Potential Effect of Spirulina Extracts on Serum Iron Reduction: Possible Application in Cancer Treatment
Download
Abstract
Background: Iron overload is a significant health concern that has been linked to various pathological conditions, including cancer and thalassemia. The relationship between iron overload and cancer has been extensively studied, particularly in the context of hepatocellular carcinoma (HCC) and other malignancies. Spirulina platensis is a blue-green algae that contains bioactive compounds such as carbohydrates, proteins, lipids, vitamins, omega-3, and omega-6. These compounds make spirulina a source of antioxidants. The compound, which acts as an antioxidant, is said to decrease or stop oxidative stress resulting from free radicals, which can lead to cancer and thalassemia. Spirulina has properties as an antioxidant, anticancer, anti-apoptotic, anti-inflammatory, immunomodulatory, and antiviral agent. In the present study, an attempt is made to evaluate the effect of spirulina extract as an antioxidant and anti-inflammatory agent on iron status in thalassemia patients and its possible application in cancer treatment.
Materials and Methods: Fifty male patients with thalassemia, aged 5–16 years, were enrolled in this research study. The serum of these patients was treated with spirulina. Afterward, the serum levels of iron, ferritin, transferrin, TIBC, and UIBC before and after treatment were evaluated.
Results: The results indicated a decrease in iron, ferritin, and transferrin saturation levels, while total iron binding capacity, unsaturated iron binding capacity, and transferrin levels were increased.
Conclusion: In conclusion, the correlation between iron overload, cancer, and thalassemia presents a complex interplay of genetic, biochemical, and environmental factors. Reducing the total body iron stored is a crucial treatment goal for thalassemia and cancer.
Introduction
Iron overload is a significant health concern linked to various pathological conditions, including cancer and thalassemia. Iron excess can be toxic to cells by generating reactive oxygen species (ROS), leading to oxidative stress, which is a central mechanism in many diseases, including cancer and thalassemia. The relationship between iron overload and cancer has been extensively studied, particularly in the context of hepatocellular carcinoma (HCC) and other malignancies. Excessive iron can catalyze the Fenton reaction, leading to the formation of highly reactive hydroxyl radicals (•OH) that cause cellular damage, DNA mutations, and ultimately, carcinogenesis [1]. The role of iron overload in cancer has gained renewed attention with studies suggesting that iron overload exacerbates the risk of liver cancer (HCC) and other malignancies through oxidative stress pathways [2].
Recent studies have highlighted the contribution of oxidative stress in thalassemia, a group of inherited hemoglobin disorders that necessitate regular blood transfusions for survival. The iron overload resulting from transfusions is a key contributor to the pathology of thalassemia, leading to increased ROS production and cellular damage. Thalassemia patients are particularly vulnerable to oxidative damage due to the combined effect of excess iron and the breakdown of unstable hemoglobin, which releases free radicals during its accelerated degradation [3]. This overproduction of ROS in thalassemia contributes to the development of organ damage, particularly in the liver, heart, and endocrine glands, which are prone to iron deposition. Additionally, studies suggest that oxidative stress exacerbates the risk of HCC in thalassemia patients, where iron accumulation combined with chronic liver disease and potential viral infections amplifies the risk [4, 5]. The molecular mechanisms linking iron overload to oxidative stress in cancer and thalassemia are complex. Iron participates in the formation of ROS through the Fenton and Haber-Weiss reactions, which catalyze the production of hydroxyl radicals, highly reactive molecules capable of damaging cellular components such as lipids, proteins, and DNA [6]. Recent studies have identified the involvement of key antioxidant defense mechanisms that are overwhelmed by the excess iron, leading to oxidative damage. The body’s natural defense systems, including enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), are often insufficient in combating the oxidative burden imposed by iron overload [7, 8].
Thalassemia is a group of inherited hemoglobin disorders that occur due to impaired synthesis of the alpha (α) or beta (β) globin protein component of hemoglobin, leading to imbalanced globin synthesis. When the body is unable to produce either of these two proteins, erythrocytes do not synthesize correctly and are unable to effectively carry oxygen. As a result, anemia can develop, leading to ongoing health issues that persist throughout life [9]. Iron overload distinguishes itself by causing serious complications for patients. Iron builds up in the body through different ways, such as ongoing blood transfusions, greater absorption in the gastrointestinal tract, persistent breakdown of red blood cells, and inadequate production of red blood cells. Iron overload is a significant health concern that has been linked to various pathological conditions, including cancer and thalassemia. Thalassemia, a group of inherited blood disorders characterized by reduced hemoglobin production, often necessitates regular blood transfusions for management. While these transfusions are critical for patient survival, they also lead to excessive iron accumulation in the body, a condition known as secondary hemochromatosis [10]. The excess iron can deposit in vital organs, including the liver, heart, and endocrine glands, leading to organ dysfunction and increased morbidity [11].
The relationship between iron overload and cancer has been extensively studied, particularly in the context of hepatocellular carcinoma (HCC) and other malignancies. Iron is a pro-oxidant that can catalyze the formation of reactive oxygen species (ROS), leading to oxidative stress, DNA damage, and ultimately, carcinogenesis [12]. In patients with thalassemia, the risk of developing liver cancer is significantly heightened due to the dual burden of iron overload and chronic liver disease, often exacerbated by viral hepatitis infections [13]. Furthermore, studies have shown that patients with thalassemia major exhibit a higher incidence of HCC compared to the general population, underscoring the need for vigilant monitoring and management of iron levels in these patients [14].
Thalassemia is a condition that can lead to increased oxidative stress due to the abnormal production of hemoglobin in red blood cells. While oxidative stress is not the primary cause of thalassemia, it plays a role in several of its pathologies. The primary causes of oxidative stress in thalassemia are the breakdown of unstable hemoglobin, which occurs at a faster rate than usual, and the excess accumulation of iron. Both of these factors prompt the generation of an overabundance of free radicals [8, 15]. To prevent the harmful effects of oxidative stress, thalassemia patients may be advised to take antioxidant supplements or consume a diet rich in antioxidants, such as fruits and vegetables [16, 17]. The prevalence is highest among individuals of Mediterranean, Middle Eastern, Indian, South-East Asian, and African descent [18]. Thalassemia is the most common genetic hemoglobin disorder worldwide, as recognized by the World Health Organization (WHO). The prevalence rate for beta-thalassemia trait is estimated to be between 2% and 6%, with approximately 240 million people being carriers of these genetic disorders [19]. The treatment for thalassemia relies on regular blood transfusions every 3–4 weeks [20]. Additionally, iron chelation therapy includes several types such as Deferoxamine (DFO) and Deferasirox (DFX) [21]. Bone marrow transplantation remains the only radical treatment option for thalassemia patients [22]. Finally, splenectomy is considered one of the treatment methods for blood transfusion-dependent thalassemia [23, 24].
Spirulina platensis algae is a blue-green algae and serves as a source of essential biologically beneficial components. Oxidative stress in thalassemia primarily stems from the rapid breakdown of unstable hemoglobin and an accumulation of iron, which both lead to an overproduction of harmful free radicals [25]. It can cultivate in both saline and fresh water and contains a variety of nutrients, including carbohydrates, proteins, lipids, vitamins, omega-3, omega-6, and minerals, as well as natural pigments such as beta carotene, chlorophyll, xanthophyll, and phycocyanins. Therefore, it can be utilized as an antioxidant [26]. It regulates the immune system and has anti-inflammatory properties by stopping oxidative stress from free radicals. This stress can lead to cellular damage, faster aging, and diseases such as cancer and thalassemia [27]. In recent studies, the significant functions of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in generating reactive oxygen species (ROS) have been revealed [28, 29]. NADPH is present in the nervous system, and when it is assembled and activated, it produces free radicals. These free radicals can lead to oxidative stress, causing damage to cells [28]. Spirulina has the ability to hinder oxidative stress by preventing NADPH oxidase and promoting mitochondrial health through encouraging an antioxidant reaction. It protects against apoptotic cell death induced by free radicals by inhibiting oxidative stress [30]. A research conducted by Gargouri, Manel, and colleagues investigated the impact of adding spirulina to the diet in protecting the kidneys from injury caused by lead acetate. This injury results in heightened oxidative stress, leading to elevated levels of creatinine and urea in the bloodstream [31]. In addition, Ferreira, Paula Benvindo, and colleagues demonstrated that rigorous strength training leads to elevated oxygen consumption, resulting in an overproduction of ROS. This surplus of reactive oxygen species heightens oxidative stress reactions, ultimately leading to uterine contractions and modifying the uterine reactivity during both contraction and relaxation phases [32].
Spirulina has antioxidant activity because it contains a number of antioxidant compounds, including beta- carotene, vitamin C, water-soluble phycocyanin pigments, carotenoids, and phenolic compounds that have antioxidant activity come from the ability to electron donating activity [33], In addition to antioxidant enzymes like superoxide dismutase, catalase, and peroxidase, blue-green algae contain a unique pigment called phycocyanin, which has been found to possess powerful antioxidants. Phycocyanin has demonstrated the ability to lower blood cholesterol levels, boost the immune system, and offer protection against the harmful effects of radiation. Moreover, it exhibits anti-inflammatory, anti-cancer, and anti-aging properties. Carotenoids, another component of blue-green algae, have multiple double bonds that can counteract free radicals and regulate inflammatory processes. They also act as free radical scavengers by neutralizing free radicals, and their antioxidant properties help to inhibit reactive oxygen species (ROS) [34].
The latest research carried out by Kumar, Agam, et al. revealed that Spirulina plays an important role as an antioxidant by utilizing the GPx enzyme to combat oxidative stress. The GPx enzyme relies on NADPH to safeguard cell membranes from lipid peroxidation. Additionally, elevated H2O2 levels were observed to cause a notable rise in the antioxidant enzyme activities within Spirulina, such as CAT, peroxidase (PX), and SOD [35]. Phenolic compounds act as antioxidants by binding to metal ions like Cu and Fe, which accelerate the production of free radicals and bolster the body’s natural antioxidant defenses. Consequently, Spirulina is recognized as an excellent source of antioxidants due to its abundant supply of phenolic molecules [36, 37].
Materials and Methods
2.1. Experimental Design
A case-study design is achieved for a total of 40 male patients. The samples were collected from the Center for Genetic Blood Diseases (Thalassemia) in AL- Diwaniyah City, Iraq, through the period from January 2023 to July 2023. A questionnaire was designed to obtain information on the detailed history of the present thalassemia, family history, weight, height, history of thalassemia, age, sex, and other anthropometric parameters that were calculated on all patients. The age range in this study was 5-16 years, which was dependent on blood transfusion as part of their treatment. The patients who undergo thalassemia were recorded in their files, and the clinical symptoms, hematological, and hemoglobin electrophoresis analysis recognized the diagnosis. Patients who were suffering from any acute illness or chronic disease such as diabetes mellitus, cardiovascular disease and malignancy, or viral hepatitis HIV and COVID-19 were not enrolled in the study. These patients were divided into two groups: group 1 (without spirulina addition) and group 2 (with spirulina addition).
2.2. Determination of IC 50 Value
The IC50, or half maximal inhibitory concentration, is a quantitative measure of a substance’s potency in inhibiting a specific biological or biochemical function. It indicates the amount of the inhibitory substance needed to inhibit a biological process or component by 50% in vitro. This substance could target an enzyme, cell, cell receptor, or microorganism, and IC50 values are usually expressed in molar concentration [38].
2.3. Determination of Optimum Time
The appropriate time was determined, and it was found that the level of iron decreased at 10 minutes when 200 µl of spirulina extract with a concentration of 0.023 mg/ml was added to the two groups.
2.4. Determination of Biochemical Parameters
2.4.1. Iron Direct Method (Ferene)
The iron direct method’s determination was identified using the iron kit produced by BIOLABO SAS, France. Following the release of iron-transferrin from an acid medium, Fe+3 was reduced to Fe+2 by ascorbic acid. Fe+2 subsequently reacted with 3-(2-Pyridyl) -5, -6-difuryl-1, -2,4-triazine-disulfonate (Ferene) to create a colored complex.
2.4.2. Ferritin Method
The human ferritin method was determined using the Ferritin kit manufactured by Bioassay, China. It was quantified using the sandwich ELISA technique. Standards and samples were added to 96-well micro ELISA plates that had been pre-coated with human ferritin antibody.
2.4.3. Total Iron Binding Capacity Level (TIBC) Method
The TIBC kit manufactured by Bioassay, China, detected the determination level of the TIBC method. The total iron binding capacity measures the serum transferrin’s capability to bind iron, and it’s linked to conditions like iron deficiency anemia and acute hepatitis.
When Fe reacts with ferrozine, it creates a bright pink compound with an absorption peak at 562nm.
2.4.4. Unsaturated Iron Binding Capacity Level (UIBC) Method
Serum level of UIBC has been calculated by the application of the following equation [39].
UIBC = TIBC – Iron
2.4.5. Calculation of Transferrin Saturation (TS%) Method
Serum level of TS% has been calculated by the following equation [40].
TS% = (Serum Iron / TIBC) × 100%
2.4.6. Calculation of Transferrin (TF) Method
Transferrin (TF) has been calculated by the using of the following equation [41].
TF = (Fe / TS) ×70.9 2.2.
Statistical Analysis
The data was analyzed using one-way analysis of variance (ANOVA) to compare different groups, and the Duncan multiple ranges test was used to identify significant differences between the groups. The statistical software SPSS was utilized for the analysis, and the statistical significance level was set at P≤ 0.05.
Results and Discussion
• Determination of IC50 Value
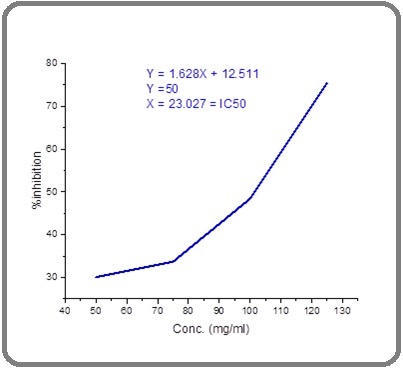
IC50 values were calculated to determine the potency of the inhibitory effects of spirulina platensis algae. IC50 values were calculated from the graphs derived from the DPPH inhibition corresponding to the concentration of extraction. The information is shown as average values plus or minus the standard deviation (SD). The lowest level of significance was determined in a linear equation:
Y = a X + b [42]
IC50 value is 23. 027, shown in Figure 1.
Figure 1. The IC50 Value of Spirulina Platensis .
• Determination of optimum time
The best timing was identified according to the information provided in Table 1, and it was determined that adding spirulina extract to group 2 after 10 minutes yielded the best results.
| Parameter | Ferritin (ng/mL) ±SD | Iron (µmol/L) ±SD |
| Serum blood without Spirulina | 198.4±88.6 | 2498.7±394.8 |
| Serum blood with Spirulina at 10 min. | 147.1±76.2 | 1334.3±728.8 |
| Serum blood with Spirulina at 30 min. | 170±80 | 2178.1±1003.1 |
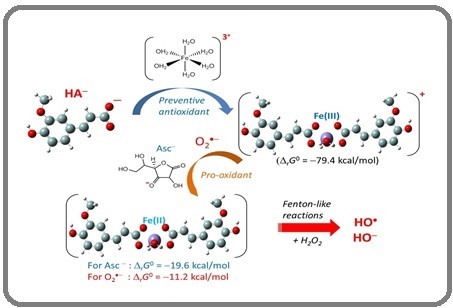
This could be attributed to the creation of a compound between iron and antioxidants that helps maintain a consistent ionic strength during the experimental tests. The stability of constant ionic strength values is influenced by the acid-base characteristics of the metal ion and ligand. Therefore, the effective stability of the compound is suggested for the reaction between iron and antioxidants [43], as shown in Figure 2.
Figure 2. The Suggested Mechanism for the Reaction between Iron and Antioxidant [44].
The data presented in Table 2 and Figure 3 indicates a considerable decrease in the mean level of iron in thalassemia patients after receiving treatment with spirulina extract.
| Parameter | Group 1 (n=50) | Group 2 (n=50) |
| Mean ± S.D | Mean ± S.D | |
| Iron (mmol/L) | 340.913±198.276 a | 138.608±93.236 b |
| Ferritin (ng/ml) | 63.5±25 a | 49.17±24.96 b |
| Transferrin (mmol/L) | 45.208±12.627 a | 48.558±12.659 a |
| TIBC (mmol/L) | 40.121±14.900 a | 49.716±13.466 b |
| UIBC (mmol/L) | 32.984±12.143 a | 48.189±14.289 b |
| TS% | 60.756±11.913 a | 47.962±13.560 b |
Different letters indicate a significant difference (P≤0.05) and similar letters indicate no significant difference
Figure 3. The Effect of Spirulina on the Level of Iron in People with Thalassemia. The value represents the mean ± SD.
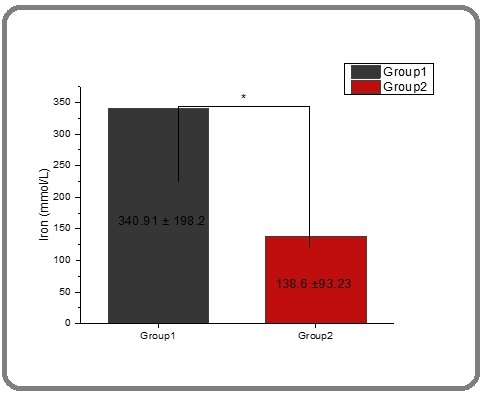
Prior to treatment, the mean iron level was recorded at 340.913 mmol/L, whereas it significantly dropped to 138.608 mmol/L post-treatment. This difference was highly significant (P≤0.05). Thalassemia patients are particularly susceptible to oxidative stress caused by iron overload, which underscores the importance of assessing their antioxidant defense to mitigate the disease’s complications [45].
Fe⁺² + H₂O₂ →Fe⁺³ + ˙ OH + OH¯ [34]
The findings of the current research aligned with those of investigations carried out by Tracz and his colleagues [46], Piga et al. [47], Kuppusamy et al. [48], which patients were found to have higher levels of iron in their bodies, a condition common in thalassemia resulting from regular blood transfusions. Thalassemia patients need frequent blood transfusions, which can lead to complications. Without proper chelation therapy, iron overload can develop [49].
As shown in Table 2 and Figure 4 there was no significant average ferritin level in thalassemia patients before they received treatment with spirulina extract, with the level being 63.5 ng/ml. However, the mean ferritin level significantly decreased to 49.17 ng/ml after treatment.
Figure 4. The Effect of Spirulina on the Level of Ferritin in People with Thalassemia. The value represents the mean ± SD.
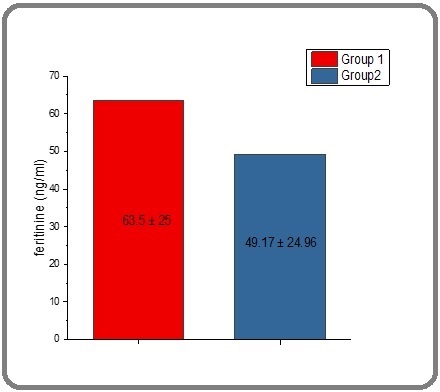
There was no significant difference between the two groups (P≤ 0.05).
Excessive iron accumulation in thalassemia patients may lead to the saturation of transferrin and the production of free iron in the blood and tissues. These reactive free iron hydroxyl radicals can cause oxidative stress by attacking lipids and forming damaging chemicals like hydroxyl radicals [50]. Many studies have evaluated serum ferritin as causing many complications, such as hepatic stiffness leading to liver fibrosis [51, 52] and [53]. In this study, Spasiano, Anna, et al. measured the serum ferritin levels that were high in thalassemia patients who depended on regular blood transfusions in Italy [54]. In another study conducted in Egypt by Elshanshory, Mohamed Ramadan et al. found a decrease in serum ferritin levels after spirulina therapy [55]. Other studies on Italian thalassemia patients explain that high levels of serum ferritin were linked to a higher risk of endocrine disorders, including thyroid and parathyroid dysfunction, liver disease, diabetes mellitus, hypothyroidism, hypogonadism hypoparathyroidism. renal and gallbladder lithiasis [56].
Transferrin It is a protein produced by the liver, and it is the main protein in the blood that binds to iron and transports it throughout the body, so it is considered a specific marker of iron overload. Depending on the structure of transferrin, one mole of transferrin can bind with two moles of iron at two affinity binding sites for ferric iron. It may be measured indirectly by its level, which is expressed as the amount of iron it is capable of binding. This is called the total iron binding capacity TIBC. Only about one-third of the iron binding sites of transferrin are occupied by Fe+3. Therefore, the serum has considerable reserve iron binding capacity. This is called the serum Unsaturated Iron Binding Capacity UIBC [57]. Table 2 and Figure 5 indicate that there was no significant difference in the average transferrin levels in thalassemia patients before treatment with spirulina extract, which measured 45.208 mmol\L. However, after treatment, the mean level significantly rose to 48.558 mmol\L.
Figure 5. The Effect of Spirulina on the Level of Transferrin in People with Thalassemia. The value represents the mean ± SD.
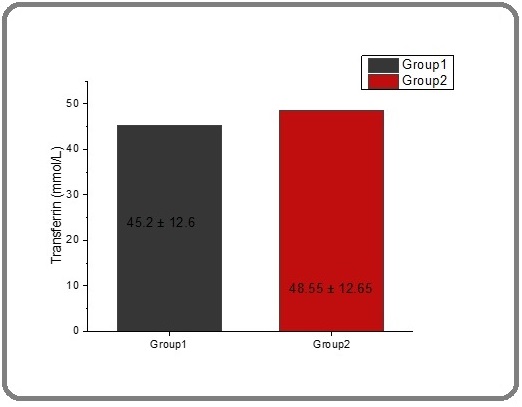
The difference between the two groups was not statistically significant (P ≤ 0.05) (Figure 5). The decreasing transferrin and increasing transferrin saturation in thalassemia for many studies resemble with the present study [58, 59] and [60]. TS is measured as a percentage; it is the value of serum iron divided by the total iron binding capacity of the available transferrin, the main protein that binds iron in the blood; this value means how much serum iron is bound to transferrin [61]. Table 2 and Figure 6 showed that the significant mean for transferrin saturation in thalassemia patients before treatment with spirulina extract was 60.75.
Figure 6. The Effect of Spirulina on the Level of TS% in People with Thalassemia. The value represents the mean ± SD..
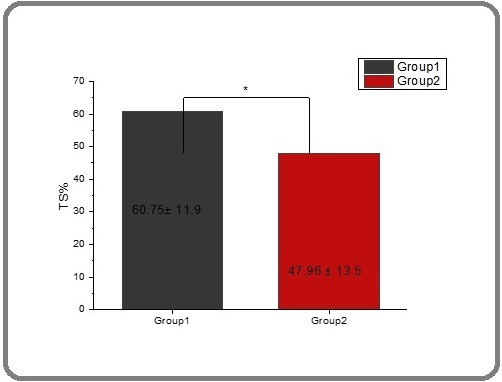
In contrast, the mean level significantly decreased in patients after treatment at 47.962. There was a significant difference between the two groups (P≤0.005) [60].
According to the results in Table 2 and Figures 7 and 8, respectively serum TIBC and UIBC (μmol/L) were found to have lower levels in thalassemia patients (40.121 and 32.984) and had a significant difference compared with patients after the therapy group, that were increased (49.716 and 48.189) respectively.
Figure 7. The Effect of Spirulina on the Level of TIBC in People with Thalassemia. The value represents the mean ± SD.
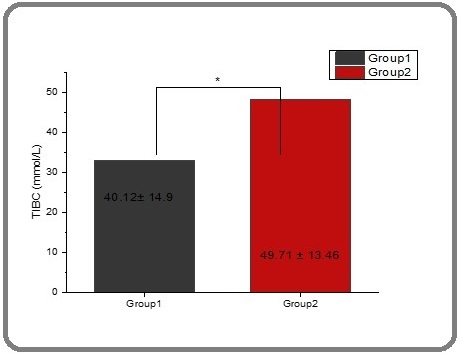
Figure 8. The Effect of Spirulina on the Level of UBIC in People with Thalassemia. The value represents the mean ± SD.
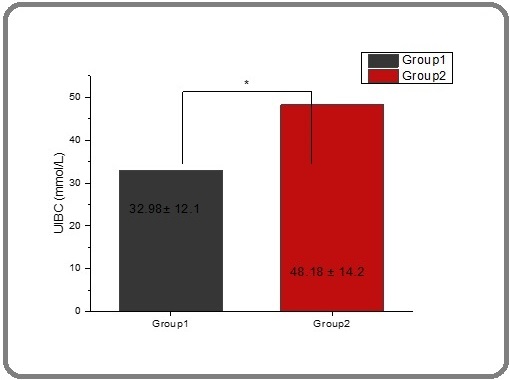
TIBC is an essential test used for the diagnosis of iron deficiency anemia and other disorders of iron metabolism. Prior studies obtained results that explain that serum TIBC and UBIC (μmol/L) are lower in thalassemia patients as compared with healthy people [62, 63] and [64]. Also, this study proved that the reason of decreasing in serum TIBC due to iron overload [41]. Spirulina’s bioactive compounds, including phycocyanin, carotenoids, and antioxidant enzymes, play a critical role in combating oxidative stress, lipid peroxidation, and cellular damage. These properties are particularly relevant in disease states like thalassemia and cancer, where oxidative stress is a key driver of pathogenesis. Spirulina’s ability to modulate oxidative stress pathways, reduce iron-induced oxidative damage, and enhance the body’s antioxidant defenses supports its potential therapeutic use in these conditions. While more clinical studies are needed, the current body of research suggests that Spirulina could serve as a promising adjunct to conventional therapies for managing oxidative stress-related diseases, including cancer and thalassemia.
The results of the study indicated notable variances in the measured biochemical parameters (iron, ferritin, transferrin, TIBC, UBIC, TS%) between patients who received spirulina extract treatment and those who did not. The patient group exhibited elevated iron, ferritin, and TS% levels, along with decreased levels of transferrin, TIBC, and UBIC. These findings underscore the necessity for further investigation into the correlation between thalassemia pathogenesis and antioxidant levels, as well as oxidative stress.
In conclusion, the correlation between iron overload, cancer, and thalassemia presents a complex interplay of genetic, biochemical, and environmental factors. Patients with thalassemia are particularly vulnerable to the adverse effects of iron accumulation due to their treatment regimens, which often involve repeated blood transfusions. The resultant iron overload not only contributes to organ damage but also significantly increases the risk of developing malignancies, particularly hepatocellular carcinoma. Ongoing research is essential to elucidate the precise mechanisms by which iron overload promotes carcinogenesis and to develop effective strategies for monitoring and managing iron levels in thalassemia patients. Implementing regular screening for liver cancer and optimizing chelation therapy can mitigate the risks associated with iron overload. Ultimately, a multidisciplinary approach that includes hematologists, oncologists, and nutritionists will be crucial in improving the quality of life and survival rates for individuals affected by thalassemia and its complications.
Acknowledgments
Statement of Transparency and Principals
Conflict of interest
The authors declare that they have no conflict of interests.
Authors Contributions
Azhaar Asker Hamadi: Methodology, Investigation, Data curation, Original draft preparation.
Ali NooryFajer: Supervision, Conceptualization, Writing- Reviewing and Editing.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethical Approval
This research protocol was evaluated and approved by Researches Ethics Committee of AL-Qadisiyah University, Iraq.
Funding
The authors declare that no funds were received during the preparation of this manuscript.
References
- Free radicals in biology and medicine: Oxford university press, USA Halliwell B, Gutteridge JM . 2015.
- Molecular pathogenesis of iron overload Trinder D., Fox C., Vautier G., Olynyk J. K.. Gut.2002;51(2). CrossRef
- Pathogenic Mechanisms in Thalassemia II: Iron Overload Ganz T, Nemeth E. Hematology/Oncology Clinics of North America.2023;37(2). CrossRef
- Thalassemia and hepatocellular carcinoma: links and risks Marsella M, Ricchi P. Journal of Blood Medicine.2019;10. CrossRef
- Hepatitis B Virus-Associated Liver Carcinoma: The Role of Iron Metabolism and Its Modulation Ali I, Muhammad S, Naqvi SSZH , Wei L, Yan W, Khan MF , Mahmood A, Liu H, Shah W. Journal of Viral Hepatitis.2024. CrossRef
- Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions Moreira AC , Mesquita G, Gomes MS . Microorganisms.2020;8(4). CrossRef
- Oxidative stress and antioxidant status in beta-thalassemia major: iron overload and depletion of lipid-soluble antioxidants Livrea M. A., Tesoriere L., Pintaudi A. M., Calabrese A., Maggio A., Freisleben H. J., D'Arpa D., D'Anna R., Bongiorno A.. Blood.1996;88(9). CrossRef
- Oxidative Stress in β-Thalassemia Fibach E, Dana M. Molecular Diagnosis & Therapy.2019;23(2). CrossRef
- Biophysical characterization of beta-thalassemic red blood cells Desouky OS , Selim NS , El-Bakrawy EM , El-Marakby SM . Cell Biochemistry and Biophysics.2009;55(1). CrossRef
- Iron-deficiency anemia Camaschella C. The New England Journal of Medicine.2015;372(19). CrossRef
- Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment Pietrangelo A. Gastroenterology.2010;139(2). CrossRef
- Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis Hartl J, Ehlken H, Sebode M, Peiseler M, Krech T, Zenouzi R, Felden J, et al . Journal of Hepatology.2018;68(4). CrossRef
- Hepatocellular carcinoma El-Serag HB . The New England Journal of Medicine.2011;365(12). CrossRef
- Imaging of Neurologic Complications in Hematologic Disorders Kanekar S. Hematology/Oncology Clinics of North America.2016;30(4). CrossRef
- Impaired oxidative stress in Thalassemia-Hemoglobin E traits after acute exhaustive exercise Nanakorn N, Chuachan S. Sport Sciences for Health.2021;18:1-9.
- Relationships Of Vitamin D And Vitamin B12 With Malonaldehyde In Patients With Beta Thalassemia Major Nama AR , Hussein WN , Saleh SS . Journal of Namibian Studies : History Politics Culture.2023;33. CrossRef
- The effect of Vitamin E and N-acetyl cysteine on oxidative status and hemoglobin level in transfusion-dependent thalassemia patients: A systematic review and meta-analysis Sezaneh H, Mahnaz HB , Omid RZ , Mohammadreza B, Mehran K, Mani R, Naeimehossadat A. 2023;15(1). CrossRef
- Iron overload in thalassemia: different organs at different rates Taher AT , Saliba AN . Hematology. American Society of Hematology. Education Program.2017;2017(1). CrossRef
- A Study on Knowledge, Attitudes, Practice and Awareness towards Pre-Marital Carrier Screening of Thalassemia among the University Students of Biological Faculty in Bangladesh: A Cross-Sectional Study Mia MA , Islam MR , Sarker A A, Shahriar EB , Hasan A, Ayon RA , Khalil MI , Hossain M, Hossain MI . European Journal of Medical and Health Sciences.2023;5(5). CrossRef
- Complications and death causes of peripheral blood stem cell transplantation in the treatment of thalassemia major Huang C, Jiang H, Li M. Chinese Journal of Tissue Engineering Research.2023;27(1):42.
- The scenario of knowledge, attitude and practice of the Bangladeshi population towards thalassemia prevention: A nationwide study Alam NE , Islam MS , Khabir MIU , Suriea , Islam MM , Mohiuddin RB , Akter S, et al . PLOS global public health.2022;2(10). CrossRef
- The Future of Gene Therapy for Transfusion-Dependent Beta-Thalassemia: The Power of the Lentiviral Vector for Genetically Modified Hematopoietic Stem Cells Rattananon P, Anurathapan U, Bhukhai K, Hongeng S. Frontiers in Pharmacology.2021;12. CrossRef
- The pros and cons of splenectomy in transfusion dependent thalassemia patient Tantiworawit A, Dumnil S, Osataphan N, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, et al . Blood.2018;132:4901.
- Long-term Results of Splenectomy in Transfusion-dependent Thalassemia Akca T, Ozdemir GN , Aycicek A, Ozkaya G. Journal of Pediatric Hematology/Oncology.2023;45(3). CrossRef
- Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy Mafi A, Rezaee M, Hedayati N, Hogan SD , Reiter RJ , Aarabi M, Asemi Z. Cell communication and signaling: CCS.2023;21(1). CrossRef
- Free radicals, antioxidants in disease and health Pham-Huy LA , He H, Pham-Huy C. International journal of biomedical science: IJBS.2008;4(2).
- Food and drug industry applications of microalgae Spirulina platensis: A review Maddiboyina B, Vanamamalai HK , Roy H, Ramaiah n, Gandhi S, Kavisri M, Moovendhan M. Journal of Basic Microbiology.2023;63(6). CrossRef
- The Potential of Spirulina platensis to Ameliorate the Adverse Effects of Highly Active Antiretroviral Therapy (HAART) Sibiya T, Ghazi T, Chuturgoon A. Nutrients.2022;14(15). CrossRef
- Intracellular and extracellular carbohydrates in microalgae Synytsya A, Sushytskyi L, Saloň I, Babayeva T, Čopíková J. Handbook of Food and Feed from Microalgae: Elsevier; 2023.;:p. 87-102.
- Oxidative Stress and Antioxidants in Neurodegenerative Disorders Olufunmilayo EO , Gerke-Duncan MB , Holsinger RMD . Antioxidants (Basel, Switzerland).2023;12(2). CrossRef
- Potential protective effects of the edible alga Arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats Gargouri M, Soussi A, Akrouti A, Magné C, El Feki A. Applied Physiology, Nutrition, and Metabolism. 2019;44(3):271-81.2019;44(3):271-81.
- Supplementation with Spirulina platensis prevents uterine diseases related to muscle reactivity and oxidative stress in rats undergoing strength training Ferreira PB , Diniz AFA , Lacerda Júnior FF , Silva MdCC , Cardoso GA , Silva AS , et al . Nutrients.2021;13(11):3763.
- Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overloaded mice, a thalassemia model Eslami S, Ebrahimzadeh MA , Biparva P. RSC advances.2018;8(46). CrossRef
- Levels of taurine, hypotaurine and homotaurine, and amino acids profiles in selected commercial seaweeds, microalgae, and algae-enriched food products Terriente-Palacios C, Castellari M. Food Chemistry.2022;368. CrossRef
- Antioxidant and phytonutrient activities of Spirulina platensis Kumar A, Ramamoorthy D, Verma DK , Kumar A, Kumar N, Kanak KR , et al . Energy Nexus.2022;6:100070.
- Antioxidant and Antidiabetic Activity of Algae Abo-Shady AM , Gheda SF , Ismail GA , Cotas J, Pereira , Abdel-Karim OH . Life (Basel, Switzerland).2023;13(2). CrossRef
- A review on antioxidant properties of Spirulina Asghari A, Fazilati M, Latifi AM , Salavati H, Choopani A. Journal of Applied Biotechnology Reports.2016;3(1):345-51.
- Antiparasitic and disease-modifying activity of Nyctanthes arbor-tristis Linn. in malaria: An exploratory clinical study Godse CS , Tathed PS , Talwalkar SS , Vaidya RA , Amonkar AJ , Vaidya AB , Vaidya ADB . Journal of Ayurveda and Integrative Medicine.2016;7(4). CrossRef
- Epidemiological and transcriptome data identify potential key genes involved in iron overload for type 2 diabetes Liu X, Hong X, Jiang S, Li R, Lv Q, Wang J, Wang X, et al . Diabetology & Metabolic Syndrome.2023;15(1). CrossRef
- Relationship between transferrin saturation and iron stores in the African American and US Caucasian populations: analysis of data from the third National Health and Nutrition Examination Survey McLaren C. E., Li K. T., Gordeuk V. R., Hasselblad V., McLaren G. D.. Blood.2001;98(8). CrossRef
- Which is the best way for estimating transferrin saturation? Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I. Renal Failure.2010;32(8). CrossRef
- Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects Karagecili H, Yılmaz MA , Ertürk A, Kiziltas H, Güven L, Alwasel SH , Gulcin İ. Molecules (Basel, Switzerland).2023;28(4). CrossRef
- A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights Malacaria L, Corrente GA , Beneduci A, Furia E, Marino T, Mazzone G. Molecules (Basel, Switzerland).2021;26(9). CrossRef
- The use of plants as phytobiotics: a new challenge Ndomou SCH , Mube HK . 2023.
- Total Antioxidant Status in Patients with Major β-Thalassemia Bazvand F, Shams S, Borji Esfahani M, Koochakzadeh L, Monajemzadeh M, Ashtiani M, Rezaei N. Iranian Journal of Pediatrics.2011;21(2).
- Physiology and pathophysiology of heme: implications for kidney disease Tracz MJ , Alam J, Nath KA . Journal of the American Society of Nephrology: JASN.2007;18(2). CrossRef
- High nontransferrin bound iron levels and heart disease in thalassemia major Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, Calabrese R, Hershko C, Cappellini MD . American Journal of Hematology.2009;84(1). CrossRef
- Chelation therapy with desferrioxamine does not normalize ferritin level but attenuates oxidative damage and improves total antioxidant level in Malaysian Chinese beta-thalassaemia major patients Kuppusamy U. R., Tan J. a. M. A.. The West Indian Medical Journal.2011;60(1).
- Total Antioxidant Capacity As Indicative of Oxidative Stress on β-Thalassemia Patients Lamia A. Medical Journal of Babylon.2007;4(3-4):51.
- Iron overload in beta thalassemia: a review Prabhu R, Prabhu V, Prabhu R. J Biosci Tech.2009;1(1):20-31.
- Common complications in beta-thalassemia patients Yaman A, Pamir I, Yarali N, Karademir S, Cetinkaya S, Ali B, et al . International journal of hematology and oncology.2013;34(1):193-9.
- Complications in patients with transfusion dependent thalassemia: A descriptive cross-sectional study Faranoush M, Faranoush P, Heydari I, Foroughi-Gilvaee MR , Azarkeivan A, Parsai Kia A, Sadighnia N, et al . Health Science Reports.2023;6(10). CrossRef
- Association between serum ferritin level, cardiac and hepatic T2-star MRI in patients with major β-thalassemia Eghbali A., Taherahmadi H., Shahbazi M., Bagheri B., Ebrahimi L.. Iranian Journal of Pediatric Hematology and Oncology.2014;4(1). CrossRef
- Setting for "Normal" Serum Ferritin Levels in Patients with Transfusion-Dependent Thalassemia: Our Current Strategy Spasiano A, Meloni A, Costantini S, Quaia E, Cademartiri F, Cinque P, Pepe A, Ricchi P. Journal of Clinical Medicine.2021;10(24). CrossRef
- Spirulina ameliorates immunity and reduces viral load in beta-thalassemia major children comorbid with hepatitis virus C: A single-arm clinical trial Elshanshory MR , Salem ML , Attia MAS , Gamal RM , El-Sheekh MM , Elshahat AA , et al . Medical Science.2020;24(103):1142-51.
- Serum ferritin level and morbidity risk in transfusion-independent patients with β-thalassemia intermedia: the ORIENT study Musallam KM , Cappellini MD , Daar S, Karimi M, El-Beshlawy A, Graziadei G, Magestro M, et al . Haematologica.2014;99(11). CrossRef
- Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity Yamanishi H, Iyama S, Yamaguchi Y, Kanakura Y, Iwatani Y. Clinical Chemistry.2003;49(1). CrossRef
- Molecular and clinical factors affecting myocardial iron retention in transfusional haemosiderosis: UCL (University College London) Garbowski MW . 2017.
- Serum ferritin and transferrin saturation levels in β⁰ and β(+) thalassemia patients Estevão I. F., Peitl Junior P., Bonini-Domingos C. R.. Genetics and molecular research: GMR.2011;10(2). CrossRef
- Correlation of Transferrin Saturation and Serum Ferritin with Bone Mass Density in Adult Transfusion Dependent Beta-Thalassemia Patients Atmakusuma TD , Tenggara JB . Journal of Blood Medicine.2021;12. CrossRef
- Iron Status in Newly Diagnosed β-Thalassemia Major: High Rate of Iron Status due to Erythropoiesis Drive Susanah S, Rakhmilla LE , Ghozali M, Trisaputra JO , Moestopo O, Sribudiani Y, Idjradinata PS , Maskoen AM . BioMed Research International.2021;2021. CrossRef
- Association Between Serum Levels of Adropin and Insulin Resistance in Patients with Beta-Thalassemia Major Madlool AK , Siadat SO , Ali HA , Ali RA , Brbber AM , Abbas AJ , et al . Egyptian Academic Journal of Biological Sciences, D Histology & Histochemistry.2023;15(1):127-38.
- Is the total number of blood transfusion in β-thalassemia major patients can be used to assess their serum ferritin levels ? Yahya Dallal Bashi A. Tikrit Journal of Pharmaceutical Sciences.2023;9(1). CrossRef
- Oxidative stress and disturbance in antioxidant balance in beta thalassemia major Ghone RA, , Kumbar K, Suryakar A, Katkam R, Joshi N. Indian Journal of Clinical Biochemistry.2008;23(4):337-340.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details