Low PD-1 mRNA Expression in Peripheral Blood of Childhood Leukemia
Download
Abstract
Background: The expression of PD-1 has been linked to prognosis and response to immune checkpoint inhibitors in solid tumors, but less is known about their role in pediatric blood cancers. Therefore, we aimed to study the levels of PD-1 mRNA in children with leukemia and explore how they relate to clinical factors.
Methods: Blood samples were collected from 15 children with leukemia and 11 healthy individuals. PD-1 mRNA expression was analyzed using real time PCR.
Results: PD-1 mRNA expression was significantly lower in childhood leukemia patients (average DCT: 12.3±2.5) compared to healthy individuals (average DCT: 10.3±0.5) (p=0.011). PD-1 mRNA expression did not correlate with gender, age, diagnosis, remission status, down syndrome, hyperleukocytosis, infection, and outcome.
Conclusion: Our findings indicated that mRNA expression of PD-1 was reduced in children with leukemia compared to healthy individuals and was not associated with all analyzed clinical factors. These results provide early insights into PD-1 expression in Indonesian pediatric leukemia patients.
Introduction
Leukemia is the most common form of cancer in children [1]. Acute lymphoblastic leukemia (ALL) constitutes around 80% of pediatric leukemia cases and has displayed significant advancements in treatment in recent decades [1]. The five-year survival rate for ALL patients has risen to over 90%, compared to just 10% in the 1960s [2]. On the other hand, acute myeloid leukemia (AML) has a less positive outlook, with 68% 5-year survival rate in young children and 57% in teenagers aged 15–19 years [2]. In low-and middle-income countries (LMICs) like Indonesia, the survival rate for childhood cancer is even lower [3]. Studies conducted at two cancer center hospitals in Indonesia, Dharmais Cancer Hospital and Dr. Sardjito Hospital, reported 5-year survival rates of 28.9% and 31.8% for childhood ALL, respectively [4]. Therefore, there is an urgent need for further improvements in leukemia treatment outcomes [5].
Immunotherapy has become an important treatment option for various types of cancer. Immune checkpoint inhibitor (ICI) is a promising immunotherapy approach for treating adult cancer. Although initial trials of ICI in childhood cancer were not very successful, current active trials indicate promising results. For instance, a trial that combined tislelizumab with decitabine and azacitidine led to a substantial improvement in outcomes, with remission achieved in two-thirds of the participants [6]. In a phase II trial, pembrolizumab treatment following high-dose cytarabine was effective in adults with refractory or relapsed AML, resulting in a 46% overall response rate [7]. Another phase II study explored the use of immune checkpoint inhibitor (ICI) therapy following autologous hematopoietic stem cell transplantation (HSCT) in post-remission AML patients with non-favorable risk. The results showed a 70% survival rate with 50% of patients sustained complete remission [8]. These findings indicate the potential efficacy of ICI in childhood cancer, warranting subsequent investigation [2, 9].
An ongoing trial in pediatric leukemia, NCT02420912, had also shown positive initial results [2]. Additionally, upcoming clinical trials, including NCT04546399, NCT03825367, and NCT02879695, are scheduled to be conducted [2]. The current and future research and clinical trials provide hope for the effectiveness of checkpoint inhibitors in treating pediatric leukemia. The results of these studies are anticipated to offer valuable insights into enhancing survival rates and treatment outcomes for pediatric leukemia patients [2].
ICI functions by using monoclonal antibodies (mAb) to hinder the interaction of PD-1 and PD-L1 [10]. The binding of PD-1 and its ligand, PD-L1, significantly reduces T-cell activity, resulting in tumor growth, promotion of metastasis, and immune suppression. Blocking this interaction enhances T-cell responses and strengthens anti-tumor immunity. PD-1 is a receptor expressed on various immune cells, including T cells, while PD-L1 is highly expressed in cancer cells [11]. PD-L1 is widely recognized as a biomarker for predicting the response to ICI in various types of adult cancer [11]. In addition to PD-L1, several studies suggest that PD-1 expression could also potentially be used as a biomarker to predict the outcome of ICI [12-18].
The role of PD-1 in hematological malignancies, particularly in pediatric cases, has not been extensively studied. Only a few studies have focused on PD-1 expression in pediatric cancers. Therefore, our objective is to analyze PD-1 expression in blood samples from childhood leukemia patients at Dharmais Hospital - National Cancer Center in Indonesia. Additionally, the relationship between PD-1 expression and various clinicopathological characteristics is investigated.
Materials and Methods
Collection of Samples
We collected peripheral blood from 15 pediatric leukemia and 11 healthy individuals as controls. None of the patients have received any treatment at the time of sample collection. The diagnosis has been conducted in the Clinical Pathology Department of Dharmais Cancer Hospital through several laboratory examinations such as bone marrow aspiration analysis, blood examination, immunophenotyping, cytogenetics, or cytochemistry. A total of 11 patients were diagnosed as ALL, and 4 patients were AML. The 15 pediatric leukemia patients comprised 8 males and 7 females with median ages of 4 years (ranging from 2 to 15 years). This study has been approved by the Ethical Committee of Dharmais Cancer Hospital.
RNA Extraction and cDNA Synthesis
The extraction of RNA was carried out using Total RNA Blood/Culture kit from Gene Aid as per the manufacturer’s instructions. The concentration and purity of RNA were assessed using a NanoDrop 2000 Spectrophotometer from Thermo Fisher Scientific. The RNA was then converted to cDNA using a High-Capacity cDNA Synthesis Kit from Applied Biosystems. Subsequently, all cDNA samples were uniformly diluted to 100 ng.
Gene Expression Analysis using Real-time PCR
Custom TaqMan Gene Expression Assay (Applied Biosystems) with assay ID APPRKYU (GAPDH) and AP9HJWE (PD-1) were used in the study. The primers and probe for PD-1 were forward primer 5’-CCAAGGCGCAGATCAAAGAGA-3’, reverse primer 5’-TGGGCTGTGGGCACTT-3’, and probe 5’- CTGCCCTTCTCTCTGTCACC-3’. The primer and probes for GAPDH were forward primer 5’-AGCCTCAAGATCATCAGCAA-3’, reverse primer 5’-ACTGTGGTCATGAGTCCTTC-3’, and probe 5’-CTGCACCACCAACTGCTTAG-3’ [19].
Real-time PCR was conducted in the Applied Biosystems™ 7500 Real-Time PCR Systems (Thermo Fisher Scientific) based on the standard protocol of the TaqMan Gene Expression assay user guide. The amount of cDNA was made equal for all samples. The ∆CT method with GAPDH as an internal control was used for data analysis. Gene expression levels were classified as low or high based on comparing the ∆CT value of each sample to the average ∆CT value of 11 healthy individuals. Samples with ΔCT below the average ΔCT of healthy individuals were classified as having high expression, while those with values above the average were classified as having low expression.
Statistical Analysis
Statistical analysis was done using SPSS v22.0 statistical software. The association between the expression of PD-1 and clinicopathological features was determined using the Fisher exact or chi-square test. A Welch’s T-test test was performed to determine the statistical difference between PD-1 expression in childhood leukemia and healthy individuals. A p-value below 0.05 (p < 0.05) was interpreted as statistically significant.
Results
Expression of PD-1 and PD-L1 in pediatric leukemia
PD-1 expression is significantly reduced in pediatric leukemia compared to healthy controls. The average ΔCT of PD-1 expression is 12.3±2.5 in pediatric leukemia, while the average ΔCT of PD-1 expression in healthy individuals is 10.3±0.5 (p=0.008) (Figure 1).
Figure 1. Expression of PD-1 mRNA in Pediatric Leukemia and Healthy Controls (HC). Lower ΔCT indicates higher mRNA expression.
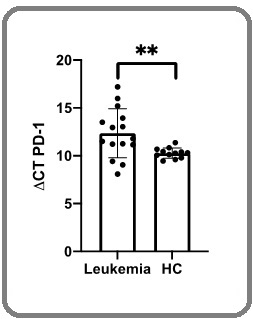
Lower values of ΔCT indicate higher mRNA expression. ΔCT of PD-1 in healthy individuals is stable across different samples, whereas ΔCT of PD-1 in childhood leukemia patients is more varied (Figure 1).
Correlation of PD-1 expression and clinicopathological characteristics of pediatric leukemia
PD-1 expression in pediatric leukemia was not associated with all clinicopathological features such as gender (p=1.000), age (p=0.516), diagnosis (p=0.154), down syndrome (1.000), remission status (p=0.06), hyperleukocytosis (p=1.000), infection (p=0.229), and outcome (p=0.242) (Table 1).
| Variables | PD-L1 expression | P-value | |
| Low n (%) | High n (%) | ||
| Gender | |||
| Male | 6 (75.0) | 2 (25.0) | 1 |
| Female | 6 (85.7) | 1 (14.3) | |
| Age | |||
| ≤ 10 Years | 10 (83.3) | 2 (16.) | 0.516 |
| >10 Years | 2 (66.7) | 1 (33.3) | |
| Diagnosis | |||
| ALL | 10 (90.9) | 1 (9.1) | 0.154 |
| AML | 2 (50.0) | 2 (50.0) | |
| Remission status | |||
| Remission | 9 (100.0) | 0 (0.0) | 0.06 |
| No remission | 2 (50.0) | 2 (50.0) | |
| Have not received therapy | 1 (50.0) | 1 (50.0) | |
| Down syndrome | |||
| No | 10 (76.9) | 3 (23.1) | 1 |
| Yes | 2 (100.0) | 0 (0.0) | |
| Hyperleukocytosis | |||
| No | 9 (81.8) | 2 (18.2) | 1 |
| Yes | 3 (75.0) | 1 (25.0) | |
| Infection | |||
| No | 6 (100.0) | 0 (0.0) | 0.229 |
| Yes | 6 (66.7) | 3 (33.3) | |
| Outcome | |||
| Life | 9 (90.0) | 1 (10.0) | 0.242 |
| Dead | 3 (60.0) | 2 (40.0) |
Discussion
Our result demonstrated that expression levels of PD-1 were significantly reduced in pediatric leukemia than in healthy individuals. There was limited research on PD-1 expression in pediatric cancer. A study by Pinto et al. [20] found that PD-1 expression was reduced in pediatric solid tumors such as Ewing sarcoma (ES), osteosarcoma (OS), neuroblastoma (NB), rhabdomyosarcoma (RMS), and Wilms’ tumor (WT), which was consistent with our findings showing lower PD-1 expression in pediatric leukemia. Additionally, in pediatric leukemia, a study by Kang et al. [21] revealed that PD-1 expression was significantly higher in ALL than in both AML and healthy volunteers. However, PD-1 expression tended to be slightly higher in healthy controls compared to AML, although the difference was not statistically significant [21]. This result demonstrated that PD-1 expression in pediatric leukemia could vary depending on the leukemia subtype. Reduced PD-1 expression in cancer may be attributed to several biological factors such as reduction of T cell immunosuppression and increased apoptosis of PD- 1+ CD4+ T cells [22-25]. Epigenetic factors, such as methylation on the PD-1, can further downregulate PD-1 expression [26]. Although ICI showed remarkable benefits in adult cancer, early trials of ICI monotherapy on childhood cancer showed poor response [9]. There are several reasons for the differences in response rates between adult and childhood cancer. Childhood leukemia generally has a lower tumor mutational burden compared to adult cancers [27]. This means there are fewer neoantigens to be recognized by the immune system, leading to a reduced immune response. The effectiveness of immune checkpoint inhibitors (ICI) in treating melanoma and non-small cell lung cancer (NSCLC) seems to be linked to the high mutational burden present in these cancers [28, 29]. Furthermore, low expression of PD-1 may contribute to the ineffectiveness of ICI. As PD-1 is the primary target of checkpoint inhibitors, low or absent expression of these proteins may reduce the effectiveness of the inhibitors. Several reports have demonstrated that low PD-1 expression are correlated with a reduced response to ICI [17, 30, 31]. In our study, we observed that the majority of pediatric leukemia patients exhibit low levels of PD-1 expression, which might explain why ICI does not show promising results in pediatric cancer. The study did not find a correlation between PD-1 expression and various clinicopathological parameters, likely due to the limited sample size. Further research with more samples is needed to establish a meaningful correlation.
In conclusion, the mRNA levels of PD-1 are lower in childhood acute leukemia patients compared to healthy individuals. These expression levels were not found to be connected to all clinicopathological features. These results offer initial insights into the expression of PD-1 in pediatric leukemia patients in Indonesia.
Acknowledgments
We extend our sincere thanks to Dharmais Hospital- National Cancer Center for their generous funding and support, which were crucial to the completion of this study.
Competing Interests
The authors declare that there are no conflicts of interest to disclose regarding this study.
References
- Progress and Prospects in Pediatric Leukemia Madhusoodhan PP , Carroll WL , Bhatla T. Current Problems in Pediatric and Adolescent Health Care.2016;46(7). CrossRef
- Checkpoint Immunotherapy in Pediatric Oncology: Will We Say Checkmate Soon? Ciurej A, Lewis E, Gupte A, Al-Antary E. Vaccines.2023;11(12). CrossRef
- Pediatric Acute Lymphoblastic Leukemia: Clinical Characteristics, Treatment Outcomes, and Prognostic Factors: 10 Years' Experience From a Low- and Middle-Income Country Ahmad I, Ghafoor T, Ullah A, Naz S, Tahir M, Ahmed S, Arshad A, Ali A, Khattack TA , Batool F. JCO global oncology.2023;9. CrossRef
- Update on Diagnosis of Childhood Acute Lymphoblastic Leukemia (ALL) in Indonesia Perdana A, Saputra F, Aisyi M. Indonesian Journal of Cancer.2020;14. CrossRef
- Profile of PD-L1 mRNA Expression in Childhood Acute Leukemia Azhar M, Chainurridha C, Aisyi M. 2021. CrossRef
- Single-center phase 2 study of PD-1 inhibitor combined with DNA hypomethylation agent + CAG regimen in patients with relapsed/refractory acute myeloid leukemia Gao X, Su Y, Li M, Jing Y, Wang J, Xu L, Zhang L, et al . Cancer immunology, immunotherapy: CII.2023;72(8). CrossRef
- Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia Zeidner JF , Vincent BG , Ivanova A, Moore D, McKinnon KP , Wilkinson AD , Mukhopadhyay R, et al . Blood Cancer Discovery.2021;2(6). CrossRef
- Phase 2 study of PD-1 blockade following autologous transplantation for patients with AML ineligible for allogeneic transplant Solomon SR , Solh M, Morris LE , Holland HK , Bachier-Rodriguez L, Zhang X, Guzowski C, Jackson KC , Brown S, Bashey A. Blood Advances.2023;7(18). CrossRef
- Immunotherapy for Pediatric Acute Lymphoblastic Leukemia: Recent Advances and Future Perspectives Lv M, Liu Y, Liu W, Xing Y, Zhang S. Frontiers in Immunology.2022;13. CrossRef
- Cancer Immunotherapy: A Review Meiliana A, Dewi NM , Wijaya A. The Indonesian Biomedical Journal.2016;8(1):1-20.
- The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. Frontiers in Immunology.2022;13. CrossRef
- PD-1 mRNA expression in peripheral blood cells and its modulation characteristics in cancer patients Wang W, Shen G, Wu S, Song S, Ni Y, Suo Z, Meng X, et al . Oncotarget.2017;8(31). CrossRef
- PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection MacFarlane AW , Jillab M, Plimack ER , Hudes GR , Uzzo RG , Litwin S, Dulaimi E, Al-Saleem T, Campbell KS . Cancer Immunology Research.2014;2(4). CrossRef
- PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S, Komatsu N, Sugawara S, Takamori S, Itoh K, Yamada A. Cancer Science.2014;105(10). CrossRef
- Prognostic value of PD-1, PD-L1 and PD-L2 deserves attention in head and neck cancer Jiang S, Li X, Huang L, Xu Z, Lin J. Frontiers in Immunology.2022;13. CrossRef
- Clinical implications of aberrant PD-1 expression for acute leukemia prognosis Ruan Y, Wang J, Zhang Q, Wang H, Li C, Xu X, Zhai Z. European Journal of Medical Research.2023;28(1). CrossRef
- PD-1 protein and gene expression as prognostic factors in early breast cancer Matikas A, Zerdes I, Lövrot J, Sifakis E, Richard F, Sotiriou C, Rassidakis G, Bergh J, Valachis A, Foukakis T. ESMO open.2020;5(6). CrossRef
- PD-1 and PD-L1 Protein Expression Predict Survival in Completely Resected Lung Adenocarcinoma Zaric B, Brcic L, Buder A, Brandstetter A, Buresch JO , Traint S, Kovacevic T, et al . Clinical Lung Cancer.2018;19(6). CrossRef
- Effect of Primary Systemic Therapy on PD-1, PD-L1, and PD-L2 mRNA Expression in Advanced Breast Cancer Karsono R, Azhar MA , Pratiwi Y, Saputra F, Nadliroh S, Aryandono T. Asian Pacific journal of cancer prevention: APJCP.2021;22(7). CrossRef
- Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors Pinto N, Park JR , Murphy E, Yearley J, McClanahan T, Annamalai L, Hawkins DS , Rudzinski ER . Pediatric Blood & Cancer.2017;64(11). CrossRef
- Expression of Immune Checkpoint Receptors on T-Cells and Their Ligands on Leukemia Blasts in Childhood Acute Leukemia Kang SH , Hwang HJ , Yoo JW , Kim H, Choi ES , Hwang SH , Cho YU , et al . Anticancer Research.2019;39(10). CrossRef
- The mRNA Expression Profile of PD-1 and PD-L1 in Peripheral Blood of Colorectal Cancer Patients Ajoedi A, Azhar MA , Nadliroh S, Hartini S, Andalusia R, Witarto AF . Indonesian Journal of Cancer.2019;13(3). CrossRef
- Profile of PD-1 and PD-L1 mRNA Expression in Peripheral Blood of Nasopharyngeal Carcinoma Azhar MAA , Nadliroh S, Prameswari K, Handoko H, Tobing DL , Herawati C. Molecular and Cellular Biomedical Sciences.2020;4(3). CrossRef
- Lower expression of PD-1 and PD-L1 in peripheral blood from patients with chronic ITP Zhong J, Chen S, Xu L, Lai J, Liao Z, Zhang T, Yu Z, et al . Hematology (Amsterdam, Netherlands).2016;21(9). CrossRef
- PD-1, PD-L1 and PD-L2 Gene Expression on T-Cells and Natural Killer Cells Declines in Conjunction with a Reduction in PD-1 Protein during the Intensive Phase of Tuberculosis Treatment Hassan SS , Akram M, King EC , Dockrell HM , Cliff JM . PloS One.2015;10(9). CrossRef
- Epigenetic Modification of PD-1/PD-L1-Mediated Cancer Immunotherapy against Melanoma Nanamori H, Sawada Y. International Journal of Molecular Sciences.2022;23(3). CrossRef
- Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden Chalmers ZR , Connelly CF , Fabrizio D, Gay L, Ali SM , Ennis R, Schrock A, et al . Genome Medicine.2017;9(1). CrossRef
- Controversies on the possible role of immune checkpoint inhibitors in pediatric cancers: balancing irAEs and efficacy Nigro O, Ferrari A, Casanova M, Orbach D, Leruste A, Gatz SA , Frappaz D, Massimino M. Tumori Journal.2021;107(4). CrossRef
- Pediatric Cancer Immunotherapy: Opportunities and Challenges Wedekind MF , Denton NL , Chen CY , Cripe TP . Paediatric Drugs.2018;20(5). CrossRef
- Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy Taube JM , Klein A, Brahmer JR , Xu H, Pan X, Kim JH , Chen L, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2014;20(19). CrossRef
- Low PD-1 Expression in Cytotoxic CD8+ Tumor-Infiltrating Lymphocytes Confers an Immune-Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value Mazzaschi G, Madeddu D, Falco A, Bocchialini G, Goldoni M, Sogni F, Armani G, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2018;24(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details