Hypoxia in Tumor Angiogenesis: An Update
Download
Abstract
Solid tumors, particularly breast cancer, undergo genetic and molecular changes that foster an immunosuppressive tumor microenvironment (TME), driven by angiogenic activation and metabolic shifts like elevated glycolysis. Angiogenesis, the formation of new blood vessels is essential for tumor survival, enabling oxygen and nutrient delivery as tumors exceed 1–2 mm³ in size. This commentary explores the mechanisms of angiogenesis in breast cancer, hypoxia’s role in driving these pathways, and emerging therapeutic strategies targeting these processes.
Introduction
Cancer is a major cause of morbidity and mortality. About 85% of cancer-related deaths are due to solid tumors [1]. Solid tumors induce various genetic modifications, such as the activation of angiogenic factors and elevated glycolysis among other etiologic factors which contribute to the immunosuppressive tumor microenvironment (TME) [2, 3]. A critical survival strategy for growing tumors is angiogenesis, the process of forming new blood vessels to supply oxygen and nutrients as tumors exceed diffusion-dependent size limits. Hypoxia, a hallmark of rapidly expanding tumors, directly fuels this process through hypoxia-inducible factor (HIF) activation [4, 5]. In oxygen-deprived environments, stabilized HIF-1α subunits dimerize with HIF-1β, triggering transcriptional upregulation of pro-angiogenic signals like VEGF, PDGF, and FGF. This HIF-mediated response enables tumors to recruit vascular networks, perpetuating growth and metastatic spread [6].
HIF-1α overexpression in breast cancer correlates with VEGF upregulation, microvessel density, and poor prognosis. Beyond VEGF, HIF-1α activates genes promoting extracellular matrix remodeling (e.g., MMPs) and endothelial cell recruitment (e.g., SDF-1/CXCR4 axis). Acidic TME conditions further stabilize HIF-1α, creating a feedforward loop that sustains angiogenesis and immunosuppression [7].
Hypoxia → HIF-1α → Angiogenic Cascade
Mechanisms of angiogenesis
Coalescent angiogenesis, first formally described in 2022, represents a novel mechanism of vascular remodeling where capillaries fuse to form larger, more efficient conduits effectively the inverse of intussusceptive angiogenesis (which splits vessels). This process transforms disorganized capillary meshes into hierarchical, tree-like structures to optimize blood flow, offering fresh insights into vascular adaptation in pathological contexts like cancer [8].
Mechanism and Novelty
Coalescent angiogenesis unfolds in three phases:
1. Isotropic Mesh: A primitive network of capillaries with uniform perfusion.
2. Preferential Pathways: Hemodynamic forces select high-flow routes, leading to capillary fusion and pillar elimination.
3. Mature Vessel Formation: Parallel capillaries merge into single vessels, reducing structural redundancy while maintaining perfusion [8] (Table 1).
| Feature | Sprouting Angiogenesis | Intussusceptive Angiogenesis | Coalescent Angiogenesis |
| Process | Endothelial tip cell migration | Vessel splitting via pillars | Capillary fusion |
| Efficiency | Slow, energy-intensive | Rapid, minimal EC proliferation | Rapid, conserves Ecs |
| Structural Outcome | New branches | Network expansion | Streamlined hierarchy |
Relevance in Tumors
While embryonic studies dominate current evidence, coalescent angiogenesis may play under recognized roles in tumor vascular adaptation such as:
Vascular Efficiency: Tumors often develop chaotic capillary meshes; coalescence could remodel these into functional hierarchies to sustain rapid growth.
Hypoxia Mitigation: By consolidating flow routes, coalescence might alleviate tumor hypoxia a key driver of VEGF over expression and metastasis.
Therapeutic Resistance: Mosaic vessels formed via coalescence could evade anti-VEGF therapies targeting sprouting angiogenesis [9].
This implies that they serve as diagnostic biomarkers; quantifying capillary fusion events in biopsies could predict tumor aggressiveness, help in therapeutic targeting; disrupting endothelial adhesion molecules (e.g., VE-cadherin) might inhibit coalescence while sparing normal vasculature as well as combination strategies; pairing anti-coalescent agents with VEGF inhibitors could overcome resistance seen in monotherapies [8].
Current limitations include the lack of direct evidence in mammalian tumors a critical gap given CAM models may not fully replicate human tumor microenvironments.
This paradigm shift underscores angiogenesis as a bidirectional process (fusion and splitting), with coalescence offering tumors a stealthy mechanism to optimize perfusion. Validating its role in malignancy could unlock precision anti-angiogenic strategies tailored to vascular remodeling modes.
Modes of angiogenesis
Angiogenesis encompasses diverse mechanisms by which blood vessels form and adapt, with several modes playing critical roles in tumor progression under hypoxic conditions as shown in Figure 1;
Figure 1. Different forms of Tumour Angiogenesis. (A) Sprouting angiogenesis: endothelial cells proliferating and migrating based on existing capillaries. (B) Bone marrow-derived angio-genesis: bone marrow-derived endothelial progenitor cells differentiate into endothelial cells to form blood vessels. (C) Vasculogenic mimicry: the vascular channels are made up of tumour cells.(D) Vessel co-option: invading normal tissues, tumour cells make use of the vascular system already in place.(E) Cancer stems cell-derived angiogenesis: tumour cells with stemness differentiate into endothelial cells. (F) Intussusceptive angiogenesis: endothelial cells split into two vessels without proliferating [9].
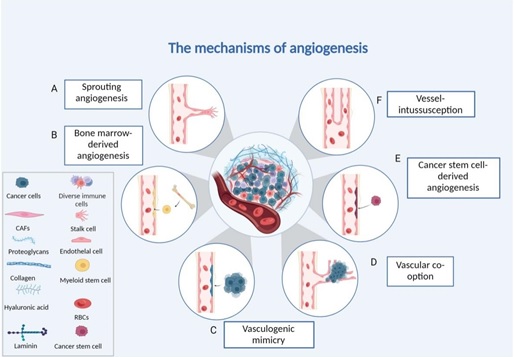
1. Vasculogenesis: De novo vessel formation from endothelial progenitor cells, primarily during embryogenesis but reactivated in tumors via hypoxia- driven recruitment of bone marrow-derived cells.
2. Post-natal angiogenesis: Hypoxia-inducible VEGF/ FGF signaling drives new vessel growth in ischemic or tumor tissues.
3. Sprouting angiogenesis (hypoxia-driven): VEGF- dominated endothelial tip/stalk cell migration creates new capillaries, essential for hypoxic tumor expansion.
4. Intussusceptive angiogenesis: Vessel splitting via pillar formation, enabling rapid vascular network expansion without extensive endothelial proliferation.
5. Coalescent angiogenesis (hypoxia-potentiated): Capillary fusion optimizes perfusion efficiency, observed in embryonic models and potentially exploited in tumor microenvironments.
6. Vessel elongation: Mechanical stretching of existing vessels, often secondary to tissue growth.
7. Vessel co-option (hypoxia-driven): Tumor cells hijack pre-existing vasculature, common in metastatic niches with limited angiogenic capacity
8. Endothelial-like differentiation (hypoxia-induced):
Cancer stem cells adopt endothelial traits to participate in vessel lining under low oxygen.
1. Vasculogenic mimicry (hypoxia-driven): Aggressive tumors form fluid-conducting channels lined by matrix-producing cancer cells, bypassing endothelial dependence.
2. Lymphangiogenesis: Hypoxia up regulates VEGF-C/D to promote lymphatic growth, facilitating metastasis [10].
Hypoxia predominantly fuels tumor-associated modes (sprouting, co-option, vasculogenic mimicry) through HIF-1α/VEGF axis activation, while coalescent and intussusceptive mechanisms may provide adaptive advantages in oxygen-starved microenvironment [11].
Hypoxia-inducible Factor
HIF-1α vs HIF-2α: Divergent Roles in Tumor Angiogenesis as shown in Figure 2.
Figure 2. Schematic Overview of HIF-induced Angiogenesis, Lymphangiogenesis and Immune Suppression.
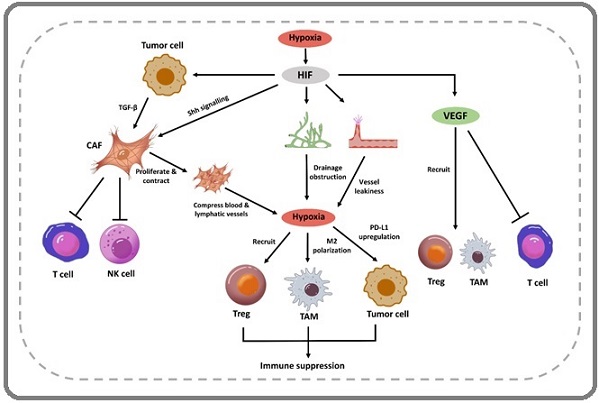
While both HIF-1α and HIF-2α are stabilized under hypoxia, they exhibit distinct regulatory roles in solid tumors [12].
• HIF-1α: Dominates acute hypoxia responses, driving angiogenesis via VEGF, FGF, and PDGF up regulation. It enhances glycolytic metabolism and endothelial cell (EC) proliferation/migration, critical for early tumor vascularization.
• HIF-2α: More prominent in chronic hypoxia, regulating VEGF and ANGPTL4 in digestive cancers (e.g., colorectal, hepatocellular). It sustains tumor stemness and resistance pathways, with stronger VEGF promoter activation than HIF-1α in certain contexts [12] (Table 2).
| Feature | HIF-1α | HIF-2α |
| Primary Role | Acute hypoxia adaptation | Chronic hypoxia adaptation |
| Key Targets | VEGF, FGF, PDGF, glycolysis enzymes | VEGF, ANGPTL4, stemness markers (e.g., OCT4) |
| Therapeutic Impact | Correlates with tamoxifen resistance. | Drives-EGFR-mediated chemo/radioresistance |
Hypoxia stabilizes HIF-1α, disrupting the angiogenic equilibrium by:
1. Pro-Angiogenic Activation: Upregulating VEGF, FGF, PDGF, and angiopoietins.
2. EC Recruitment: Enhancing EC proliferation, migration, and tube formation via VEGF/VEGFR2 signaling.
3. Matrix Remodeling: Inducing MMPs and plasminogen activators to degrade basement membranes This “angiogenic switch” transforms dormant vasculature into leaky, disorganized networks that fuel tumor growth and metastasis [3].
Autocrine VEGF Signaling: Beyond Endothelial Targeting
HIF-driven VEGF not only acts on endothelial cells but also directly promotes tumor cell survival and therapy resistance. Pancreatic and mesothelioma cancers express VEGFR1/2 on tumor cells, enabling VEGF to stimulate proliferation via MAPK/c-FOS pathways.
Tumor cells bypass VEGF inhibition by leveraging autocrine signaling for survival and HIF-2α upregulates EGFR in breast cancer, exacerbating endocrine therapy resistance [3].
HIF Pathways and Therapy Resistance
HIF-driven angiogenesis contributes to treatment failure through:
1. Chemotherapy Resistance: HIF-2α upregulates ANGPTL4 and CCNG2, shielding colorectal tumors from 5-FU.
2. Radioresistance: HIF-1α enhances glycolysis, reducing ROS-mediated DNA damage in hypoxic niches.
3. Anti-Angiogenic Therapy Limitations:
- Tumors exploit autocrine VEGF to sustain growth despite VEGF inhibitors.
- HIF-2α promotes alternative angiogenic pathways (e.g., ANGPTL4) in liver hemangiomas [13].
Emerging Strategies to Overcome Resistance
1. Dual HIF Inhibition: Combining HIF-1α (e.g., digoxin) and HIF-2α (e.g., PT2399) blockers disrupts compensatory signaling as shown in Figure 2 [14].
2. VEGFR2/EGFR Co-Targeting: Neutralizing tumor cell VEGFR2 alongside EGFR reduces autocrine survival signals [15].
3. Hypoxia Modulation: Hyperbaric oxygen or HIF-1α siRNA sensitizes tumors to radiotherapy.
By dissecting HIF isoform-specific roles and their downstream effectors, precision therapies can be designed to counteract resistance while normalizing tumor vasculature [9] (Figure 2).
Microvessel Density
Microvessel density (MVD) has been proposed as a surrogate marker of tumor angiogenesis and a potential prognostic indicator for breast cancer since the early 1990s. By evaluating MVD, clinicians can identify patients at an early stage of their disease, allowing for more tailored and effective treatments, such as adjuvant chemotherapy or specific anti-angiogenic therapies. MVD is typically assessed using anti-pan-endothelial antibodies like CD31 and von Willebrand Factor (vWF), which help visualize the density of microvessels within tumor tissues [16].
However, the use of MVD as a prognostic tool is not without its challenges. Variability in results can arise from differences in microvessel counting methods, the antibodies used for assessment, and the subjective nature of vessel counting. Technical limitations also include the influence of field size on MVD measurements; smaller field areas have been shown to provide more accurate prognostic information compared to larger fields, which may dilute the concentration of vessels and underestimate MVD. Additionally, there are inconsistencies in how MVD correlates with established prognostic factors such as tumor size, grade, and lymph node involvement [16]. Emerging biomarkers are being explored to complement or potentially replace MVD in predicting patient outcomes. For instance, circulating endothelial cells (CECs) can provide insights into the dynamic state of tumor vasculature and may serve as indicators of disease progression. Furthermore, hypoxia-related microRNAs (miRNAs) are gaining attention for their roles in regulating angiogenesis and could offer additional prognostic value by reflecting the hypoxic status of tumors [16].
While MVD remains a valuable marker for assessing angiogenesis in breast cancer, its limitations necessitate further research into standardized methodologies and the exploration of emerging biomarkers that could enhance prognostic accuracy and treatment strategies.
Anti-angiogenic therapies
Anti-angiogenic therapies have become a cornerstone in cancer treatment, particularly through the targeting of the vascular endothelial growth factor (VEGF) pathway. These therapies aim to inhibit the formation of new blood vessels, thereby depriving tumors of essential nutrients and oxygen. The development of these agents has led to significant advancements in treating various malignancies, though challenges remain, particularly regarding resistance mechanisms [17].
Mechanisms of Anti-Angiogenic Therapies
Anti-angiogenic agents can be categorized based on their mechanisms of action:
1. Monoclonal Antibodies
- Bevacizumab (Avastin): This is a monoclonal antibody that binds to VEGF, preventing it from interacting with its receptors on endothelial cells. Clinical studies have shown that bevacizumab improves progression-free survival (PFS) in several cancers, including breast cancer (median PFS increase of approximately 5 months) and colorectal cancer (median PFS increase of about 4 months)
- Aflibercept (Zaltrap): This agent acts as a decoy receptor for VEGF, effectively trapping it and preventing angiogenesis [18].
2. Tyrosine Kinase Inhibitors (TKIs)
- Sunitinib (Sutent): This TKI targets multiple receptors, including VEGFRs and PDGFRs, and has shown improved PFS in renal cell carcinoma (median PFS of about 11 months)
- Sorafenib (Nexavar): Another multi-targeted TKI that inhibits VEGFRs and has demonstrated efficacy in hepatocellular carcinoma, with a median PFS increase of approximately 3 months.
3. Monoclonal Antibodies Targeting VEGF Receptors
Ramucirumab (Cyramza): This monoclonal antibody specifically targets VEGFR2 and has been utilized in gastric cancer and non-small cell lung cancer, showing a median PFS improvement of around 2 months in certain contexts [17].
Eficacy Data
The efficacy of these anti-angiogenic agents varies across different cancers:
• Breast Cancer: Bevacizumab combined with chemotherapy has shown modest improvements in PFS but variable overall survival benefits due to resistance mechanisms.
• Colorectal Cancer: Bevacizumab has consistently improved PFS when used alongside standard chemotherapy regimens.
• Renal Cell Carcinoma: Sunitinib has become a standard first-line treatment, significantly improving PFS compared to placebo.
• Hepatocellular Carcinoma: Sorafenib has been shown to extend PFS compared to untreated controls [19].
Resistance Mechanisms
Despite the initial success of anti-angiogenic therapies, resistance remains a significant challenge. Several mechanisms contribute to this phenomenon:
1. Compensatory Pathways: Tumors may activate alternative angiogenic pathways involving factors such as FGF or PDGF when VEGF signaling is inhibited.
2. Vascular Co-option: Tumor cells can proliferate near existing blood vessels, effectively bypassing the need for new vessel formation.
3. Vasculogenic Mimicry: Cancer cells can form vessel-like structures without endothelial involvement, further complicating treatment efficacy [19].
Combination Therapies
To overcome resistance, combining anti-angiogenic therapies with immunotherapy has emerged as a promising strategy. By addressing both tumor vasculature and immune evasion, these combinations aim to enhance overall treatment efficacy. For instance, the dual targeting of VEGF and angiopoietin-2 (Ang-2) is being explored in clinical trials to improve outcomes in resistant tumors [19, 20].
In summary, while anti-angiogenic therapies have transformed cancer treatment landscapes by targeting tumor blood supply, understanding and overcoming resistance mechanisms will be crucial for maximizing their therapeutic potential across various malignancies. Recent advances in therapies targeting VEGF pathways have highlighted several key mechanisms, including hypoxia-inducible factors (HIFs), platelet-derived growth factors (PDGFs), and Notch signaling. Novel agents such as Dll4 inhibitors and TGF-β antagonists are currently undergoing clinical trials to enhance anti-tumor efficacy and overcome resistance to existing treatments. Future directions in cancer therapy emphasize personalized medicine, leveraging genetic profiles to tailor treatments based on individual responses to angiogenic therapies. This approach aims to improve patient outcomes by considering specific genetic variations that influence treatment efficacy [1, 21-23].
Examples of Personalized Medicine
1. VEGF Polymorphism Screening: Genetic screening for polymorphisms in the VEGF gene can help predict patient responses to anti-VEGF therapies. Variations in VEGF levels may correlate with treatment efficacy, allowing for more tailored therapeutic strategies.
2. Liquid Biopsies: The use of liquid biopsies to monitor circulating tumor DNA (ctDNA) and circulating endothelial cells (CECs) provides real-time insights into tumor dynamics and angiogenesis. This non-invasive approach can help identify patients who are likely to benefit from anti-angiogenic treatments and monitor their response over time [24].
Ongoing Clinical Trials
Dll4 Inhibitors: Clinical trials investigating Dll4 inhibitors, such as those listed under NCT identifiers, are exploring their potential to enhance anti-tumor effects by disrupting the Notch signaling pathway, which is crucial for maintaining the balance of angiogenesis and tumor growth [20]. Hypoxia-targeted therapies are gaining traction as a means to enhance the efficacy of anti- angiogenic treatments. HIF inhibitors, such as belzutifan, are designed to inhibit HIF-1α activity, thereby reducing the expression of pro-angiogenic factors like VEGF in hypoxic tumor environments [17, 25].
In conclusion, the future of anti-angiogenic therapy is increasingly focused on personalized approaches that integrate genetic profiling and innovative biomarkers with emerging therapeutic strategies. These approaches aim to identify patients who are more likely to benefit from specific treatments, thus enhancing therapeutic efficacy. Our understanding of the role of hypoxia on tumor environments and integrating biomarker-driven strategies with innovative therapeutic approaches would be essential in overcoming the resistance and enhancing the effectiveness of anti-angiogenic therapies in cancer treatment.
Acknowledgments
None
Conflict of Interest
Author declares no conflict of interest.
References
- The current knowledge concerning solid cancer and therapy Najafi M, Majidpoor J, Toolee H, Mortezaee K. Journal of Biochemical and Molecular Toxicology.2021;35(11). CrossRef
- Angiogenesis in Breast Cancer: A Review Gamde MS , Ogenyi OD . Asian Pacific Journal of Cancer Biology.2024;9(1). CrossRef
- A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumors: An Update Magar AG , Morya VK , Kwak MK , Oh JU , Noh KC . International Journal of Molecular Sciences.2024;25(6). CrossRef
- Tumor angiogenesis: Current challenges and therapeutic opportunities Al-Ostoot FH , Salah S, Khamees HA , Khanum SA . Cancer Treatment and Research Communications.2021;28. CrossRef
- On coalescent angiogenesis and the remarkable flexibility of blood vessels Pezzella F, Kerbel RS . Angiogenesis.2022;25(1). CrossRef
- Pathological angiogenesis: mechanisms and therapeutic strategies Dudley AC , Griffioen AW . Angiogenesis.2023;26(3). CrossRef
- Survival strategies: How tumor hypoxia microenvironment orchestrates angiogenesis Yang M, Mu Y, Yu X, Gao D, Zhang W, Li Y, Liu J, Sun C, Zhuang J. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2024;176. CrossRef
- Coalescent angiogenesis-evidence for a novel concept of vascular network maturation Nitzsche B, Rong WY , Goede A, Hoffmann B, Scarpa F, Kuebler WM , Secomb TW , Pries AR . Angiogenesis.2022;25(1). CrossRef
- Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy Zhang M, Zhang Y, Ding Y, Huang J, Yao J, Xie Z, Lv Y, Zuo J. Cells.2022;11(18). CrossRef
- Mechanisms of angiogenesis in tumour Zhang R, Yao Y, Gao H, Hu X. Frontiers in Oncology.2024;14. CrossRef
- Basic and Therapeutic Aspects of Angiogenesis Updated Eelen G, Treps L, Li X, Carmeliet P. Circulation Research.2020;127(2). CrossRef
- Targeting HIF-2α in the Tumor Microenvironment: Redefining the Role of HIF-2α for Solid Cancer Therapy Davis L, Recktenwald M, Hutt E, Fuller S, Briggs M, Goel A, Daringer N. Cancers.2022;14(5). CrossRef
- Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain Bui BP , Nguyen PL , Lee K, Cho J. Cancers.2022;14(24). CrossRef
- Regulation of redox signaling in HIF-1-dependent tumor angiogenesis Manuelli V, Pecorari C, Filomeni G, Zito E. The FEBS journal.2022;289(18). CrossRef
- The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF Ghalehbandi S, Yuzugulen Ja, Pranjol MZI , Pourgholami MH . European Journal of Pharmacology.2023;949. CrossRef
- Study of microvascular density in carcinoma of breast Rainy R, Shah N, Babina S, Hussain SMA , Debnath M, Singh SJ . ResearchGate.2024. CrossRef
- Anti-Angiogenic Therapy: Current Challenges and Future Perspectives Lopes-Coelho F, Martins F, Pereira SA , Serpa J. International Journal of Molecular Sciences.2021;22(7). CrossRef
- Antiangiogenic cancer treatment: The great discovery and greater complexity (Review) Maj E, Papiernik D, Wietrzyk J. International Journal of Oncology.2016;49(5). CrossRef
- Resistance Mechanisms to Anti-angiogenic Therapies in Cancer Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, Shamseddine A. Frontiers in Oncology.2020;10. CrossRef
- Anti-angiogenesis in cancer therapeutics: the magic bullet Oguntade AS , Al-Amodi F, Alrumayh A, Alobaida M, Bwalya M. Journal of the Egyptian National Cancer Institute.2021;33(1). CrossRef
- Changing landscape of anti-angiogenic therapy: Novel approaches and clinical perspectives Gacche BN . Biochimica Et Biophysica Acta. Reviews on Cancer.2023;1878(6). CrossRef
- The Role of Anti-angiogenesis in the Treatment Landscape of Non-small Cell Lung Cancer - New Combinational Approaches and Strategies of Neovessel Inhibition Daum S, Hagen H, Naismith E, Wolf D, Pircher A. Frontiers in Cell and Developmental Biology.2020;8. CrossRef
- Exploring the Past, Present, and Future of Anti-Angiogenic Therapy in Glioblastoma Zhang AB , Mozaffari K, Aguirre B, Li V, Kubba R, Desai NC , Wei D, Yang I, Wadehra M. Cancers.2023;15(3). CrossRef
- Genetic polymorphisms of vascular endothelial growth factor (VEGF) associated with gastric cancer recurrence after curative resection with adjuvant chemotherapy Jo IH , Kim YJ , Chung WC . Journal of Clinical Oncology.2019;37(4_suppl):9. CrossRef
- Microvessel density as a prognostic indicator of prostate cancer: A systematic review and meta-analysis Feng G, Wang K, Jiang Z. Open Medicine.2021;16(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details