathways including programmed death 1/programmed death ligand 1 (PD-1/ PD-L1) pathway [5]. According to previous studies; a higher level of TILs and PD-L1 positive expression are associated with improved pathologic complete response (pCR) and better survival rates in breast cancer patients [6].
Cancer stem cells (CSCs) are a subpopulation of cancer cells that share some properties with normal stem cells, such as the ability to proliferate and self-renew. They play a critical role in tumor development and progression. CSCs also contribute to tumor heterogeneity and dissemination. Furthermore, CSCs are hypothesized to be more resistant to radiotherapy and chemotherapy, resulting in relapse following the treatment. By identifying and characterizing CSCs, targeted therapeutic agents against these aggressive cells may be developed, leading to increased efficacy of anticancer drugs and improved outcomes [7]. According to previous reports, breast cancer stem cells (BCSCs) express more pro-invasive genes and have highly invasive characteristics [8].
Several genes and biomarkers have been reported to be correlated with BCSCs. Among these biomarkers is aldehyde dehydrogenase 1 (ALDH1), which belongs to the ALDH superfamily of enzymes. It is primarily located on chromosome 9q21. It is found mainly in the cytoplasm of liver cells, where it functions as an isoenzyme of acetaldehyde dehydrogenase. It is responsible for the conversion of acetaldehyde to harmless acetic acids through oxidation [9]. ALDH1 has a critical role in many cellular biological processes, including cell differentiation and resistance to chemotherapy and radiotherapy. Furthermore, clinical studies reported a positive correlation between tumor progression and high expression of the ALDH1 gene signature. Additionally, recent studies have reported that ALDH1 may be used as a stem cell marker for a variety of solid tumors. Overexpressed ALDH1 in CSCs has been reported to promote tumor progression, metastasis, immune escape, and resistance to anti-cancer therapeutic agents [10].
ALDH1 comprises three main isotypes, including ALDH1A1, ALDH1A2, and ALDH1A3. It was observed that ALDH1 activation depends mainly on ALDH1A1, which is hypothesized to be the main isozyme of ALDH1. ALDH1A1 is responsible for the oxidation of retinaldehyde to retinoic acid. It controls the expression of the genes involved in the maintenance of CSCs characteristics and hence promotes tumor growth and drug resistance. Recent studies have shown that a high level of ALDH1A1 is associated with an unfavorable prognosis. Many therapeutic agents have been developed against CSCs by targeting ALDH1A1 [11]. Significantly, ALDH1A1 has been implicated in reducing intracellular pH in breast cancer cells, inhibiting antitumor immune response, and promoting the breast cancer progression. This emphasizes its potential as an immunotherapy synergistic target [12]. This study was designed to evaluate ALDH1A1 expression status in invasive mammary carcinoma, correlating such expression with the different clinicopathological features of the included cases, focusing on its correlation to the level of TILs to clarify its potential prognostic role.
Materials and Methods
Clinical data and specimens’ collection
A retrospective study was conducted on 72 samples of invasive mammary carcinoma. Paraffin tissue blocks were retrieved from the archived material at the Pathology Laboratory at Sohag University Hospital over the last five years. In accordance with local ethical guidelines, the patients underwent modified radical mastectomy or breast-conserving surgery at the Department of General Surgery at the same hospital, and the specimens were referred to the Pathology Laboratory. After receiving the acceptance of the Sohag University Faculty of Medicine’s Medical Research Ethics Committee, we selected 72 paraffin tissue blocks that fulfill the inclusion criteria. Two tissue sections were prepared from each tissue block and processed for staining with hematoxylin and eosin (H&E) stain and an immunostain using an anti-human ALDH1A1 antibody. Sections stained with H&E stain were reexamined to confirm the diagnosis and reevaluate the histological parameters, including histological subtype, degree of differentiation, LVI, and perineural invasion. The pathological stage was also assessed whenever possible using the eighth edition of the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) staging system. Based on the AJCC staging system, tumors were divided into three groups according to their size: T1≤2cm, T2 >2 and ≤5 cm, and T3 >5 cm [13]. The hospital records of the patients were the source of the clinical information for the selected specimens. Additionally, reports about the expression of estrogen receptors (ER), progesterone receptors (PR), and HER2, as well as Ki67 index, were also available and included. Ki67 staining was classified as low or high based on a 14% level [14]. The molecular subtype for each case was defined based on ER, PR, Her2, and Ki-67 [15].
Nottingham Prognostic Index (NPI) was calculated using the following formula: NPI = tumor size (cm) x (0.2) + lymph node stage (1, 2, or 3) + histological grade (1, 2, or 3). According to this index, cases were divided into three categories as follows: good prognosis (a score of ≤ 3.4), moderate prognosis (a score of 3.41–5.4), and poor prognosis (a score of > 5.4) [16].
Tumor infiltrating lymphocytes (TILs)
The percentage of the stromal TILs was evaluated according to the international TIL Working Group Consensus guidelines, and defined as the area occupied by mononuclear inflammatory cells over the total intra-tumoral stromal area. According to the predefined criteria, it is scored as low and high, where > 50% indicates a high level [17].
Inclusion and exclusion criteria
The selection of specimens is based mainly on the availability of clinical data and adequate tissue material suitable for IHC. Specimens with pre-operative chemo or radiotherapy, insufficient clinical data, and those with insufficient tissue material or extensive tissue necrosis were excluded.
Ethical statement
Under IRB registration number: Soh-Med-23-09- 21PD; this study was granted approval by the Sohag University Ethics Committee and conducted in accordance with its guidelines.
Immunohistochemical staining
For the immunostaining procedure, tissue sections of 4µm thick were prepared from each specimen, mounted on positive-charged glass slides, and incubated overnight at room temperature to ensure that the tissues adhered correctly to the slides. The sections were deparaffinized by immersion in two changes of xylol (10 minutes each), followed by rehydration in ethyl alcohol using series of descending concentrations. Endogenous peroxidase activity was blocked by applying 3% hydrogen peroxide (H2O2) for 15 minutes at room temperature, followed by two washes in phosphate buffer solution (PBS). 15 minutes of boiling in a sodium citrate buffer (pH 6), divided into 3 cycles, was used to retrieve the antigen. The buffer level was checked, and buffer was added if necessary to prevent sections from drying out. The sections were rinsed in distilled water, then in PBS for 5 minutes. 10% normal goat serum was used for 10 minutes, to prevent the non- specific protein binding. Following washing twice in PBS, tissue sections were incubated overnight with the primary anti-ALDH1A1 recombinant rabbit monoclonal antibody (BLR089G) at a dilution of 1/200 (Catalog# MA5-44369, ThermoFisher SCIENTIFIC Corporation, Fremont, USA). Tissue slices were treated with a biotinylated goat secondary antibody at room temperature for half an hour after being washed in PBS. Following this, the tissue sections were rinsed twice in PBS. After 10 minutes of streptavidin peroxidase incubation, Two PBS washes were performed on tissue sections. Finally, diaminobenizidine (DAB) chromogen was prepared by combining substrate with DAB chromogen at a ratio of 1:25. Each tissue section received fifty microlitres, which were then left to incubate at room temperature until the positive control began to exhibit staining. After that, distilled water was used to rinse the sections. Tissue sections were rinsed in Mayer’s hematoxylin stain for 30 seconds, and then, to remove extra dye, immediately rinsed with tap water. Using up-graded dilutions of alcohol, tissue sections were dehydrated. After two changes of xylol (two minutes each), the slides were cleaned, and DPX was used to mount cover slips to each slide.
Positive and negative controls
To ensure the specificity of the ALDH1A1 antibody; positive and negative controls were included in each staining run. A section of invasive ductal carcinoma of the breast, known to be positive for ALDH1A1, was used as a positive control as recommended by the manufacturer. Negative controls were prepared by omitting the primary antibody from the steps of immunostaining.
Evaluation of ALDH1A1 immunostaining
Using an Olympus light microscope, semi-quantitative evaluation of ALDH1A1 immunostaining in tumor cells was done by two pathologists blinded to the clinicopathological data. The cytoplasmic staining of tumor cells was considered positive, and staining within necrotic foci was ignored. The percentage of positive tumor cells as well as the intensity of staining was scored. The percentage of positive cells ranged from an undetectable level (0%), to homogeneous staining (100%). The staining intensity was scored on a scale ranging from 0-3 as follows: (score 0= no stain, 1= weak, 2= moderate and 3= strong staining). The final score was obtained using the Quick score (Q), which is calculated by multiplying the percentage of positive cells by the intensity score. The final score ranged from 0-300. For statistical purposes and due to the relatively large number of positive cases, the final scores were subdivided into 2 groups: negative expression (score ≤ 10) and positive expression (score > 10) [18].
Statistical analysis
The findings were tabulated and statistically analyzed using the Statistical Software Package for Social Science version 26 for Windows (SPSS26, Chicago, IL, USA). Mean, median, and range were used to portray quantitative data while number and percentage were used for qualitative data. The expression rate between the categories was compared using the Chi square test (χ2) or the Fisher’s exact test whenever indicated. Statistical significance was determined if P<0.05.
Results
Patients’ characteristics
The current study included 72 specimens of invasive mammary carcinoma, of which 70 were of ductal origin and only 2 were of lobular origin, which were excluded from statistical analysis and discussed separately. Regarding IDC cases, the patients’ ages ranged from 25 to 72 years, with a mean ±SD of 49.4 ±9.1 and a median of 50 years. The patients were grouped into two age groups: the first age group includes patients who were <50 years old, and the second group includes patients who were ≥50 years old.
The specimens were surgically removed either by modified radical mastectomy in 54 (77.1%) cases or breast-conserving surgery in 16 (22.9%) cases.
The resected tumors ranged in size from 1 to 10 cm, with a mean ±SD and a median of 5.9 ±2.6 and 5.5, respectively. Tumor size was ≤2cm in 9 (12.9%) specimens, >2 and ≤5 cm in 26 (37.1%) specimens, and >5 cm in 35 (50%) specimens. The clinical data for the included IDC patients was displayed in Table 1.
| Variable | No. of cases (%) |
| Age/ Year | |
| Range | 25-72 years. |
| Mean ±SD | 49.4 ±9.1 |
| Menopausal status | |
| Premenopausal | 28 (40) |
| Postmenopausal | 42 (60) |
| Tumor size/cm. | |
| Range | 1-10 cm. |
| Mean ±SD | 5.9 ±2.6 |
| Laterality | |
| Right breast | 29 (41.4) |
| Left breast | 41 (58.6) |
Different histologic subtypes were included, the tumors were classified histologically as infiltrating ductal carcinoma of no specific type (IDC-NST) in 59 (84.3%) and rare special subtypes in 11 (15.7%), including mucinous, mixed ductal/lobular, micropapillary, tubular, cribriform, and metaplastic carcinomas in 3, 2, 2, 2, 1, and 1 case, respectively.
Among the investigated cases, 8 (11.4%) were classified as grade I, 40 (57.1%) as grade II, and 22 (31.4%) as grade III. According to the TNM staging system of the AJCC (8th edition), 17 (24.3%) cases were stage I, 34 (48.6%) cases were stage II, and 19 (27.1%) cases were stage III. LVI and metastatic spread to the axillary lymph nodes were detected in 38 (54.3%) and 36 (51.4%) of the included cases, respectively.
Upon categorizing the examined cases based on NPI, most of the studied patients (35.7%) were among the moderate prognostic index group, followed by the good prognostic index group (32.9%), and the smallest percentage was in the poor prognosis index group (31.4%).
The investigated cases were classified according to the molecular subtype as follows: 26 (37.1%) of cases were luminal A, 19 (27.1%) were luminal B, 11 (15.7%) were Her2 type and 14 (20%) had a triple negative phenotype. The histopathological features of the included IDC patients were displayed in Table 2.
| Variable | No. of cases | Percentage |
| Histological variant | ||
| IDC-NST | 59 | 84.3 |
| Rare special subtypes | 11 | 15.7 |
| Histologic grade | ||
| Grade I | 8 | 11.4 |
| Grade II | 40 | 57.1 |
| Grade III | 22 | 31.4 |
| Tumor size | ||
| T1 (≤ 2 cm) | 9 | 12.9 |
| T2 (>2 and ≤5 cm) | 26 | 37.1 |
| T3 (> 5 cm) | 35 | 50 |
| Lymph node status | ||
| N0 (0) | 34 | 48.6 |
| N1 (1-3) | 14 | 20 |
| N2 (4-9) | 12 | 17.1 |
| N3 (> 9) | 10 | 14.3 |
| AJCC stage | ||
| I | 17 | 24.3 |
| II | 34 | 48.6 |
| III | 19 | 27.1 |
| NPI | ||
| Good | 23 | 32.9 |
| Moderate | 25 | 35.7 |
| Poor | 22 | 31.4 |
| LVI | ||
| Present | 38 | 54.3 |
| Absent | 32 | 45.7 |
| TILs | ||
| High | 32 | 45.7 |
| Low | 38 | 54.3 |
| ER | ||
| Positive | 45 | 64.3 |
| Negative | 25 | 35.7 |
| PR | ||
| Positive | 43 | 61.4 |
| Negative | 27 | 38.6 |
| HER2/neu | ||
| Positive | 19 | 27.1 |
| Negative | 51 | 72.9 |
| Ki-67 | ||
| Low | 28 | 40 |
| High | 42 | 60 |
| Molecular subtypes | ||
| Luminal A | 26 | 37.1 |
| Luminal B | 19 | 27.1 |
| Her2+ | 11 | 15.7 |
| TNBC | 14 | 20 |
Immunohistochemical expression of ALDH1A1
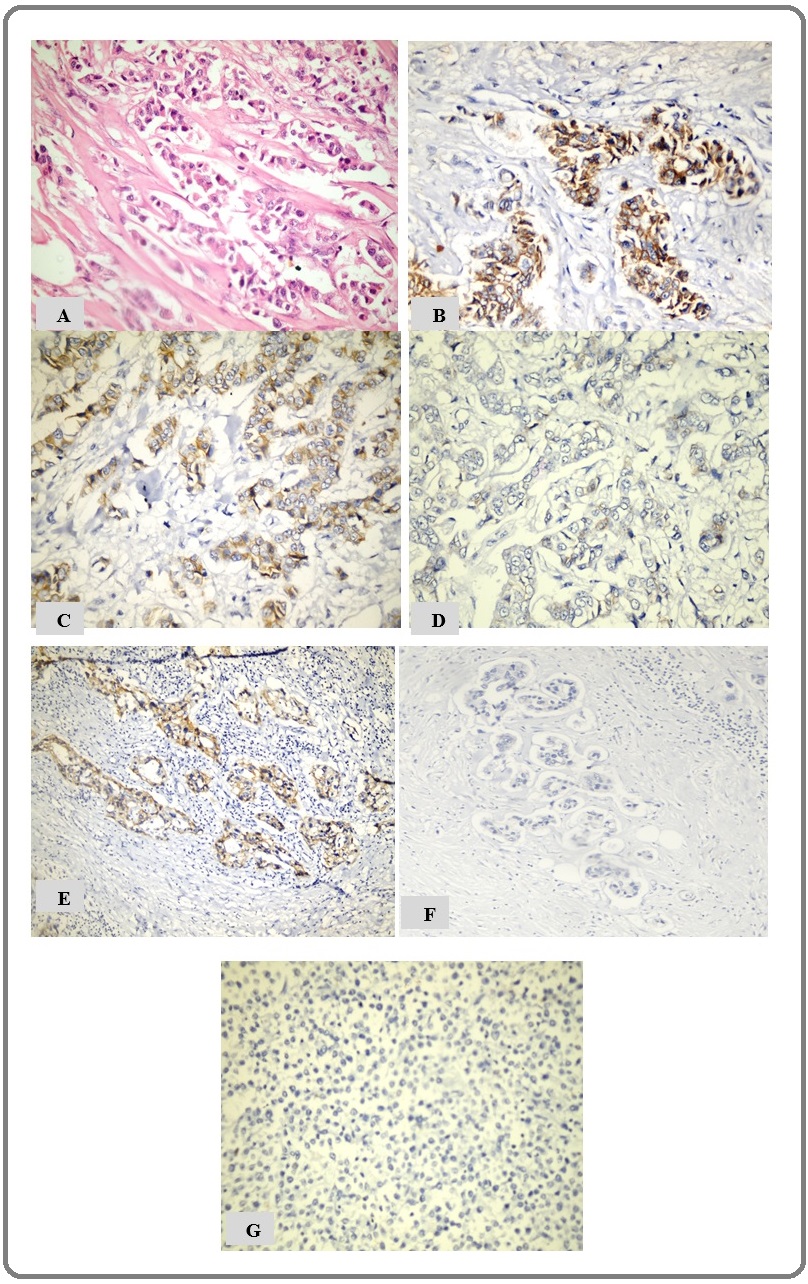
Positive cytoplasmic ALDH1A1 expression (score >10) was detected within the tumor cells in 38 (54.3%) of cases, while 32 (45.7%) cases were scored as negative (score ≤ 10). (Figure 1) showed different expression levels of ALDH1A1.
Figure 1. (A) Poorly differentiated IDC-NST (H and E X200), (B) High positive cytoplasmic expression of ALDH1A1 in poorly differentiated IDC-NST (IHC, X200), (C) Positive ALDH1A1 expression in moderately differentiated IDC-NST (IHC, X200), (D) Negative ALDH1A1 expression in well-differentiated IDC-NST with low level of TILs (IHC, X200); (E) Positive ALDH1A1 expression in well-differentiated IDC-NST with high level of TILs (IHC, X200);(F) Negative ALDH1A1 expression in histologically normal breast tissue (IHC, X200); (G) Negative ALDH1A1 expression in invasive lobular carcinoma (IHC, X200).
Correlation of ALDH1A1 expression in tumor cells with clinicopathological parameters
The immunohistochemical expression of ALDH1A1 within the tumor cells was correlated with the available clinicopathological data. According to the statistical analysis, Positive ALDH1A1 expression was significantly correlated with larger tumor size (p = 0.007), less differentiated tumors (p = 0.001), advanced stage (p < 0.001), and poor NPI (p = 0.01). Moreover, we observed that tumors with positive ALDH1A1 expression exhibited higher levels of TILs with a statistically significant correlation (p= 0.001).
A highly significant inverse correlation was noted between ALDH1A1 expression and ER and PR positivity (P<0.001), while ALDH1A1 expression was directly correlated with Her2/neu positivity (P=0.01). The rate of ALDH1A1 positivity varies significantly among the different molecular subtypes (p<0.001). The highest expression rate was observed in the triple negative phenotype (31.5%), followed by the Her2-enriched subtype (26.3%).
The correlation of ALDH1A1 expression with the invasive potential of tumor cells was also assessed. Its expression was shown to have a statistically significant positive correlation with the presence of lymphovascular tumor emboli (p=0.03) and LNM (p=0.04).
However, there was no statistically significant difference found when ALDH1A1 expression was analyzed in relation to patients’ age, menopause status, tumor laterality, and histologic subtype. Table (3) & (4) showed the statistical analysis of ALDH1A1 expression in relation to the clinical and histopathological characteristics.
| Clinical | ALDH1A1 expression (%) | P value | |
| Parameters | Negative | Positive | |
| N=32 | N=38 | ||
| Age/ Year | |||
| <50 | 16 (50) | 15 (39.5) | 0.377 |
| ≥50 | 16 (50) | 23 (60.5) | |
| Menopausal status | |||
| Premenopausal | 15 (46.9) | 13 (34.2) | 0.281 |
| Postmenopausal | 17 (53.1) | 25 (65.8) | |
| Laterality | |||
| Right breast | 15 (46.9) | 14 (36.8) | 0.396 |
| Left breast | 17 (53.1) | 24 (63.2) |
| Histopathological | ALDH1A1 expression (%) | ||
| parameters | Negative | Positive | P value |
| N=32 | N=38 | ||
| Histological variant | |||
| IDC-NST | 27 (84.4) | 32 (84.2) | 0.178 |
| Rare special subtypes | 5 (15.6) | 6 (15.8) | |
| Histologic grade | |||
| Grade I | 7 (21.9) | 1 (2.6) | |
| Grade II | 21 (65.6) | 19 (50) | 0.001 |
| Grade III | 4 (12.5) | 18 (47.4) | |
| Tumor size | |||
| T1 (≤ 2 cm) | 8 (25) | 1 (2.6) | 0.007 |
| T2 (>2 and ≤5 cm) | 13 (40.6) | 13 (34.2) | |
| T3 (> 5 cm) | 11 (34.4) | 24 (63.2) | |
| Lymph node status | |||
| N0 (0) | 20 (62.5) | 14 (36.8) | 0.04 |
| N1 (1-3) | 7 (21.9) | 7 (18.4) | |
| N2 (4-9) | 4 (12.5) | 8 (21.1) | |
| N3 (> 9) | 1(3.1) | 9 (23.7) | |
| AJCC stage | |||
| I | 14 (43.8) | 3 (7.9) | |
| II | 16 (50) | 18 (47.4) | <0.001 |
| III | 2 (6.2) | 17 (44.7) | |
| NPI | |||
| Good | 16 (50) | 7 (18.4) | 0.01 |
| Moderate | 10 (31.3) | 15 (39.5) | |
| Poor | 6 (18.7) | 16 (42.1) | |
| LVI | |||
| Present | 13 (40.6) | 25 (65.8) | 0.03 |
| Absent | 19 (59.4) | 13 (34.2) | |
| TILs | |||
| High | 8 (25) | 24 (63.2) | 0.001 |
| Low | 24 (75) | 14 (36.8) | |
| ER | |||
| Positive | 29 (90.6) | 16 (42.1) | <0.001 |
| Negative | 3 (9.4) | 22 (57.9) | |
| PR | |||
| Positive | 28 (87.5) | 15 (39.5) | <0.001 |
| Negative | 4 (12.5) | 23 (60.5) | |
| HER2 | |||
| Positive | 4 (12.5) | 15 (39.5) | 0.01 |
| Negative | 28 (87.5) | 23 (60.5) | |
| Ki67 | |||
| Low | 18 (56.3) | 10 (26.3) | |
| High | 14 (43.7) | 28 (73.7) | 0.01 |
| Molecular subtypes | |||
| Luminal A | 18 (56.3) | 8 (21.1) | <0.001 |
| Luminal B | 11 (34.4) | 8 (21.1) | |
| Her2 type | 1 (3.1%) | 10 (26.3%) | |
| TNBC | 2 (6.2%) | 12 (31.5%) |
P value is calculated by Pearson Chi-Square test or the Fisher’s exact test whenever indicated, P<0.05 is considered significant.
The two studied cases of infiltrating lobular carcinoma (ILC) were aged 45 and 53 years old. The sizes of their tumors were 3cm and 5cm in the largest diameter. Axillary lymph nodes were involved by the tumor in both cases. No available information about distant metastasis. Histologically, the two cases were classical ILC. ALDH1A1 expression was negative in both cases (Figure 1G).
Discussion
Breast cancer remains a life-threatening malignant tumor in females worldwide, despite the advances in breast cancer therapy [1]. ALDH1 has been widely investigated due to its strong CSC properties; it has been identified as a putative marker for BCSCs. It plays a crucial role in tumor progression and spread. Emerging evidence reports that ALDH1 may serve as a diagnostic and therapeutic target for CSC in various solid tumors, such as breast cancer, colorectal cancer, and ovarian cancer, providing new insights and new strategies for effective anti-cancer treatment [9].
Many studies have been conducted recently to evaluate the clinicopathological and prognostic significance of ALDH1A1 in various human tumors. Although several previous studies have addressed the relationship between ALDH1A1 expression and clinicopathological characteristics to clarify its prognostic value in breast cancer, its role in breast cancer outcomes remains controversial [19].
In this study, we detected positive ALDH1A1 expression within tumor cells in 54.3% of cases. It was rarely expressed in adjacent histologically normal breast tissues. This finding was similar to the results recorded by Sarker et al. (2018), where ALDH1A1 expression was observed in 54.6% of cases [20]. However, Pan et al. (2015) detected positive ALDH1A1 in 93% of cases [21]. These variations in expression may be explained by differences in cutoff points and sample size.
Correlating IHC expression of ALDH1A1 with the available clinicopathological parameters, we found that its expression was significantly correlated with larger tumor size, poorly differentiated tumors, advanced T stage, and N stage. These findings is in line with prior study conducted by Abd El-Fattah et al. (2019), who concluded that ALDH1A1 expression in IDC was more frequent in larger tumors, higher grades, and advanced TNM stage [22]. Additionally, previous studies by Pan et al. (2015) and Liu et al. (2013) reported that ALDH1A1 positive expression was more frequently observed in large-sized and higher-grade tumors [21, 23]. On the other hand, Ma and his colleague demonstrated that ALDH1A1 immunoreactivity was considerably related to increased tumor size and advanced TNM staging [18]. Also, Althobiti and his colleague concluded that ALDH1A1 positivity was significantly associated with high-grade tumors, advanced stages, and LNM. Moreover, consistent with our findings, their study demonstrated that there was a significant correlation between ALDH1A1 and NPI [24]. Furthermore, we detected that ALDH1A1 positive cases were significantly more likely to be associated with lymphovascular tumor emboli. A similar finding was reported by Althobiti and his colleague, who found a significant positive correlation between ALDH1A1 and LVI [24]. In contrast, Demir et al., (2018) and Farrag et al. (2022) reported that there was no association between ALDH1A1 expression and LVI [25, 26].
Positive ALDH1A1 expression showed a significant correlation to high levels of TILs. Our findings were in agreement with those of López Flores and his colleagues, who demonstrated that positive ALDH1A1 expression in breast cancer is more frequent in tumors with high TILs. To the best of our knowledge, limited data is available about the correlation between ALDH1A1 expression and TILs in different neoplasms, especially breast cancer [27]. We observed that positive ALDH1A1 expression was significantly correlated with ER/PgR negativity, HER2 positivity, and a higher Ki-67 index. This is in agreement with the findings shown by Abdelaziz and his colleague [28]. We compared ALDH1A1 expression in different molecular subtypes of BC; we noticed that its expression was higher in TNBC and HER2 type compared to luminal A and luminal B types. These findings were similar to those of Kim et al. (2015) and Kida et al. (2016), who showed that ALDH1A1 expression was significantly correlated with HER2 type and TNBC [29,30]. Additionally, Demir et al. (2018) and Abdelaziz (2022) reported that ALDH1A1 expression was significantly associated with breast cancer molecular subtypes [25, 28].
Consistent with our findings, Liu and his colleague’s meta-analyses of 15 previous papers revealed that ALDH1A1 expression was linked to increased tumor size, less differentiation, advanced stage, higher HER2 expression, and lower ER expression [31]. Meta-analysis conducted by Liu et al. (2015), which comprised 21 publications, demonstrated a significant correlation between ALDH1A1 expression and ER expression as well as histological grade. However, there was no clear correlation found between ALDH1A1 expression and any of the following: age, tumor size, lymph node status, LVI, or HER2 expression [32].
Our study identified that ALDH1A1 expression in breast cancer cases showed no significant association with age, menopause status, tumor laterality, and histological subtypes.
In conclusion, ALDH1A1 is a putative marker for BCSCs that play a critical role in breast cancer progression. ALDH1A1 positivity was correlated with different unfavorable prognostic indices and indicators of poor outcome. Moreover, high levels of TILs was associated with ALDH1A1 positive expression. Therefore, using therapeutic agents that target this molecule may be a helpful therapeutic strategy for patients with breast cancer. Limitations of the study
The statistical power of the results was impacted by the insufficient sample size, the few number of included histologic variants, and the exclusion of important histologic subtypes of breast cancer like ILC from statistical analysis. In addition to these limitations, the retrospective nature of the study.
Recommendations
- Investigating ALDH1A1 expression on a large scale of different breast lesions is recommended. Follow-up of patients is recommended to highlight the correlation between ALDH1A1 expression and patient survival and disease outcome.
- Future studies are required to evaluate the correlation between ALDH1A1 expression and PD-L1 expression to explore its implication on neoadjuvant treatment response in certain molecular subtypes of breast cancer, as it could be possible target for immunotherapy.
Acknowledgments
Statement of Transparency and Principals Declarations
Ethical approval and consent to participate
The study protocol was reviewed and approved by Ethical Committee of Sohag Faculty of Medicine.
Consent for publication
Not applicable.
Availability of data and material
All data and materials were available.
Competing interests
The authors declare that they have no conflict of interest.
Funding
No fund was received for this work.
Authors Contribution
Noha ED Hassab El-Naby and Nagwa Abd EL-Sadek Ahmed carried out manuscript writing & design, figures analysis & manipulation and histopathological diagnosis with archival revision of paraffin blocks and slides. Maisa Hashem Mohammed assisted with the design of the study and analysis of the data, collected the clinical data, and edited the manuscript.
Abbreviations
ALDH1A1: Aldehyde dehydrogenase1A1, BCSCs: Breast cancer stem cells, PBS: Phosphate buffer solution, CSCs: Cancer stem cells, ER: Estrogen receptors, H&E: hematoxylin and eosin, IDC: Infiltrating ductal carcinoma, IDC-NST: Infiltrating ductal carcinoma of no specific type, LNM: Lymph node metastasis, LVI: lymphovascular invasion, NPI: Nottingham prognostic index, PR: Progesterone receptors, TNBC: Triple- negative breast cancer.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- Cancer Burden Among Arab-World Females in 2020: Working Toward Improving Outcomes Mahdi H, Mula-Hussain L, Ramzi ZS , Tolba M, Abdel-Rahman O, Abu-Gheida I, Khorshid O, et al . JCO global oncology.2022;8. CrossRef
- Heterogeneity of Tumors in Breast Cancer: Implications and Prospects for Prognosis and Therapeutics Tuasha N, Petros B. Scientifica.2020;2020. CrossRef
- Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy Denkert C, Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI , Weber KE , Budczies J, et al . The Lancet. Oncology.2018;19(1). CrossRef
- PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients Tsang JYS , Au W, Lo K, Ni Y, Hlaing T, Hu J, Chan S, et al . Breast Cancer Research and Treatment.2017;162(1). CrossRef
- Predictive and prognostic value of PDL1 protein expression in breast cancer patients in neoadjuvant setting Wu Z, Zhang L, Peng J, Xu S, Zhou L, Lin Y, Wang Y, et al . Cancer Biology & Therapy.2019;20(6). CrossRef
- Cancer stem cells (CSCs) in cancer progression and therapy Najafi M, Farhood B, Mortezaee K. Journal of Cellular Physiology.2019;234(6). CrossRef
- Breast Cancer Stem Cells Crabtree JS , Miele L. Biomedicines.2018;6(3). CrossRef
- ALDH1: A potential therapeutic target for cancer stem cells in solid tumors Wei Y, Li Y, Chen Y, Liu P, Huang S, Zhang Y, Sun Y, et al . Frontiers in Oncology.2022;12. CrossRef
- Aldehyde dehydrogenase in solid tumors and other diseases: Potential biomarkers and therapeutic targets Xia J, Li S, Liu S, Zhang L. MedComm.2023;4(1). CrossRef
- Aldehyde dehydrogenase 1A1 in stem cells and cancer Tomita H, Tanaka K, Tanaka T, Hara A. Oncotarget.2016;7(10). CrossRef
- ALDH1A1 Activity in Tumor-Initiating Cells Remodels Myeloid-Derived Suppressor Cells to Promote Breast Cancer Progression Liu C, Qiang J, Deng Q, Xia J, Deng L, Zhou L, Wang D, et al . Cancer Research.2021;81(23). CrossRef
- Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer Giuliano AE , Edge SB , Hortobagyi GN . Annals of Surgical Oncology.2018;25(7). CrossRef
- Value of Breast Cancer Molecular Subtypes and Ki67 Expression for the Prediction of Efficacy and Prognosis of Neoadjuvant Chemotherapy in a Chinese Population Wang J, Sang D, Xu B, Yuan P, Ma F, Luo Y, Li Q, et al . Medicine.2016;95(18). CrossRef
- Presentation of the Molecular Subtypes of Breast Cancer Detected By Immunohistochemistry in Surgically Treated Patients Kondov B, Milenkovikj Z, Kondov G, Petrushevska G, Basheska N, Bogdanovska-Todorovska M, Tolevska N, Ivkovski L. Open Access Macedonian Journal of Medical Sciences.2018;6(6). CrossRef
- A Better Perspective of Nuclear Atypia in Nottingham Prognostic Index: Study on Breast Carcinoma Patients Samanta D, Senapati S, Sahoo T, Khuntia P, Mishra S, Satapathy S, Mallick R. Journal of Cancer and Tumor International.2016;13:1-13.
- The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014 Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2015;26(2). CrossRef
- Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC) Ma F, Li H, Li Y, Ding X, Wang H, Fan Y, Lin C, Qian H, Xu B. Medicine.2017;96(14). CrossRef
- The role of ALDH1A1 in contributing to breast tumour aggressiveness: A study conducted in an African population Gyan E, Green A, Ahenkorah-Fondjo L, Jackson A, Toss MS , Akakpo PK , Derkyi-Kwarteng L, Rahman GA , Owiredu W. Annals of Diagnostic Pathology.2021;51. CrossRef
- Correlations of aldehyde dehydrogenase-1 (ALDH1) expression with traditional prognostic parameters and different molecular subtypes of breast carcinoma Sarkar P, Basu K, Sarkar P, Chatterjee U, Mukhopadhyay M, Choudhuri MK , Srakar DK . Clujul Medical (1957).2018;91(2). CrossRef
- Aldehyde dehydrogenase 1 expression correlates with the invasion of breast cancer Pan H, Wu N, Huang Y, Li Q, Liu C, Liang M, Zhou W, Liu X, Wang S. Diagnostic Pathology.2015;10. CrossRef
- Significance of Tumoral and Stromal ALDH1A1 Expression in Breast Invasive Duct Carcinoma in Egyptian Female Patients Abd El-Fattah GA , Ibrahim E, Nasif SN . Egypt J Pathol.2019;39:151-158.
- Stem cell marker aldehyde dehydrogenase 1 (ALDH1)-expressing cells are enriched in triple-negative breast cancer Li H, Ma F, Wang H, Lin C, Fan Y, Zhang X, Qian H, Xu B. The International Journal of Biological Markers.2013;28(4). CrossRef
- The prognostic significance of ALDH1A1 expression in early invasive breast cancer Althobiti M, El Ansari R, Aleskandarany M, Joseph C, Toss MS , Green AR , Rakha EA . Histopathology.2020;77(3). CrossRef
- Prognostic value of aldehyde dehydrogenase 1 (ALDH1) in invasive breast carcinomas Demir H, Dulgar O, Gulle BT , Turna H, Ilvan S. Bosnian Journal of Basic Medical Sciences.2018;18(4). CrossRef
- Aldehyde dehydrogenase 1 expression in invasive breast carcinoma and its correlation with other clinicopathological parameters Farrag Mayada Sa , Elmetwally Mohamedb Farrag , Nesrine Sc , Ibrahiem Afaf Td . Egyptian Journal of Pathology.2022;42(2):117-124. CrossRef
- Relationship between Aldehyde Dehydrogenase, PD-L1 and Tumor-Infiltrating Lymphocytes with Pathologic Response and Survival in Breast Cancer López Flores M, Honrado Franco E, Sánchez Cousido LF , Minguito-Carazo C, Sanz Guadarrama O, López González L, Vallejo Pascual ME , et al . Cancers (Basel).2022 Sep 11;14(18):4418. CrossRef
- The prognostic significance of ALDH-1 and SOX9 expression in early breast cancer Abdelaziz LA , Harb OA , Abdelbary AM , Mohammed AA , Hamdey Rashed Elkalla HM . Middle East J Cancer.2022;13(4):581-592. CrossRef
- Prognostic Impact and Clinicopathological Correlation of CD133 and ALDH1 Expression in Invasive Breast Cancer Kim SJ , Kim YS , Jang ED , Seo KJ , Kim JS . Journal of Breast Cancer.2015;18(4). CrossRef
- Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype Kida K, Ishikawa T, Yamada A, Shimada K, Narui K, Sugae S, Shimizu D, et al . Breast Cancer Research and Treatment.2016;156(2). CrossRef
- ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis Liu Y, Lv D, Duan J, Xu S, Zhang J, Yang X, Zhang X, et al . BMC cancer.2014;14. CrossRef
- Aldehyde dehydrogenase 1 expression correlates with clinicopathologic features of patients with breast cancer: a meta-analysis Liu J, Xia P, Hu W, Wang D, Xu X. International Journal of Clinical and Experimental Medicine.2015;8(6).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details