Utilizing Niosome Nanoparticles for the Combined Treatment of Curcumin and Cisplatin in Oral Cancer
Download
Abstract
Overview: Oral cancer remains a significant health challenge due to its aggressive nature and limited treatment options. This study investigates the use of niosome nanoparticles to deliver a combination of curcumin and cisplatin, a natural anti-inflammatory and anti-cancer agent and a widely used chemotherapeutic drug, respectively.
Methods: Niosome nanoparticles were formulated and optimized for encapsulation efficiency and stability. The physicochemical properties of the nanoparticles were characterized, including particle size, zeta potential, and polydispersity index (PDI). In vitro cytotoxicity assays were conducted using oral cancer cell lines to evaluate the efficacy of the combined treatment.
Results: The niosome formulations with a mean particle size of approximately 150 nm, a favorable zeta potential of 24.6 ± 3.2 mV, and a low PDI of 0.23 ± 0.05. The release profile showed a controlled and sustained release of both curcumin and cisplatin over 48 hours, with a cumulative release of 51% for curcumin and 48% for cisplatin. In vitro studies revealed that the combined treatment significantly reduced cell viability compared to individual treatments, with a synergistic effect observed at specific concentrations.
Conclusion: The findings suggest that niosome nanoparticles can effectively deliver a combination of curcumin and cisplatin, enhancing the therapeutic potential against oral cancer. This innovative approach may pave the way for more effective treatment strategies, ultimately improving patient outcomes in oral cancer therapy
Introduction
Oral cancer is a devastating disease that affects millions of people worldwide, with a high mortality rate due to its aggressive nature and limited treatment options [1-3]. Cisplatin, a widely used chemotherapeutic agent, has been the cornerstone of oral cancer treatment for decades. However, its use is often hampered by severe side effects, including nephrotoxicity, neurotoxicity, and ototoxicity, which can significantly impact patients’ quality of life. Furthermore, the development of resistance to cisplatin is a major concern, reducing its effectiveness and necessitating the exploration of alternative treatment strategies [4-7]. Curcumin, a natural anti-inflammatory and anti-cancer agent, has been shown to have synergistic effects when combined with chemotherapeutic agents, including cisplatin [8]. Curcumin’s anti-cancer properties are attributed to its ability to inhibit cell proliferation, induce apoptosis, and modulate various signaling pathways. However, its poor water solubility and limited bioavailability hinder its therapeutic potential [9-10]. Recent advances in nanotechnology have led to the development of novel drug delivery systems, such as niosome nanoparticles, which can enhance the therapeutic efficacy of both cisplatin and curcumin [11-13]. Niosome nanoparticles are non-ionic surfactant-based vesicles that can encapsulate both hydrophilic and lipophilic compounds, improving their solubility and bioavailability [14-15]. The combination of curcumin and cisplatin in niosome nanoparticles offers a promising approach to the treatment of oral cancer. By encapsulating both agents in a single nanocarrier, we can potentially reduce the side effects associated with cisplatin while enhancing its therapeutic efficacy. Moreover, the synergistic effects of curcumin and cisplatin may lead to improved anti-cancer activity, reduced drug resistance, and enhanced patient outcomes [16].
In this study, we investigate the use of niosome nanoparticles for the combined treatment of curcumin and cisplatin in oral cancer. By exploring the physicochemical properties of niosome nanoparticles, their ability to encapsulate and release both agents, and their anti-cancer effects in vitro, we aim to shed light on the potential of this innovative approach for improving oral cancer treatment. The use of niosome nanoparticles to deliver a combination of curcumin and cisplatin offers several advantages. These benefits include enhanced bioavailability and solubility of both agents, reduced side effects associated with cisplatin, improved therapeutic efficacy and anti-cancer activity, potential to overcome drug resistance, and enhanced patient outcomes. By harnessing the potential of niosome nanoparticles, we may be able to develop a more effective and safer treatment strategy for oral cancer, ultimately improving the quality of life for patients affected by this devastating disease. Considering these benefits, using niosome nanoparticles can be a significant step forward in developing more effective treatments for oral cancer. This innovative technology can help us provide more personalized and targeted treatments for patients with oral cancer, ultimately improving their quality of life.
Materials and Methods
Materials
Acquired from the Sigma Corporation Were cisplatin, curcumin, Span 60, Cholesterol, Polyethylene Glycol 200, culture solution RPMI 16-40, Ethanol. Additionally, the CAL 27 cell line was supplied courtesy of the Cell Bank affiliated with the Iranian Pasteur Institute.
Synergistic effect of cisplatin and curcumin in CAL 27 cells
The sensitivity of CAL 27 cells to individual drugs and the drug combination was determined using a cytotoxicity assay. Briefly, cells were plated in 96-well at a density of 1 × 104 cells per well, each well with 100 μL medium, and incubated for 24 h in a CO2 incubator, followed by the addition of Cisplatin, curcumin, or Cisplatin: curcumin (1:4, 1:8). Following a 24 h incubation, the relative effect on the presence of viable cells was determined by MTT assay. The median-effect algorithm analysed a synergy of the two drugs over a range of drug ratios and concentrations.
Preparation of nanoparticles containing drugs
In summary, to form a mixture, AgNO3 (100 mg, 0.45 mmol) was added to a suspension of cisplatin in double-distilled water. This mixture was then heated at 45 °C for 2 hours and stirred overnight in a flask, kept in the dark with aluminum foil covering. Afterwards, the mixture was centrifuged at 10,000 g for 10 minutes to remove the AgCl precipitate. The solution was subsequently filtered through a 0.2 μm syringe filter. The concentration of cisplatin was determined using a SpectrAA-24OFS Atomic Absorption Spectrometer (Varian, USA) and adjusted to 100 mM. In the next step, a cisplatin solution (100 μL, 150 mM) was mixed into a cyclohexane/Igepal CO-520 (75, 33 V: V, 8 mL) and cyclohexane/triton-X100/ hexanol blend to create a well-dispersed water-in-oil reverse micro-emulsion. Tween 20 (40 μL, 12 mM) was added to the Cisplatin-containing microemulsion and stirred for 15 min. Separately, another reverse micro-emulsion containing KCl was prepared by adding a KCl solution into a similar reverse micro-emulsion. The two emulsions were combined, and the reaction proceeded for 30 minutes. Afterwards, ethanol (15 mL) was added to the mixture and vortexed, followed by centrifugation at 10,000 g for at least 10 minutes to remove the cyclohexane. The resulting pellets were washed with ethanol 2 times, then re-dispersed in chloroform (3.0 mL). To prepare cisplatin niosomes, span 60 (8 mg), cholesterol (14 mg), PEG200 (14 mg), and NPC nanoparticle cores solution (1.5 mL, 1 μg/mL) were dispersed in ethanol, and the ethanol was removed by rotary evaporation. The remaining niosomes were then dispersed in water. For curcumin niosome preparation, span 60 (8 mg), cholesterol (14 mg), PEG200 (14 mg), and a curcumin solution (1.5 mg/mL, 1.5 mL) were mixed in ethanol. Once the ethanol was evaporated, the residual niosomes were dispersed in PBS (3 mL). To create liposomes co-loaded with cisplatin and curcumin (Cis/CUR-NPs), span 60 (8 mg), cholesterol (14 mg), and PEG200 (11 mg) were combined with NPC nanoparticle core solution (1.5 mL at 1 μg/mL) and a curcumin solution (1.5 mL at 1.5 mg/mL) in 20 mL of ethanol. This mixture was then subjected to rotary evaporation to remove the chloroform. The remaining lipids were subsequently suspended in 3.0 mL of PBS at pH 7.
Characterization of Niosomes
The particle size and zeta potential, PDI of the niosomal formulations were analyzed using a Zeta PALS analyzer from Brookhaven Instruments Corporation (Austin, TX). Measurements were performed at room temperature, with each parameter recorded three times. The average values and standard deviations were then calculated. Niosomal Cisplatin and Curcumin concentrations were quantified using an atomic absorption spectrometer and an F-4500 fluorescence spectrophotometer (Hitachi, Japan).
In Vitro drug release
The in vitro release of Cisplatin and Curcumin from cisplatin/curcumin nanoparticles was evaluated using the dynamic dialysis method. A 2 mL sample of cisplatin/ curcumin nanoparticles was placed into a pre-treated dialysis bag (MWCO: 10 kDa). The bag was then submerged in a brown bottle containing 200 mL of dialysis solution, which consisted of 25% (v/v) ethanol in PBS. The solution was stirred at 140 rpm at 37 °C. At specific time intervals (1 h, 2 h, 4 h, 6 h, 9 h, 12 h, 24 h, and 48 h), two 10 μL samples were withdrawn from the dialysis bag. HPLC analyzed curcumin concentration after demulsification with methanol, and Cisplatin concentration was measured after digesting the sample with concentrated nitric acid.
In vitro cell cytotoxicity
The cytotoxicity of Cisplatin-Sol (free cisplatin), Cisplatin-niosomes, Curcumin-Sol (free curcumin), Curcumin-niosomes, Cisplatin/Curcumin-Sol (a solution of free cisplatin and curcumin at a 1:4 ratio), and Cisplatin/ Curcumin nanoparticles were assessed using the MTT assay. In brief, cells were seeded into 96-well plates at a density of 1 × 10⁴ cells per well, each well containing 100 μL of medium, and incubated for 24 hours in a CO2 incubator. Different formulations were then added at varying concentrations. After an additional 48-hour incubation, the viability of the cells was measured using the MTT assay, following the instructions in the user manual. Cell viability for each group was expressed as a percentage relative to the untreated control cells. Based on the viability data, the IC50 was calculated.
Statistics analysis
Data values were expressed as mean ± S.D. Unless otherwise specified, statistical analyses were performed using analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test, utilizing GraphPad Prism Version 5 software (San Diego, CA, USA). P-values.
Results
In vitro synergistic effect of cisplatin and curcumin combinations
To identify the optimal combination ratio, an MTT assay was conducted to assess the viability of CAL 27 cells after 24 hours of treatment with Cisplatin and Curcumin, either individually or in combination at various molar ratios. The median-effect method and combination index (CI) were employed to evaluate the synergistic inhibitory effects (Figure 1).
Figure 1. Screening of the Optimal Combination Ratio of Cisplatin and Curcumin in CAL-27 Cells.
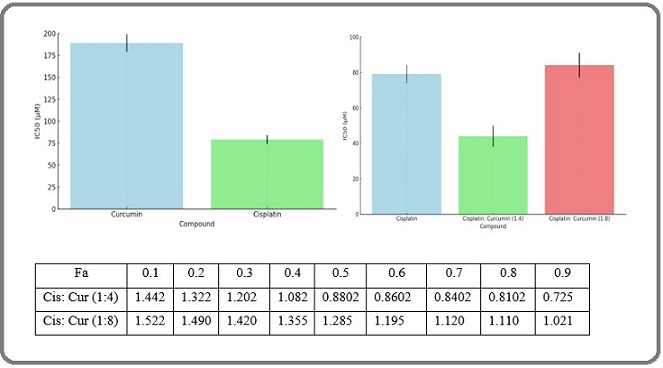
The combination index (CI) was plotted as a function of the fraction affected (fa) in CAL 27cells, where fa values corresponded to growth inhibition. The CI values indicated synergism (CI < 1), additivity (CI = 1), or antagonism (CI > 1). As shown in Figure 1, synergistic activity was observed at higher levels of growth inhibition with the combinations of Cisplatin at ratios of 1:4. However, when the Curcumin proportion increased to a Cisplatin ratio of 1:8, the combination exhibited an antagonistic effect (CI > 1), with the antagonism becoming more pronounced at higher levels of growth inhibition. Figure 1 demonstrates that at a Cisplatin ratio of 1:4, the synergistic effect became significant when inhibition exceeded 50% (fa = 0.5) and continued to increase in the higher inhibitory range (fa = 0.9), indicating that this ratio provides a more effective anti-tumor synergy compared to other ratios. Therefore, this combination ratio was selected for further study in the preparation and evaluation of co-loaded nano-niosomes.
Preparation and characterization of Nanoparticles
Niosomes co-loaded with Cisplatin and Curcumin, as well as separate niosomes for each drug, were formulated using a mixture of Span 60, Chol, and PEG200 in the ratio 60:20:4. Dynamic Light Scattering (DLS) measurements revealed that the average particle size of these Cisplatin/ Curcumin nanoparticles was 150.9 nm, with a zeta potential of 24.6 ± 3.2 mV. The polydispersity index was 0.23, indicating the particles’ uniform size and good dispersion. Release profiles, detailed in Figure 2, showed a two-phase release for both drugs: an initial burst within the first 4 hours, followed by a sustained release over the next 24 hours, with cumulative releases of 48% for Cisplatin and 51% for Curcumin at 48 hours.
Figure 2. Preparation and Characterization of Cis/CUR- nanoparticles. In vitro release of Cisplatin and CUR in 48h.
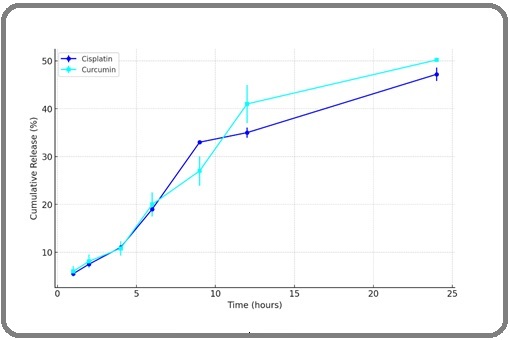
The release rates throughout the period were consistent, suggesting that the encapsulation allowed for a sustained and simultaneous release of both drugs, supporting their synergistic potential.
In vitro cytotoxicity and synergistic effect of Nanoparticles in CAL 27 cells
An MTT assay was utilized to assess the cytotoxic effects of various formulations on CAL 27 cells. Cis-NPs exhibited notably higher cytotoxicity against CAL 27 cells compared to Cis-Sol at equivalent concentrations, with Cis-Sol displaying a higher IC50 than Cis-NPs, suggesting that niosomal encapsulation of Cisplatin might facilitate greater uptake by cancer cells, thereby enhancing its cytotoxic impact. The cytotoxic effects of CUR-Sol and CUR-NPs were comparable, with no significant differences noted (p < 0.05) at the tested concentrations. Both Cis/CUR-Sol and Cis/CUR-NPs showed the most significant cytotoxic effects on CAL 27 cells. When compared to the single-drug formulations of Cis-Sol and Cis-NPs, the combination of Cis and CUR in a niosomal carrier significantly increased the anticancer efficacy synergistically. Consequently, the co-loaded liposomes demonstrated enhanced cytotoxicity compared to their respective free drug solutions (Table 1).
| Compounds | IC50 (µM) |
| Cis-Sol | 178±10.4 |
| Cis-NPs | 79±6.6 |
| CUR-Sol | 114±8.8 |
| CUR-NPs | 98±7.2 |
| Cis-CUR-Sol | 66±2.9 |
| Cis-CUR-NPs | 32±1.9 |
Discussion
Oral cancer poses a significant health challenge globally due to its aggressive nature and limited treatment options. Traditional therapies, including surgery, radiation, and chemotherapy, often fall short in terms of efficacy and patient tolerance. The development of innovative drug delivery systems is essential to improve therapeutic outcomes [17]. Niosome nanoparticles have emerged as a promising carrier system for delivering hydrophobic drugs, enhancing bioavailability, and providing targeted delivery [18]. This study investigates the combined use of curcumin, a natural anti-inflammatory and anti-cancer agent, with cisplatin, a widely used chemotherapeutic drug, utilizing niosome nanoparticles for effective treatment against oral cancer [19-23]. In this research, niosome nanoparticles were formulated and optimized. The characterization of these nanoparticles showed a mean particle size of approximately 150 nm, a zeta potential of 24.6 ± 3.2 mV, and a low polydispersity index (PDI) of 0.23 ± 0.05. These characteristics are crucial for ensuring the stability of the niosomes in biological fluids and enhancing their uptake by cancer cells. Smaller particles with a favorable zeta potential tend to have improved dispersion and reduced aggregation, allowing for better penetration into tissues [24-27]. The controlled release profile of curcumin and cisplatin over 48 hours demonstrated the potential of niosomes to deliver these drugs effectively. A cumulative release of 51% ± 5.2% for curcumin and 48% ± 4.5% for cisplatin indicates that the niosome formulation can maintain drug availability at the target site over an extended period. This sustained release is advantageous in minimizing the frequency of dosing and enhancing patient compliance, ultimately contributing to better treatment outcomes [8, 28-29]. The combination of curcumin and cisplatin presents a powerful therapeutic strategy against oral cancer. Curcumin possesses anti-inflammatory, antioxidant, and anti-cancer properties, which can enhance the effectiveness of cisplatin. Studies have shown that curcumin can sensitize cancer cells to cisplatin by inhibiting survival pathways and enhancing apoptosis. This synergistic effect is particularly important in the context of oral cancer, where resistance to chemotherapy is a significant concern. The findings from in vitro cytotoxicity assays revealed that the combined treatment significantly reduced cell viability compared to individual treatments, further underscoring the potential of this combination in enhancing therapeutic efficacy [19-20, 23, 30] While both niosomes and liposomes serve as effective drug delivery systems, they have distinct characteristics that influence their performance. Liposomes are composed of phospholipid bilayers and can encapsulate both hydrophilic and hydrophobic drugs, providing flexibility in formulation. However, their stability can be compromised by environmental factors such as temperature and pH, leading to premature drug release. Niosomes, on the other hand, are formed from non-ionic surfactants, offering greater stability in biological environments and potentially lower production costs [15, 31-36]. Furthermore, niosomes can be engineered to achieve specific targeting, enhancing the accumulation of drugs in tumor tissues. The smaller size and modified surface characteristics of niosomes may facilitate better penetration through biological barriers compared to liposomes. This is particularly relevant in the treatment of solid tumors, such as oral cancer, where effective drug delivery to the tumor site is crucial for therapeutic success [31-39]. Despite their advantages, the use of niosome nanoparticles in drug delivery is not without limitations. One significant concern is the biodistribution and pharmacokinetics of niosomes. They can be rapidly eliminated from the bloodstream by the reticuloendothelial system (RES), reducing their circulation time and therapeutic concentration at the tumor site. Additionally, niosomes may face challenges related to tissue penetration, as their size and surface properties can impact their ability to reach and accumulate in tumor tissues effectively [40-44].
The human body’s complex structure encompassing biology, mechanisms, chemistry, and more continuously interacts with technological advancements. With the increasing prevalence of various diseases, including cancers, heart conditions, mental disorders, and others that have persistently impacted human life, innovative solutions like artificial intelligence, advanced pharmaceutical formulations, and expert psychological support are proving invaluable. These advancements offer significant potential to enhance disease management and treatment, fostering a better quality of life [45-72]. We examine important diseases and health issues. Drug delivery systems have significantly improved transporting of essential biological materials [73]. Cancer is one of the leading causes of death globally, and chemotherapy has been extensively used as a treatment method over the past few decades [74]. Oral squamous cell carcinoma (OSCC) is a common and impactful form of oral cancer with widespread health consequences globally [75]. Apart from cancer, the COVID-19 pandemic can be mentioned as it endangered the lives of people worldwide [76]. For example, the coronavirus has created numerous challenges for healthcare teams, especially for nurses [77]. Among the health issues that threaten human life, stroke can be mentioned, and research has been conducted in this area [78]. One of the diseases that becomes more prevalent with ageing in society is Alzheimer’s [79]. Recent advancements include medical modeling, which has become an important innovation in the field [80]. Technology holds a special place in today’s diagnostics and treatment; for example, artificial intelligence provides significant assistance in achieving these goals [81]. Among technological advancements, vaccine development stands out as it has effectively contributed to improving quality of life [82]. Individual and environmental factors significantly influence a person’s growth and skill development [83].
In conclusion, the findings of this study suggest that niosome nanoparticles can effectively deliver a combination of curcumin and cisplatin, significantly enhancing their therapeutic potential against oral cancer. The synergistic effects of curcumin and cisplatin represent a promising approach to overcoming resistance and improving treatment outcomes. While niosomes offer several advantages over traditional liposomes, addressing the limitations related to drug clearance, stability, and tissue penetration is essential for translating this technology into clinical practice. Future research should focus on optimizing niosome formulations and exploring advanced targeting strategies to maximize the clinical benefits of niosome-mediated drug delivery systems in oral cancer therapy.
Acknowledgements
None.
Data availability
Not applicable as we used information from previously published articles.
Approved by any scientific Body
Not applicable as the manuscript is not a part of any student thesis or study.
Ethical issue and approval
Not applicable as we used information from previously published articles.
Consent for publication
All authors have given consent for publication.
Conflict of interest
The authors declare no potential conflict of interest.
References
- Scaffold Application for Bone Regeneration with Stem Cells in Dentistry: Literature Review Saberian E, Jenča A, Zafari Y, Jenča A, Petrášová A, Zare-Zardini H, Jenčová J. Cells.2024;13(12). CrossRef
- Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study Saberian E, Jenča A, Seyfaddini R, Jenča A, Zare-Zardini H, Petrášová A, Jenčová J. Biomolecules.2024;14(6). CrossRef
- Enhancing Cisplatin Delivery via Liposomal Nanoparticles for Oral Cancer Treatment Ghanbarikondori P, Aliakbari R, Saberian E, Jenca A, Petrášová A, Jencova J, Khayavi A. Indian Journal of Clinical Biochemistry.2024. CrossRef
- Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects Florea A, Büsselberg D. Cancers.2011;3(1). CrossRef
- The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma Cheng Y, Li S, Gao L, Zhi K, Ren W. Frontiers in Oncology.2021;11. CrossRef
- Cisplatin in cancer therapy: molecular mechanisms of action Dasari S, Tchounwou PB . European Journal of Pharmacology.2014;740. CrossRef
- Cisplatin: The first metal based anticancer drug Ghosh S. Bioorganic Chemistry.2019;88. CrossRef
- Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma Cheng Y, Zhao P, Wu S, Yang T, Chen Y, Zhang X, He C, et al . International Journal of Pharmaceutics.2018;545(1-2). CrossRef
- Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells Srivastava NS , Srivastava RAK . Phytomedicine: International Journal of Phytotherapy and Phytopharmacology.2019;52. CrossRef
- Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling Bai C, Zhao J, Su J, Chen J, Cui X, Sun M, Zhang X. Life Sciences.2022;306. CrossRef
- Dual-Responsive Curcumin-Loaded Nanoparticles for the Treatment of Cisplatin-Induced Acute Kidney Injury Lan T, Guo H, Lu X, Geng K, Wu L, Luo Y, Zhu J, et al . Biomacromolecules.2022;23(12). CrossRef
- Preparation, Optimization and In-Vitro Evaluation of Curcumin-Loaded Niosome@calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment Akbarzadeh I, Shayan M, Bourbour M, Moghtaderi M, Noorbazargan H, Eshrati Yeganeh F, Saffar S, Tahriri M. Biology.2021;10(3). CrossRef
- Nanoparticle formulations of cisplatin for cancer therapy Duan X, He C, Kron SJ , Lin W. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology.2016;8(5). CrossRef
- Nano-niosomes as nanoscale drug delivery systems: an illustrated review Moghassemi S, Hadjizadeh A. Journal of Controlled Release: Official Journal of the Controlled Release Society.2014;185. CrossRef
- An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications Masjedi M, Montahaei T. 2020. CrossRef
- Co-Delivery of Cisplatin and Curcumin Using Mesoporous Silica Nanoparticles to Improve their Anticancer Effects Dizaj SM , Ebrahimi SSB , Jafari S, Basiri A, Yazdani J, Sharifi S, Abdolahinia ED . http://www.eurekaselect.com..
- Application of Scaffold-Based Drug Delivery in Oral Cancer Treatment: A Novel Approach Saberian E, Jenča A, Petrášová A, Zare-Zardini H, Ebrahimifar M. Pharmaceutics.2024;16(6). CrossRef
- Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery Momekova DB , Gugleva VE , Petrov PD . ACS omega.2021;6(49). CrossRef
- Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects Hussain Y, Islam L, Khan H, Filosa R, Aschner M, Javed S. Phytotherapy research: PTR.2021;35(12). CrossRef
- Curcumin-Encapsulated Nanomicelles Improve Cellular Uptake and Cytotoxicity in Cisplatin-Resistant Human Oral Cancer Cells Kumbar VM , Muddapur U, Bin Muhsinah A, Alshehri SA , Alshahrani MM , Almazni IA , Kugaji MS , et al . Journal of Functional Biomaterials.2022;13(4). CrossRef
- Curcumin Nanoparticle Enhances the Anticancer Effect of Cisplatin by Inhibiting PI3K/AKT and JAK/STAT3 Pathway in Rat Ovarian Carcinoma Induced by DMBA Sandhiutami NMD , Arozal W, Louisa M, Rahmat D, Wuyung PE . Frontiers in Pharmacology.2020;11. CrossRef
- Anticancer evaluation of methotrexate and curcumin-coencapsulated niosomes against colorectal cancer cell lines Mousazadeh N, Gharbavi M, Rashidzadeh H, Nosrati H, Danafar H, Johari B. Nanomedicine (London, England).2022;17(4). CrossRef
- Synergistic Effects of New Curcumin Analog (PAC) and Cisplatin on Oral Cancer Therapy Semlali A, Beji S, Ajala I, Al-Zharani M, Rouabhia M. Current Issues in Molecular Biology.2023;45(6). CrossRef
- Maximizing the Therapeutic Efficacy of Imatinib Mesylate-Loaded Niosomes on Human Colon Adenocarcinoma Using Box-Behnken Design Kassem MA , El-Sawy HS , Abd-Allah FI , Abdelghany TM , El-Say KM . Journal of Pharmaceutical Sciences.2017;106(1). CrossRef
- High-concentration zeta potential measurements using light-scattering techniques Kaszuba M, Corbett J, Watson FM , Jones A. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences.2010;368(1927). CrossRef
- Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies El-Nabarawi MA Mohamed Ahmed, Abd El Rehem RT , Teaima M, Abary M, El-Mofty HM , Khafagy MM , Lotfy NM , Salah M. Drug Development and Industrial Pharmacy.2019;45(6). CrossRef
- Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes Wichayapreechar P, Anuchapreeda S, Phongpradist R, Rungseevijitprapa W, Ampasavate C. Journal of Liposome Research.2020;30(2). CrossRef
- Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate Gugleva V, Michailova V, Mihaylova R, Momekov G, Zaharieva MM , Najdenski H, Petrov P, et al . Pharmaceutics.2022;14(4). CrossRef
- Folate-Targeted Curcumin-Loaded Niosomes for Site-Specific Delivery in Breast Cancer Treatment: In Silico and In Vitro Study Honarvari B, Karimifard S, Akhtari N, Mehrarya M, Moghaddam ZS , Ansari MJ , Jalil AT , et al . Molecules (Basel, Switzerland).2022;27(14). CrossRef
- The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro Elattar T. M., Virji A. S.. Anticancer Research.2000;20(3A).
- Niosomes, an alternative for liposomal delivery Bartelds R, Nematollahi MH , Pols T, Stuart MCA , Pardakhty A, Asadikaram G, Poolman B. PloS One.2018;13(4). CrossRef
- Do niosomes have a place in the field of drug delivery? Muzzalupo R, Mazzotta E. Expert Opinion on Drug Delivery.2019;16(11). CrossRef
- Niosomes: novel sustained release nonionic stable vesicular systems--an overview Mahale N. B., Thakkar P. D., Mali R. G., Walunj D. R., Chaudhari S. R.. Advances in Colloid and Interface Science.2012;183-184. CrossRef
- Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications Chen S, Hanning S, Falconer J, Locke M, Wen J. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V.2019;144. CrossRef
- Niosomes from 80s to present: the state of the art Marianecci C, Di Marzio L, Rinaldi F, Celia C, Paolino D, Alhaique F, Esposito S, Carafa M. Advances in Colloid and Interface Science.2014;205. CrossRef
- Effects of Micro-environmental pH of Liposome on Chemical Stability of Loaded Drug Shao X, Wei X, Zhang S, Fu N, Lin Y, Cai X, Peng Q. Nanoscale Research Letters.2017;12(1). CrossRef
- Niosomes Functionalized with a Synthetic Carbohydrate Binding Agent for Mannose-Targeted Doxorubicin Delivery Burrini N, D'Ambrosio M, Gentili M, Giaquinto R, Settimelli V, Luceri C, Cirri M, Francesconi O. Pharmaceutics.2023;15(1). CrossRef
- Current Trends of Targeted Drug Delivery for Oral Cancer Therapy Zhang M, Liang J, Yang Y, Liang H, Jia H, Li D. Frontiers in Bioengineering and Biotechnology.2020;8. CrossRef
- Niosomes: A Novel Carrier Drug Delivery System Kauslya A, Borawake PD , Shinde JV , Chavan RS . Journal of Drug Delivery and Therapeutics.2021;11(1). CrossRef
- Overcoming in vivo barriers to targeted nanodelivery Chrastina A, Massey KA , Schnitzer JE . Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology.2011;3(4). CrossRef
- Targeted Fusogenic Liposomes for Effective Tumor Delivery and Penetration of Lipophilic Cargoes Han H, Jung J, Lee H, Lee J, Jang S, Goh U, Yoon J, Park J. ACS biomaterials science & engineering.2023;9(4). CrossRef
- Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues Villegas MR , Baeza A, Vallet-Regí M. ACS applied materials & interfaces.2015;7(43). CrossRef
- NIR II-Excited and pH-Responsive Ultrasmall Nanoplatform for Deep Optical Tissue and Drug Delivery Penetration and Effective Cancer Chemophototherapy Zhu L, Gao D, Xie L, Dai Y, Zhao Q. Molecular Pharmaceutics.2020;17(10). CrossRef
- ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: Light-triggered size-reducing and enhanced tumor penetration Jin H, Zhu T, Huang X, Sun M, Li H, Zhu X, Liu M, et al . Biomaterials.2019;211. CrossRef
- Survival Analysis of Young Triple-Negative Breast Cancer Patients Horestani FJ , Schwarz G. arXiv preprint arXiv:2401.08712.2024. CrossRef
- Prediction of Breast Cancer Recurrence With Machine Learning. In M. Khosrow-Pour, D.B.A. (Ed.), Encyclopedia of Information Science and Technology, Owrang OMM , Schwarz G, Horestani FJ . Sixth Edition. Advance online publication.2025. CrossRef
- Integrating Protein Structure Prediction and Bayesian Optimization for Peptide Design. InNeurIPS 2023 Generative AI and Biology (GenBio) Workshop Manshour N, He F, Wang D, Xu D. 2023. https://openreview.net/ forum?id=CsjGuWD7hk.
- Feasibility of Mri-guided Left Heart Catheterization on a Commercially Available 0.55T Scanner Platform and Readily Available Invasive Pressure Monitoring Hardware Unal HB , Zeynali S, Anttila E, Roll J, Kreutz R, Frick K, Raman S, et al . Journal of Cardiovascular Magnetic Resonance.2024;26. CrossRef
- Environmental Determinants of Oral Cancer Development: An Overview Maddahi M, Ghanbarikondori P, Amiri F, Jahromi A, Pour N, Allahyartorkaman M, Moazzam F. Asian Pacific Journal of Environment and Cancer.2024. CrossRef
- Maxillary Sinus Volume in Cleft Lip and Palate Patients with and without an Oronasal Fistula using CBCT Kiaei B, Hafezi L, Karani M, Amiri F, Jamilian A. Stomatology Edu Journal.2021;8. CrossRef
- Oral Cancer and HPV: Review Article Pirmoradi Z, Nazari K, Shafiee N, Nikoukar N, Minoo S, Ghasemi H, Ghanbarikondori P, Allahyartorkaman M. Asian Pacific Journal of Cancer Biology.2024;9. CrossRef
- tRNA-derived fragments: Key determinants of cancer metastasis with emerging therapeutic and diagnostic potentials Salehi M, Kamali MJ , Rajabzadeh A, Minoo S, Mosharafi H, Saeedi F, Daraei A. Archives of Biochemistry and Biophysics.2024;753. CrossRef
- High-Resolution Ultrasound Imaging for Non-Invasive Characterization of Acute Wound Healing in Radiation Injury on Guinea Pig Skin Tissue Hormozi-Moghaddam Z, Mokhtari-Dizaji M, Nilforoshzade , Bakhshande M, Zare S. Frontiers in Biomedical Technologies.2024;11(1):104-112.
- Ultrasound Waves Effect on the Proliferation of Fibroblast Cells: Collagen Type I Expression Hormozi Moghaddam Z, Mokhtari-Dizaji M, Nilforoshzadeh MA , Bakhshandeh M. Journal of Biomedical Physics and Engineering.2023. CrossRef
- Synergistic anticancer effects of doxorubicin and metformin combination therapy: A systematic review Jalali F, Fakhari F, Sepehr A, Zafari J, Sarajar BO , Sarihi P, Jafarzadeh E. Translational Oncology.2024;45. CrossRef
- Enhancing breast cancer diagnosis accuracy through genetic algorithm-optimized multilayer perceptron. Multiscale and Multidisciplinary Modeling Talebzadeh H, Talebzadeh M, Satarpour M, Jalali, F, Farhadi B, Vahdatpour MS . Experiments and Design.2024;:1-17.
- The AI Diagnostician: Improving Medical Diagnosis with Artificial Intelligence Farrokhi M, Taheri F, Adibnia E, Mehrtabar S, Rassaf Z, Tooyserkani SH , Rajabloo Y, et al . Kindle.2024;4(1):1-219.
- Investigating the mortality trend of gastrointestinal cancers in Babol, North Iran (2013-2021) Ebrahimi P, Karami M, Delavari S, Shojaie L, Hosseini-Berneti SH , Bayani F, Moghaddasi M, et al . BMC gastroenterology.2024;24(1). CrossRef
- A snapshot of SARS-CoV-2 viral RNA throughout wastewater treatment plants in Arkansas Long A, Loethen K, Behzadnezhad A, Zhang W. Water Environment Research: A Research Publication of the Water Environment Federation.2024;96(2). CrossRef
- Batch adsorption of methyl tert-butyl ether (MTBE) from aqueous solution by combined CNT and zeolite Behzadnezhad A, Ebadi T, Taheri S, Kowsari E. DESALINATION AND WATER TREATMENT.2020;191. CrossRef
- A sequential Ugi–Smiles/transition-metal-free endo-dig Conia–ene cyclization: the selective synthesis of saccharin substituted 2,5-dihydropyrroles Seyrani H, Ramezanpour S, Vaezghaemi A, Kobarfard F. New Journal of Chemistry.2023;47(46). CrossRef
- Development of an electrochemical sensor for detection of lupron as a drug for fibroids treatment and uterine myoma in pharmaceutical waste and water sources | Request PDF Movahed F, Ehymayed H, Kalavi S, Shahrtash SA , Al-Hijazi A, Daemi A, Mahmoud H, et al . ResearchGate.2024;18. CrossRef
- Beta lactam allergy and risk of surgical site infection: A systematic review and meta-analysis Salehi SA , Hajishah H, Amini MJ , Movahed F, Ebrahimi A, Rihani FSS , Dehbozorgi M, et al . Current Problems in Surgery.2024;61(11). CrossRef
- Ethical Considerations and Equipoise in Cancer Surgery Vakili-Ojarood M, Naseri A, Shirinzadeh-Dastgiri A, Saberi A, HaghighiKian SM , Rahmani A, Farnoush N, et al . Indian Journal of Surgical Oncology.2024;15(Suppl 3). CrossRef
- Hardy-Weinberg Equilibrium in Meta-Analysis Studies and Large-Scale Genomic Sequencing Era Neamatzadeh H, Dastgheib SA , Mazaheri M, Masoudi A, Shiri A, Omidi A, Rahmani A, et al . Asian Pacific journal of cancer prevention: APJCP.2024;25(7). CrossRef
- A comprehensive review of etiology, pathophysiologyepidemiology, and management of hair loss and its correlation with COVID-19 Faraz Changizi , Maryam Abdolmaleki , Mina Farjam , Laya Ohadi . International Journal of Medicine.2024;12(2):41-47. CrossRef
- The Effect of Pharmacist Interventions on the Antimicrobial Prevention Pattern in Vascular and Gastrointestinal Surgeries: A Prospective Study Yazd H, Ohadi L, Abdolmaleki M, Farsi Y, Pishgahi M. J Case Rep Med Hist.2024;4(4).
- Dynamic control of contractile resistance to iPSC-derived micro-heart muscle arrays Schuftan D, Kooh YKZ , Guo J, Sun Y, Aryan L, Stottlemire B, Berkland C, et al . Journal of Biomedical Materials Research. Part A.2024;112(4). CrossRef
- Serum level measurement of progranulin in relapsing-remitting multiple sclerosis and neuromyelitis optica patients Azadani NN , Norouzi F, Hajizadeh M, Parsa S, Khalighinejad F. American Journal of Clinical and Experimental Immunology.2019;8(3).
- Examine the impact of mental planning on building mental health and cognitive beliefs of bank employees Ghahjavarestani A. International Journal of Science and Research Archive.2024;12. CrossRef
- Redictive Role of Personality Dimensions on Quality of Life and Satisfaction in Patients With Gender Identity Disorder after Gender Reassignment Surgery Ghahjavarestani AHM. The scientific heritage.2024. https://orcid. org/0000-0002-0440-0509;135. CrossRef
- Plasma NT1 tau is associated with hypometabolism in Alzheimer’s disease continuum Ghahri Lalaklou Z, Montazeri Ghahjavarestani A, Pishkari Y, Emami D. Neurology Letters, 3(Special Issue (Diagnostic and Therapeutic advances in Neurodegenerative diseases)).2024;:8-13. CrossRef
- The effects of tumor-derived exosomes enriched with miRNA-211a on B16F10 cells Atashzar MR , Ataollahi MR , Asad AG , Doroudgar P, Amani D. Contemporary Oncology.2024;28(2). CrossRef
- Anti-cancer Activity of New Phosphoramide-functionalized Graphene Oxides: An Experimental and Theoretical Evaluation Gholivand K, Faraghi M, Pooyan M, Babaee LS , Malekshah RE , Pirastehfar F, Vahabirad M. Current Medicinal Chemistry.2023;30(30). CrossRef
- Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review Mesgari H, Esmaelian S, Nasiri K, Ghasemzadeh S, Doroudgar P, Payandeh Z. Cancers.2023;15(23). CrossRef
- A Multistage Stochastic Optimization Model for Resilient Pharmaceutical Supply Chain in COVID-19 Pandemic Based on Patient Group Priority. 2024 Systems and Information Engineering Design Symposium (SIEDS) (pp. 382-387) Mahdavimanshadi M, Anaraki MG , Mowlai M, Ahmadirad Z. IEEE.2024. CrossRef
- A qualitative approach to the ethical challenges of Iranian nurses during the COVID-19 pandemic Rajabipoor Meybodi A, Mohammadi M, Arjmandi H. Journal of Preventive and Complementary Medicine.2022;1(3):156-162. CrossRef
- Effect of a visual dual task on postural stability-A comparative study using linear and nonlinear methods Ghamari N, Ghaderpanah R, Sadrian SH , Fallah N. Health Science Reports.2023;6(8). CrossRef
- A Review on the Comparison of Working Memory Performance, Cognitive Function, and Behavioral, and Psychological Symptoms across Normal Aging, Mild Cognitive Impairment, and Alzheimer's Disease Ghayedi Z, Banihashemian K, Shirdel S, Salarvand R, Zare M, zeinali S, Lalaklou Z. Neurology Letters.2024;3. CrossRef
- Design of Microfluidic Sensing and Transport Device Asgharighajari M, Amziah N, Sulaiman N, Sodeinde SA . Journal of Biomedical Engineering and Technology.2015;3(1):15-20. CrossRef
- Examination of AI’s role in Diagnosis, Treatment, and Patient care. In Gupta, M., Kumar, R., & Lu, Z. (Eds.), Transforming Gender-Based Healthcare with AI and Machine Learning (1st ed., pp. 221- 238) Sajjadi Mohammadabadi SM , Seyedkhamoushi F, Mostafavi M, Borhani Peikani M. CRC Press.2024. CrossRef
- The potential use of therapeutics and prophylactic mRNA vaccines in human papillomavirus (HPV) Movahed F, Darzi S, Mahdavi P, Mahdi M, Allela O, Sameer H, Adil M, et al . Virology Journal.2024;21. CrossRef
- Evaluating the effect of cochlear implantation age on pragmatic abilities before and after age of 3 Nikrah P, Ghareh Chahie R, Ghazvini A, Hajizadeh A. Applied Neuropsychology. Child.2024. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details