Relationship of Tumor-Associated Neutrophil Expression and Neutrophil-to-Lymphocyte Ratio with Clinical Response to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer
Download
Abstract
Introduction: Breast cancer (BC) is a serious health concern. Neoadjuvant chemotherapy (NAC) is a crucial therapy for managing BC, but further research is needed to identify biomarkers that can help to predict the efficacy of this therapy. The presence and functional activity of tumor-associated neutrophils (TANs) are capable of serving as predictive biomarkers for therapeutic outcomes. Elevated neutrophil-to-lymphocyte ratio (NLR) is linked to high tumor stage and low histological grade, indicating a more aggressive disease profile. This study analyzed the relationship between TAN expression and NLR with clinical response to NAC in locally advanced BC (LABC).
Methods: This was a cohort study of 41 patients with BC. TAN expression was examined using immunohistochemistry, NLR was obtained from routine blood tests, and chemotherapy response was assessed using the RECIST method. The relationship between TAN expression and NLR with chemotherapy response in LABC was tested using chi-square tests.
Results: High and low TAN expression was observed in 80.5% and 19.5% of patients, respectively. For NLR before chemotherapy, 48.8% and 51.2% of patients exhibited a high and low NLR, respectively; after chemotherapy, 51.2% and 48.8% of patients exhibited a high and low NLR, respectively. Moreover, 90.2% patients responded to NAC. A significant relationship was observed between TAN expression and chemotherapy response in BC, with a moderately strong negative correlation.
Conclusion: TAN expression is an important potential predictor of chemotherapy response in LABC. By identifying biomarkers like TAN, clinicians may be better able to customize treatment plans and enhance outcomes for patients with LABC.
Introduction
In Indonesia, among women, breast cancer (BC) ranks first for cancer incidence, with 65,858 cases (30.8%), and cervical cancer follows distantly at 17.2%. The estimated incidence of BC in Indonesia is 50 per 100,000 women [1]. Moreover, in Indonesia, BC patients mostly present at advanced stages, with 63% diagnosed at stage III and IV [2]. A study conducted at Wahidin Sudirohusodo Hospital from January 2002 to December 2019 revealed that BC was the highest-incidence solid cancer, with 1,008 patients (12.9%) [3]. Neoadjuvant chemotherapy (NAC) is preoperative systemic chemotherapy and has become the standard treatment for advanced-stage BC and the preferred therapy for early-stage operable BC [4-6]. The primary goal of NAC is to reduce tumor size (downsizing), prevent micrometastasis, assess chemotherapy response, and increase breast conservation rates [4, 5].
Neutrophils that penetrate the tumor microenvironment are frequently designated as tumor-associated neutrophils (TANs). The phenotypic attributes and functional roles of TANs may differ contingent upon variables such as the particular subtype of BC and the progression stage of the disease. Certain TANs may manifest an N1 phenotype characterized by anti-tumor activity, whereas alternative TANs may exhibit an N2 phenotype associated with pro-tumorigenic properties. The presence and functional activity of TANs are capable of serving as predictive biomarkers for therapeutic outcomes in BC. The equilibrium between pro-tumorigenic and anti-tumorigenic functions of neutrophils may significantly affect patients’ responses to particular treatment modalities, including targeted therapy, immunotherapy, or chemotherapy [7].
The neutrophil-to-lymphocyte ratio (NLR) has emerged as a significant prognostic marker in BC, reflecting systemic inflammation and correlating with local immune responses [8]. NLR correlates with tumour-infiltrating lymphocytes and tumour-associated macrophages, and serves as an independent prognostic marker for overall survival, BC-specific survival, and disease-free survival in BC patients undergoing neoadjuvant chemotherapy [9]. Elevated NLR is linked to high tumor stage and low histological grade, indicating a more aggressive disease profile [10, 11]. The roles of TANs and the NLR in BC warrant further investigation to validate their prognostic effectiveness and understand their role in cancer progression, tumorigenesis, and response to chemotherapy in diverse populations. This study, conducted in Makassar, thus investigated the relationship between TAN expression and the NLR value concerning the clinical response to NAC in locally advanced BC (LABC).
Methods
This preliminary study was an analytical observational cohort study (prospective, longitudinal) to assess the relationship between TAN expression and NLR on the clinical response of NAC in LABC. The study was conducted at Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital in Makassar, Indonesia. The research period was from January 2023 to December 2023. This study was and was approved by the Medical Research Ethics Commission of the Medical Faculty of Hasanuddin University (number: 602/UN4.6.4.5.31/PP36/2023) and has been reported in accordance with the Strengthening the reporting of cohort studies in surgery (STROCSS) guidelines [12].
The population consisted of all patients diagnosed with LABC who were treated at the Hospitals. The research sample comprised all individuals meeting the sample criteria, and the sample was obtained based on the order of admission to the Hospitals (consecutive random sampling). Blood and breast tumor specimens were taken from women diagnosed with BC (clinical examination, histopathological examination, chest X-ray examination, and abdominal ultrasound examination).
The inclusion criteria were being a woman with LABC who had not received prior therapy, having the histopathological type of invasive ductal carcinoma of the breast, being a woman with BC receiving NAC, and being willing to participate in this study. The exclusion criteria were being a woman with bilateral BC, being a woman with BC accompanied by other malignant diseases, and having tumor tissue samples taken for immunohistochemical examination that were damaged or not representative.
Research Procedures
Patients were provided with information, and informed consent was obtained. Anamnesis was then conducted to complete the recording of identities and examination results according to the research form prepared. The collection of breast tissue biopsy/surgery material from patients was performed under sterile conditions, and the samples were then placed in bottles containing 10% formalin buffer solution and sent to the Department of Pathological Anatomy, Faculty of Medicine, Hasanuddin University. Tissue preparations were made using 10% formalin buffer fixation, ethanol, and paraffin.
Immunohistochemical Examination
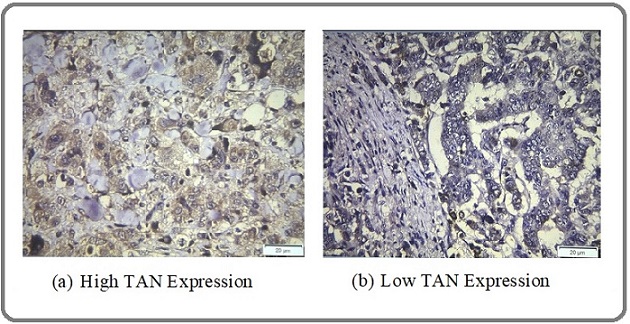
Immunohistochemical staining of TAN on tissue was performed using CEACAM8 Polyclonal Antibody brand Elabscience (Houston, Texas, USA), catalog number E-AB-18217. TAN expression was assessed in terms of staining intensity and extent. Staining intensity was assessed as negative (0), weak (1), moderate (2), and strong (3), and the extent of staining was assessed as 0% (0), 1%–25% (1), 26%–50% (2), 51%–75% (3), and 76%–100% (4). Assessments of the intensity scale and percentage scale were then performed, and the final scale score was obtained by adding the percentage scale and intensity scale. The TAN expression level was divided into low (negative; score 0–2) and high (positive; score >2). An example of staining is shown in Figure 1.
Figure 1. Immunohistochemical Staining of TAN Expression.
NLR
NLR is the ratio between the number of neutrophils [neutrophil count (109/L)] and lymphocytes [lymphocyte count (109/L)] measured in peripheral blood. The baseline NLR was defined as NLR1 (pre) and was calculated from the complete blood count routinely performed within 1 week before the first cycle of chemotherapy. NLR2 (post) was defined as the NLR calculated from the complete blood count within 1 week after the third cycle of chemotherapy. No clinical signs of infection, such as fever, were evident among the patients on the day of blood sample collection [4].
NAC
NAC refers to the administration of cytotoxic drugs to patients with BC as neoadjuvant therapy at intervals of 3 weeks for three cycles of chemotherapy.
Clinical Response
Clinical response refers to the reduction of a tumor mass assumed to be representative of the sensitivity of all tumor cells to clinically administered chemotherapy. Clinical response was measured using calipers, with tumor size measured before the first cycle of chemotherapy and 3 weeks after the third cycle of chemotherapy. Clinical response was assessed according to the RECIST criteria: Clinical complete response (CR), clinical partial response (PR), clinical stable disease (SD), and progressive disease (PD) [13-15].
Statistical Analysis
The collected data were grouped based on objectives and data types and were then analyzed using SPSS version 26.0 (Armonk, NY: IBM Corp.). The chi-square test was used to analyze the data of independent variables with a ratio scale, with dependent variables subject to an ordinal scale (in the form of classification) when the data were not normally distributed. The decision concerning the hypothesis testing results was considered significant if p < 0.05. Receiver operating characteristic (ROC) curve analysis was used to determine the NLR cut-off value.
Results
A total of 41 research participants with the characteristics shown in Table 1 were enrolled, consisting of 37/41 (90.2%) subjects responsive to NAC and 4/41 (9.8%) who were nonresponsive. For the histopathological grade of the 41 samples, 3/41 (7.3%) samples were grade I, 19/41 (46.3%) samples were grade II, and 19/41 (46.3%) samples were stage III.
| Characteristics | n | % | Range (Median) | Mean + SD | |
| Age (years) | |||||
| 25–34 | 1 | 2.4 | 27–73 (51) | 49.3 + 10.2 | |
| 35–44 | 14 | 34.1 | |||
| 45–54 | 16 | 39 | |||
| 55–64 | 5 | 12.2 | |||
| 65–74 | 5 | 12.2 | |||
| Menopausal Status | |||||
| Premenopause | 26 | 63.4 | |||
| Menopause | 15 | 36.6 | |||
| Histopathological grade | |||||
| I | 3 | 7.3 | |||
| II | 19 | 46.3 | |||
| III | 19 | 46.3 | |||
| Pretreatment tumor size (cm) | 41 | 100 | 3–27 (11) | 12.6 + 6.7 | |
| Posttreatment tumor size (cm) | 41 | 100 | 0–21 (11) | 6.6 + 4.5 | |
| NLR1 Pre | |||||
| Low 2 | 21 | 51.2 | 0.54–24.1 (2.4) | 3.6 + 3.9 | |
| High 3 | 20 | 48.8 | |||
| NLR1 Post | |||||
| Low 2 | 20 | 48.8 | 0.1–12.6 (2.4) | 3.6 + 3.3 | |
| High 3 | 21 | 51.2 | |||
| TAN Expression | |||||
| Low | 8 | 19.5 | |||
| High | 33 | 80.5 | |||
| Chemotherapy Regimen | |||||
| TAC | 18 | 43.9 | |||
| TAP | 11 | 26.8 | |||
| TA | 9 | 21.9 | |||
| CAF | 1 | 2.4 | |||
| AC | 1 | 2.4 | |||
| Chemotherapy Response | |||||
| Responsive | 37 | 90.2 | |||
| Nonresponsive | 4 | 9.8 |
Note, 1Neutrophil-to-lymphocyte ratio; 2NLR <2.49; 3NLR >2.49; TAC: taxane, adriamycin, cyclophosphamide; TAP: taxane, adriamycin, cisplatinum; TA: taxane, adriamycin; CAF: cyclophosphamide, adriamycin, 5-FU; AC: adriamycin, cyclophosphamide.
Figure 2 shows the ROC curve of NLR with an AUC (area under the curve) value of 0.561, p = 0.693.
Figure 2. ROC Analysis of NLR.
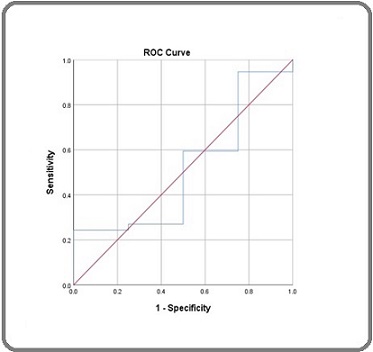
When the above NLR data were considered, variations in sensitivity and specificity intersected at the value of 2.49. NLR > 2.4 was considered high, and NLR < 2.4 was considered low. The expression of TAN (Table 2) tends to influence the response to chemotherapy in BC. High TAN expression tended to be responsive to NAC in 32/41 cases (78.0%), and low TAN expression also tended to be responsive to NAC in 5/41 cases (12.0%). Statistically, a significant association was observed with a p-value of 0.003 (p < 0.05).
| TAN Expression | Chemotherapy Response | |
| Responsive | Nonresponsive | |
| n (%) | n (%) | |
| High TAN | 32 (78.0) | 1 (2.4) |
| Low TAN | 5 (12.0) | 3 (7.6) |
Note, X2, 8,690; df, 1; p, 0.003 (p < 0.05)
Table 3 shows the percentage of High and Low Pre and Post NLR Values. Both for NLR values before (pre) and after (post) NAC, the percentage of patients with high and low NLR values was almost equally high. This suggests no significant difference existed in the distribution of NLR before and after chemotherapy.
| NLR value pre | Chemotherapy Response | |
| Responsive | Nonresponsive | |
| n (%) | n (%) | |
| High | 19 (46.3) | 2 (4.9) |
| Low | 18 (43.9) | 2 (4.9) |
| NLR value post | Chemotherapy Response | |
| Responsive | Responsive | |
| High | 19 (46.3) | 2 (4.9) |
| Low | 18 (43.9) | 2 (4.9) |
Note: X2, 0.003; df, 1; p, 0.959 (p > 0.05)
For the relationship between Pre-NLR and Chemotherapy Response, the high p-value (0.959) indicates no significant relationship was evident between prechemotherapy NLR values and chemotherapy response. In other words, no evidence supported the premise that prechemotherapy NLR values influence how well patients respond to chemotherapy.
Table 4 displays the results of the test on the relationship between TAN expression, low pre-NLR, and chemotherapy response using chi-square test, which yielded a p-value of 0.05, indicating that the relationship between TAN expression, low pre-NLR value, and chemotherapy response is considered significant.
| TAN Expression | Chemotherapy Response | p value | ||
| Responsive | Nonresponsive | |||
| n (%) | n (%) | |||
| Pre-NLR High | TAN High | 17 (81) | 1 (4.8) | 0.27 |
| TAN Low | 2 (9.5) | 1 (4.8) | ||
| Pre-NLR Low | TAN High | 15 (75) | 0 (0) | 0.05 |
| TAN Low | 3 (15) | 2 (10) | ||
| Post-NLR High | TAN High | 18 (85.7) | 1 (4.8) | 0.18 |
| TAN Low | 1 (4.8) | 1 (4.8) | ||
| Post-NLR Low | TAN High | 14 (70) | 0 (0) | 0.07 |
| TAN Low | 4 (20) | 2 (10) |
Discussion
In a sample of 41 patients with BC, monitoring of the influence of TAN expression and NLR on NAC response was conducted, revealing that 37/41 (90.2%) were responsive and 4/41 (9.8%) were nonresponsive. This study, based on clinical response to NAC, found a responsiveness rate of 37/41 (90.2%), which is higher than those found by Rahman et al. and Stamatovic et al. who used anthracycline-based NAC in patients with LABC and revealed clinical response rates of 65.3% and 70%, respectively, demonstrating benefits from such treatment when followed by adjuvant chemotherapy in terms of reducing local recurrence and metastasis [16, 17]. The study demonstrated a significant relationship between TAN and chemotherapy response, with a p-value of 0.003 (p < 0.05). Further, based on the relationship between TAN expression, low pre-NLR, and chemotherapy response using the chi-square test, a p-value of 0.05 was obtained, indicating significance. This suggests that the relationship between TAN expression, low pre-NLR value, and chemotherapy response is significant.
The tumor microenvironment (TME) influences not only the effects of immunotherapy but also the effects of anticancer drugs and their treatment outcomes. Many anticancer drugs have immunosuppressive effects and are not very compatible with immunotherapy, but increased immunology and elimination of immunosuppression can occur. Mechanisms for increasing antitumor immunity include increasing immune escape on the cancer cell side, inducing immunogenic cell death, and increasing immune escape on the host side. The administration of 5-FU and paclitaxel increases the sensitivity of cytotoxic T cells, and the addition of cyclophosphamide and anthracycline drugs can induce immunogenic cell death in tumor cells. Further, paclitaxel can inhibit Treg cells, and 5-FU can inhibit myeloid-derived suppressor cells [18].
Galdiero et al. revealed that higher TAN density indicates a better response to 5-FU-based chemotherapy in patients with colorectal cancer [19]. Wang et al. also observed that neutrophils in the tumor parenchyma is an independent factor for a poor prognosis in patients with BC [20]. Wang et al. revealed that neutrophils induce epithelial-mesenchymal transition (EMT) in BC through the tissue inhibitor metalloproteinase-1 (TIMP-1) [21]. Conversely, BC cells undergoing EMT increase TIMP-1 neutrophil secretion by CD90 through cell contact. Research has revealed a close relationship between EMT and resistance to chemotherapy agents [21]. Kajiyama et al. identified a relationship between paclitaxel resistance and EMT induction in epithelial ovarian carcinoma [22]. Adriamycin treatment induced EMT and apoptosis simultaneously in a cell-cycle- dependent manner in BC cells. Only cells undergoing EMT showed increased invasion/metastasis and multidrug resistance. Thus, EMT can contribute to the malignant phenotype of epithelial cancer in response to chemotherapy. The understanding of the exact molecular mechanisms mediating chemotherapy-induced EMT is still evolving [23].
Shaul and Fridlender hypothesized that TANs play a crucial role in recruiting and maintaining the balance between immune system activation and suppression in cancer [24]. Wang et al., in their study on patients with stage III and IV bile duct cancer, revealed that patients with high TAN levels did not benefit from adjuvant chemotherapy (p = 0.415). However, patients with low TAN levels could significantly benefit from adjuvant chemotherapy (p = 0.047) [20].
Patients with high TAN levels should be carefully considered in the selection of chemotherapy administration and the for prevention of excessive toxicity. Further, the unfavorable role of neutrophils in cancer suggests the potential for new immunotherapies targeting TANs to enhance sensitivity and response to chemotherapy. This approach could improve the survival of cancer patients with high TAN levels, which is consistent with findings that neutrophil inhibition by C-X-C chemokine receptor type 2 (CXCR2) blockade can enhance tumor inhibition in chemotherapy. As previously reported, neutrophils can suppress antitumor immune responses mediated by CD8+ T cells through inducible nitric oxide synthase and arginase 1 [5, 20]. Wang et al. concluded that high TAN levels correlated with immunosuppression in the TME, which may account for poor prognosis and chemotherapy efficacy [20].
The complexity within tumors depends not only on the inherent properties of tumor cells but also on the interactions among transformed tumor cells and various components of the TME that contribute to tumor growth, development, and even metastasis. The tumorigenicity of BC is the result of interactions between breast tumor cells and the surrounding stroma. Phenotypic and genetic changes of these cells composing the TME contribute to tumor initiation, development, metastasis, and drug resistance. Metastasis and therapeutic resistance are the main causes of BC-related deaths; thus, chemotherapy resistance is an important area of concern and requires further investigation. Therefore, the interaction between tumor cells and the TME has become a key point in various studies, and this knowledge can open up new therapeutic boundaries for treating BC. Despite significant advances in the molecular characterization of heterogeneity in BC, this work has not exerted a substantial influence on clinical practice. Well-designed future clinical trials, including of new combinational or sequential therapies including biological and immunological agents targeting cancer cells and their microenvironment, are needed to identify the best treatment options for patients with BC [25, 26].
The limitations of this study include, first, this preliminary study with a relatively small number of patients; second, needing long-term follow-up; third, NAC being performed for only three cycles and aimed at downsizing; fourth, pCR (pathological complete response) was not achieved in this study; five, the measurement of dependent variables using RECIST criteria by manual methods with calipers is rough; more detailed tumor size measurements can be made using computed tomography/ magnetic resonance imaging (CT/MRI) assistance; and six, although CD66b has a sensitivity for identifying TAN, CD66b is not a highly specific marker for neutrophils. Therefore, these results need to be validated with future prospective multicenter randomized trials with a larger population. The optimal NLR cutoff value varies between studies. Some studies use median values, others use mean values, and others use ROC curves to obtain optimal values. The cutoff value determined in this study requires further verification.
On the other hand, this study’s strength lies in using a prospective cohort method, starting from identifying predictive factors and following up until chemotherapy response outcomes are obtained. This study has supplemented the existing knowledge with supporting evidence that TAN as a potential predictive biomarker is associated with NAC response in BC. We hope future research can further validate and confirm the use of TAN biomarkers in BC.
In conclusion, although no significant relationship was found between NLR values and chemotherapy response in LABC, the study highlighted the potential of TAN as a potential predictive biomarker for treatment outcomes. The expression of TANs can be considered a potential predictive biomarker for NAC response in LABC. Further research with prospective multicenter randomized trials with a larger population, long-term follow-up, and chemotherapy response measurements using CT/MRI or biomarkers with the polymerase chain reaction method is needed to support this study further.
Acknowledgements
None
Author contributions
Conception: ABD, IND, DJF, BN, and BDA; Interpretation or analysis of data: ABD, IND, DJF, BN, YA, and MF; Preparation of the manuscript: ABD, IND, and MF; Revision for important intellectual content: ABD, IND, DJF, BN, YA, and MF; Supervision: IND, DJF, and BN. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
Self-funding
References
- An overview of precision oncology basket and umbrella trials for clinicians Park JJH , Hsu G, Siden EG , Thorlund K, Mills EJ . CA: a cancer journal for clinicians.2020;70(2). CrossRef
- Comparison of outcomes in patients with luminal type breast cancer treated with a gonadotropin-releasing hormone analog or bilateral salpingo-oophorectomy: A cohort retrospective study Andriyanto DR , Prihantono n, Syamsu SA , Kusuma MI , Hendarto J, Indra n, Smaradania N, et al . Annals of Medicine and Surgery (2012).2022;77. CrossRef
- Cancer Incidence and Mortality in a Tertiary Hospital in Indonesia: An 18-Year Data Review Prihantono n, Rusli R, Christeven R, Faruk M. Ethiopian Journal of Health Sciences.2023;33(3). CrossRef
- Prognostic Roles of Neutrophil-to-Lymphocyte Ratio and Stromal Tumor-Infiltrating Lymphocytes and Their Relationship in Locally Advanced Triple-Negative Breast Cancer Treated with Neoadjuvant Chemotherapy Dong X, Liu C, Yuan J, Wang S, Ding N, Li Y, Wu Y, Xiao Z. Breast Care (Basel, Switzerland).2021;16(4). CrossRef
- Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer Lin F, Zhang L, Xie S, Huang H, Chen X, Jiang T, Guo L, Lin H. Frontiers in Oncology.2022;12. CrossRef
- Breast Cancer Chemotherapy Response in Wahidin Sudirohusodo Hospital, Makassar Prihantono P, Haryasena H, Sampepajung D. Nusantara Medical Science Journal.2016;I(1). CrossRef
- Exploring neutrophil functionality in breast cancer progression: A review Obeagu EI , Obeagu GU . Medicine.2024;103(13). CrossRef
- Relationship Between the Neutrophil to Lymphocyte Ratio, Stromal Tumor-infiltrating Lymphocytes, and the Prognosis and Response to Neoadjuvant Chemotherapy in Triple-negative Breast Cancer Pang J, Zhou H, Dong X, Wang S, Xiao Z. Clinical Breast Cancer.2021;21(6). CrossRef
- Neutrophil-lymphocyte ratio reflects tumour-infiltrating lymphocytes and tumour-associated macrophages and independently predicts poor outcome in breast cancers with neoadjuvant chemotherapy Li JJX , Ni SYB , Tsang JYS , Chan WY , Hung RKW , Lui JWH , Ng SWY , et al . Histopathology.2024;84(5). CrossRef
- Determining the Association of Neutrophil to Lymphocyte Ratio with Clinicopathological Features in Breast Cancer Soomro R, Cho N. New Horizons in Medicine and Medical Research Vol. 1.2022. CrossRef
- Neutrophil-lymphocyte Ratio as Independent Prognostic Factor among Breast Cancer Patients in a Tertiary Care Hospital, Kolkata, India. Mayur N, Roy , Banik L. Journal Of Clinical And Diagnostic Research.2022. CrossRef
- STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. International Journal of Surgery (London, England).2019;72. CrossRef
- Assessment of tumor response to neoadjuvant chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1.0 Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Nagoya Journal of Medical Science.2018;80(2). CrossRef
- Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria Chung W, Park M, Shin SJ , Baek S, Kim Y, Choi JY , Kim M. AJR. American journal of roentgenology.2012;199(4). CrossRef
- New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eisenhauer E. A., Therasse P., Bogaerts J., Schwartz L. H., Sargent D., Ford R., Dancey J., et al . European Journal of Cancer (Oxford, England: 1990).2009;45(2). CrossRef
- Profiles of molecular subtypes and clinical responses to anthracycline-based neoadjuvant chemotherapy in locally advanced breast cancer at Dr. Soetomo General Hospital, Surabaya Rahman H, Ali I, Hari Susilo D. Bali Medical Journal.2024;13(2):654-658. CrossRef
- The influence of breast cancer subtypes on the response to anthracycline neoadjuvant chemotherapy in locally advanced breast cancer patients Stamatovic L, Susnjar S, Gavrilovic D, Minic I, Ursulovic T, Dzodic R. Journal of B.U.ON.: official journal of the Balkan Union of Oncology.2018;23(5).
- Predictive Value of Neutrophil/Lymphocyte Ratio for Efficacy of Preoperative Chemotherapy in Triple-Negative Breast Cancer Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, Ohsawa M, Kitagawa S, Hirakawa K. Annals of Surgical Oncology.2016;23(4). CrossRef
- Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer Galdiero MR , Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, et al . International Journal of Cancer.2016;139(2). CrossRef
- Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer Wang J, Bo X, Suo T, Liu H, Ni X, Shen S, Li M, et al . Cancer Science.2018;109(7). CrossRef
- Tumor-Contacted Neutrophils Promote Metastasis by a CD90-TIMP-1 Juxtacrine-Paracrine Loop Wang Y, Chen J, Yang L, Li J, Wu W, Huang M, Lin L, Su S. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2019;25(6). CrossRef
- Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. International Journal of Oncology.2007;31(2).
- Involvement of NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells Li Q, Chen Z, Cao X, Xu J, Xu J, Chen Y, Wang W, et al . Cell Death and Differentiation.2011;18(1). CrossRef
- Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function Shaul ME , Fridlender ZG . The FEBS journal.2018;285(23). CrossRef
- Tumor microenvironment promotes breast cancer chemoresistance Mehraj U, Dar AH , Wani NA , Mir MA . Cancer Chemotherapy and Pharmacology.2021;87(2). CrossRef
- Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with breast cancer receiving neoadjuvant chemotherapy: a meta-analysis Zhou Q, Dong J, Sun Q, Lu N, Pan Y, Han X. BMJ open.2021;11(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details