Enhanced Therapeutic Potential of Paclitaxel-Loaded Niosomes on Ovarian Cancer Cell Line
Download
Abstract
Background: Paclitaxel is a widely used chemotherapeutic agent for ovarian cancer treatment; however, its clinical application is limited by poor solubility and severe side effects. Niosomes, non-ionic surfactant vesicles, have emerged as a promising nanocarrier for targeted drug delivery. This study investigates the enhanced therapeutic potential of paclitaxel-loaded niosomes in ovarian cancer cell line.
Methods: Paclitaxel-loaded niosomes were prepared using the thin-film hydration method and characterized for size, zeta potential, and polydispersity index (PDI). The morphology of the niosomes was evaluated by scanning electron microscopy (SEM). Cytotoxicity of paclitaxel-loaded niosomes was assessed using the MTT assay on ovarian cancer cell line (A2780S) after 24 and 48 hours of incubation. The results were compared with free paclitaxel to evaluate the effect of the niosomal formulation on drug efficacy.
Results: The paclitaxel-loaded niosomes exhibited a mean size of approximately 285 nm, a PDI of 0.44, and a negative zeta potential of -21 mV. SEM images confirmed the spherical morphology of the niosomes. The MTT assay results showed a significant increase in cytotoxicity in the niosomal formulation compared to free paclitaxel at both 24 and 48 hours (p < 0.05, p < 0.01), indicating enhanced therapeutic efficacy.
Conclusion: Paclitaxel-loaded niosomes demonstrate improved drug delivery and enhanced cytotoxicity in ovarian cancer cell lines. The results suggest that niosomal paclitaxel could be a promising strategy for improving the therapeutic potential of paclitaxel in ovarian cancer treatment. Further in vivo studies are warranted to confirm these findings and explore the clinical applicability of niosomal formulations.
Introduction
Therapeutic methods for addressing physical and mental problems have made significant progress with the advancement of technology [1-13]. Technological advancements have significantly transformed the landscape of healthcare, particularly through the integration of artificial intelligence (AI), blockchain, and data-driven decision-making models. These innovations enhance efficiency in medical supply chains by improving transparency, security, and operational effectiveness, ultimately leading to better healthcare outcomes [14]. Additionally, AI and deep learning have played a crucial role in deciphering immune system complexities, allowing for more precise immunotherapy applications, particularly in the treatment of autoimmune diseases [15]. Furthermore, emerging research suggests that existing pharmaceuticals, such as metformin, could have neuroprotective effects, as seen in mitigating microstructural changes in the white matter of Alzheimer’s patients [16]. The growing intersection of AI and healthcare also extends to the study of disease pathogenesis, where computational models are enhancing our understanding of T cell specificity and immune responses, paving the way for improved diagnostic and therapeutic approaches [17]. As these technologies continue to evolve, their integration into clinical practice will not only optimize treatment strategies but also foster innovations that advance global healthcare accessibility and effectiveness [18]. With the advancement of technology, newer and more serious problems are clearly emerging [19]. Various diseases have threatened human life and can emerge as pandemics [20]. Many diseases only harm individuals’ physical bodies, but many others affect their mental and emotional well-being [21, 22]. Cancer is a complex disease that is increasing day by day [23]. Ovarian cancer remains one of the most aggressive and challenging gynecological malignancies, ranking as the fifth leading cause of cancer-related deaths among women worldwide [24]. Despite advances in diagnostic techniques and the development of treatment strategies, the overall prognosis for ovarian cancer patients is still poor, primarily due to late-stage diagnosis, resistance to chemotherapy, and the severe side effects associated with conventional treatment regimens [25-27]. Standard chemotherapy, typically involving agents such as paclitaxel and cisplatin, remains the cornerstone of treatment; however, the clinical effectiveness of these drugs is often compromised by several inherent challenges [28, 29]. Paclitaxel, a potent chemotherapeutic agent, is widely used in the treatment of ovarian cancer due to its ability to inhibit cancer cell division by stabilizing microtubules [30]. Despite its proven efficacy, paclitaxel suffers from significant drawbacks, including poor aqueous solubility, low bioavailability, and rapid systemic clearance, which contribute to its limited therapeutic window and the high incidence of severe side effects, such as neurotoxicity, myelosuppression, and cardiotoxicity [31, 32]. These limitations highlight the need for novel drug delivery systems that can improve the pharmacokinetic profile of paclitaxel, enhance its therapeutic index, and reduce the associated toxicity [33]. In recent years, nanotechnology has emerged as a promising approach to overcome the limitations of traditional drug delivery systems [34]. Among various nanocarriers, niosomes non-ionic surfactant-based vesicles have attracted significant attention due to their unique properties [35]. Niosomes are biocompatible, stable, and capable of encapsulating both hydrophilic and hydrophobic drugs, making them ideal candidates for delivering paclitaxel [36, 37]. Their ability to improve the solubility and bioavailability of paclitaxel, prolong its circulation time in the bloodstream, and target drug release to cancer cells offers a compelling strategy to enhance treatment efficacy while minimizing systemic toxicity [38, 39]. The formulation of paclitaxel-loaded niosomes has the potential to significantly improve the delivery and therapeutic action of paclitaxel in ovarian cancer treatment [40]. Niosomes can not only serve as an effective carrier for paclitaxel but also offer controlled drug release, which may reduce the frequency and severity of adverse effects [41]. Furthermore, their size, surface charge, and composition can be tailored to optimize their interaction with cancer cells, providing a more targeted and effective treatment strategy [42, 43]. This study aims to explore the therapeutic potential of paclitaxel-loaded niosomes in ovarian cancer treatment. Specifically, we investigate the physicochemical properties of the niosomal formulation, including particle size, zeta potential, and morphology, as well as its cytotoxicity against ovarian cancer cell lines (A2780S). Using the MTT assay, we will evaluate the effectiveness of paclitaxel-loaded niosomes at different time intervals (24 and 48 hours) and compare the results to free paclitaxel. We hypothesize that encapsulating paclitaxel in niosomes will enhance its cytotoxicity, improve its therapeutic efficacy, and reduce its side effects. Through this research, we aim to provide a deeper understanding of the potential benefits of paclitaxel-loaded niosomes as a novel therapeutic strategy for ovarian cancer. The findings from this study could pave the way for the clinical application of niosomal drug delivery systems, potentially transforming the treatment landscape for ovarian cancer patients by improving outcomes and reducing treatment-related toxicity.
Materials and Methods
Materials
All necessary reagents, including Paclitaxel, Span 40, Cholesterol, Polyethylene Glycol 3350, RPMI 1640 culture medium, Ethanol, Isopropanol, and Diethyl Ether, were meticulously acquired from the Sigma Corporation to ensure the highest quality standards for the experiments. Additionally, the A2780S ovarian cancer cell line utilized in this study was generously supplied by the Cell Bank affiliated with the Iranian Pasteur Institute, guaranteeing a reliable and consistent source of cellular material for our research purposes.”
Preparation of nanoparticles
Initially, a precise combination of 80 milligrams of Span 40, 30 milligrams of Cholesterol, and 25 milligrams of Polyethylene Glycol 3350 (a molar ratio of about 25:10:1) was prepared in a solvent system consisting of 40 milliliters of Diethyl Ether, ensuring proper dissolution of the components. To this mixture, two separate aliquots of Ethanol (96%), each containing 14 milligrams of Paclitaxel, were gradually introduced over time, with each addition carefully monitored to ensure uniform dispersion of the drug. Once all components were thoroughly mixed, the solution was subjected to a gentle agitation process for a duration of one hour at 37°C, maintaining a consistent speed of 300 rotations per minute to promote complete solubilization and homogenization of the ingredients. Once fully dissolved, the resulting solution was slowly poured into 14 ml of phosphate buffer (pH 7.2) that was preheated to 70°C and continuously stirred. Due to the temperature difference between the two phases, the ether quickly evaporated, leading to the formation of niosomes. To ensure the homogeneity and proper encapsulation of the active ingredient, the mixture was subsequently processed using a sonicator at room temperature for five minutes, providing sufficient energy to produce uniform vesicles.
Determination of size of nanoniosomes
To determine the mean diameter of nanoniosomes, a formulation was prepared using a 1:50 nanoniosome- to-PBS ratio at pH 7.2. Nanoparticle concentration was measured by absorbance at 633 nm, while size and surface charge were analyzed with a Malvern Nano ZS3600 zetasizer. Morphology was examined using a Philips XL30 SEM. For SEM analysis, 200 µL nanoparticle suspensions were centrifuged at 13,000 RPM for 30 minutes at 4°C to obtain pellets. These pellets were resuspended in 200 µL of 15 mg/mL sucrose as a cryoprotectant, lyophilized, coated with a thin gold layer, and then imaged with the SEM.
MTT test
The cytotoxicity of the Paclitaxel formulation was evaluated using the MTT assay and compared to the standard drug. A2780S cells were seeded in a 96-well plate and cultured for 24 hours before being treated with varying concentrations of either the drug formulation or free drug for 24 and 48 hours. After treatment, MTT solution was added and incubated for one hour, then replaced with isopropanol to dissolve the formazan crystals. Absorbance was measured at 540 nm using an ELISA reader.
Cytotoxicity (%) was calculated as:
Cytotoxicity (%) = [1 - (Absorbance of treated cells / Absorbance of control)] × 100
Cell viability was determined as 100 minus the cytotoxicity percentage. The IC₅₀ value was calculated using the Pharm program.
Statistical analysis
The data were statistically analyzed using SPSS version 11, and all phases of toxicity were evaluated with Pharm software.
Results
Characterization of nanoparticles
Paclitaxel-loaded niosomes were characterized to determine their physicochemical properties. The formulations exhibited a mean particle size of 285 nm and a polydispersity index (PDI) of 0.440, indicating a moderately broad size distribution. The zeta potential was measured at -21 mV, suggesting sufficient colloidal stability (Table 1).
| Parameter | Value | Unit |
| Mean Particle Size | 285±14 | nm |
| Polydispersity Index (PDI) | 0.44±0.9 | - |
| Zeta Potential | -21±1.8 | mV |
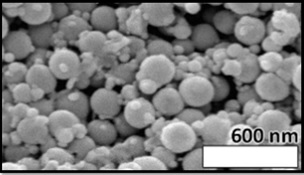
Additionally, scanning electron microscopy (SEM) images confirmed the spherical morphology of the niosomes, demonstrating uniform and consistent structural characteristics (Figure 1).
Figure 1. Scanning Electron Microscopy (SEM) of Nanoparticles.
In vitro cytotoxicity assay
The cytotoxicity of paclitaxel-loaded niosomes was evaluated in comparison to conventional paclitaxel using A2780S ovarian cancer cells. Cells were incubated with equivalent doses of either the niosomal formulation or the free drug for 24 and 48 hours at 37°C. Figure 2 illustrates the dose-response curves for both formulations, highlighting the lower IC₅₀ values achieved by the niosomal paclitaxel at each incubation period.
Figure 2. Comparative Cytotoxicity of Paclitaxel-loaded Niosomes and Conventional Paclitaxel on A2780S Cells after 24 and 48 hours of Incubation. Error bars represent standard deviations. Significant differences are indicated with asterisks (p < 0.05, p < 0.01) .
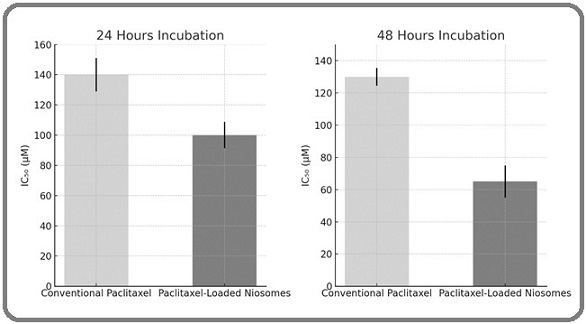
Specifically, the IC₅₀ for the niosomal formulation decreased from 100 µM at 24 hours to 65 µM at 48 hours, whereas the free paclitaxel maintained higher IC₅₀ values of 140 µM and 130 µM at the respective time points. These results demonstrate that encapsulating paclitaxel within niosomes significantly enhances its cytotoxicity against A2780S cells, potentially due to improved cellular uptake and sustained drug release. The increased efficacy of the niosomal formulation over conventional paclitaxel underscores its potential as a more effective therapeutic strategy for ovarian cancer treatment (Table 2).
| Treatment | IC₅₀ (µM), 24 Hours incubation | IC₅₀ (µM), 48 Hours incubation |
| Conventional Paclitaxel | 140±11.1 µM | 130±5.5 µM |
| Paclitaxel-Loaded Niosomes | 100±8.7 µM | 65±10.0 µM |
*Values represent the mean ± standard deviation of three independent experiments. p < 0.05 vs. conventional paclitaxel at 24 hours, p < 0.01 vs. conventional paclitaxel at 48 hours.
Discussion
The use of niosomes as drug carriers in cancer treatment represents an innovative advancement in nanotechnology. These non-ionic surfactant-based vesicles can encapsulate both hydrophilic and lipophilic drugs, enhancing treatment efficacy by improving targeting precision while reducing side effects. For example, a study by Nowroozi et al. (2018) demonstrated that theranostic niosomes containing doxorubicin and Ag2S quantum dots, when directly injected into tumors, significantly increased drug accumulation in the tumor and inhibited tumor growth by 71.7%. This method highlighted the high efficacy of direct intratumoral injection in breast cancer models [44]. Additionally, Akbarzadeh et al. (2021) developed niosomes loaded with curcumin for breast cancer treatment. These niosomes significantly increased cell death and apoptosis in MDA-MB231 and SKBR3 cancer cell lines, effectively delivering hydrophobic drugs to target cells while minimizing side effects. This innovative formulation highlights the potential of niosomes in enhancing the efficacy of cancer therapies [45]. Due to their high stability and ability to target specific cells, niosomes are recognized as a powerful tool in delivering anticancer drugs, offering significant improvements to current treatments. This study investigates the improved therapeutic efficacy of paclitaxel-loaded niosomes in an ovarian cancer cell line (A2780S), highlighting the potential benefits of niosome encapsulation in enhancing drug delivery and cytotoxic effects. The results demonstrate that the niosomal formulation of paclitaxel significantly reduces IC₅₀ values at both 24 and 48 hours when compared to the free drug, indicating enhanced cytotoxicity against ovarian cancer cells. This improvement in drug efficacy suggests that niosomes enhance drug solubility, stability, and cellular uptake, allowing for more efficient drug delivery directly to cancer cells [46, 47]. Consequently, niosomes may reduce the required dosage of paclitaxel, potentially lowering systemic toxicity. The niosomes’ mean size of 285 nm and negative zeta potential indicate good stability and suitability for biological interactions, while the moderately broad PDI suggests a relatively uniform size distribution, essential for consistent drug delivery and bioavailability [48-51]. The spherical morphology observed in SEM further supports the effective encapsulation and expected cellular uptake behaviors. This study positions niosomes as a promising nanocarrier for paclitaxel, particularly due to their ability to address the drug’s poor water solubility and severe side effects. By enhancing drug delivery and therapeutic outcomes, niosomes may reduce the limitations of traditional formulations that often require higher doses, increasing the risk of adverse side effects. Although this study provides essential insights, further research, particularly in vivo studies, is needed to assess the pharmacokinetics, biodistribution, and long-term safety of niosomal paclitaxel. Moreover, understanding the molecular mechanisms underlying the enhanced drug uptake and efficacy of niosomes could provide a deeper understanding of their therapeutic potential. Ultimately, the promising findings from this study suggest that niosomal paclitaxel could offer a safer and more effective alternative for ovarian cancer treatment, with future clinical trials critical to validating these results and establishing niosomal paclitaxel as a standard treatment option.
In conclusion, Humanity, through technological advancements, has consistently contributed to combating a wide range of diseases [52-62]. For example, the use of paper-based sensors for detecting cancer markers offers rapid, cost-effective, and accurate diagnostic methods that could play a crucial role in enhancing health outcomes and treatment efficacy [63]. Cancer is a complex, multifactorial disease characterized by the uncontrolled proliferation of malignant cells that can invade healthy tissues and disrupt the normal function of various organs [64-69]. Recent studies employing advanced analytical techniques such as hybrid metaheuristic machine learning for obesity risk prediction [70], finite mixture modeling for investigating health risk behavior disparities [71], and cardiac marker evaluation as predictors of mortality in methanol toxicity [72] underscore the critical role of data-driven approaches in enhancing our understanding of complex health determinants and improving public health outcomes. This study underscores the potential of niosomal encapsulation to significantly enhance the therapeutic efficacy of paclitaxel, presenting a viable advancement in the treatment of ovarian cancer. The improved delivery and increased cytotoxicity observed in vitro lay a strong foundation for further development and clinical investigation of niosomal drug delivery systems.
Acknowledgements
None.
Data availability
Not applicable as we used information from previously published articles.
Approved by any scientific Body
Not applicable as the manuscript is not a part of any student thesis or study.
Ethical issue and approval
Not applicable as we used information from previously published articles.
Consent for publication
All authors have given consent for publication.
Conflict of interest
The authors declare no potential conflict of interest.
References
- Evaluation of the difficulty of laparoscopic cholecystectomy during COVID-19 pandemic using externally validated prediction models: A retrospective cohort study Hatampour K, Ebrahimian M, Zamani A, Zardoui A, Ramezani A, Ghahremanloo K, Mirhashemi SH , et al . International Journal of Surgery Open.2023;61. CrossRef
- Prevalence of cataract and its contributing factors in Iranian elderly population: the Gilan eye study Ramezani A, Sabbaghi H, Katibeh M, Ahmadieh H, Kheiri B, Yaseri M, Moradian S, et al . International Ophthalmology.2023;43(12). CrossRef
- A rare case of cutaneous mucormycosis in the forearm: A case report Shadidi-Asil R, Kialashaki M, Fateh A, Ramezani A, Zamani A, Ebrahimian M. International Journal of Surgery Case Reports.2022;94. CrossRef
- Plasma NT1 tau is associated with hypometabolism in Alzheimer’s disease continuum Lalaklou ZG , Ghahjavarestani AM , Pishkari Y, Emami D. Neurology Letters.2024.
- Redictive Role of Personality Dimensions on Quality of Life and Satisfaction in Patients With Gender Identity Disorder after Gender Reassignment Surgery Ghahjavarestani A. The scientific heritage.2024. https://orcid. org/0000-0002-0440-0509;135.
- The effect of transcranial alternating current stimulation on cognitive flexibility and attention of children with intellectual disability: a case report Ghahri Lalaklou Z, Haghighat-Manesh E, Montazeri Ghahjavarestani A, Ahmadi E. Journal of Medical Case Reports.2024;18(1). CrossRef
- Berberis Vulgaris Fruit Crude Extract As A Novel Anti-Leukaemic Agent Saedi T. A., Ghafourian S., Jafarlou M., Sabariah M. N., Ismail P., Eusni R. M. T., Othman F.. Journal of Biological Regulators and Homeostatic Agents.2015;29(2).
- High cholesterol diet increases expression of cholesterol 24-hydroxylase and BACE1 in rat hippocampi: implications for the effect of diet cholesterol on memory Nikasa M, Karimi P, Rajavand H, Afshari F, Jafarlou M, Soltanali M. Iranian Red Crescent Medical Journal.2016;18(12):e35677. CrossRef
- Inflammatory reflex disruption in COVID-19 Hajiasgharzadeh K, Jafarlou M, Mansoori B, Dastmalchi N, Baradaran B, Khabbazi A. Clinical & Experimental Neuroimmunology.2022. CrossRef
- The Combination of PD-L1 and CTLA-4 Suppression Significantly Decreased the Expression Levels of Cancer Stem Cell Factors in the Pancreatic Cancer Cell Line Alizadeh N, Kazemi T, Hemmat N, Jafarlou M, Baradaran B.. ImmunoAnalysis.2023.
- Regulation of Dendritic Cell Functions by Vitamins as Promising Therapeutic Strategy for Immune System Disorders Ghahramanipour Z, Alipour S, Masoumi J, Rostamlou A, Hatami-Sadr A, Heris JA , Naseri B, Jafarlou M, Baradaran B. Advanced Biology.2023;7(12). CrossRef
- Immune Checkpoints and CAR-T Cells: The Pioneers in Future Cancer Therapies? Hosseinkhani N, Derakhshani A, Kooshkaki O, Abdoli Shadbad M, Hajiasgharzadeh K, Baghbanzadeh A, Safarpour H, et al . International Journal of Molecular Sciences.2020;21(21). CrossRef
- Mitochondrial RNAs in Oncology: Review of Interventions and Innovative Diagnostic Approaches in the Biogenesis of Human Cancers Shafiei Asheghabadi P, Delavari Dosar A, Hashemi M. 3.2024;3(International Journal of BioLife Sciences (IJBLS)):202-207.
- Evaluation and prioritization of artificial intelligence integrated block chain factors in healthcare supply chain: A hybrid Decision Making Approach. Seifi N, Ghoodjani E, Majd SS , Maleki A, Khamoushi S. Computer and Decision Making: An International Journal.2025;2:374-405. CrossRef
- AI and Deep Learning in Understanding the Etiology and Pathogenesis of Autoimmune Diseases. Kindle Rigi A, Harati K, Abbaspour M, Fattahpour SF , Hosseini P, Moghadam Fard A, Hobbi M, et al . 2024;4(1):1-182. Retrieved from https://preferpub.org/index.php/kindle/article/view/Book46.
- Metformin attenuates white matter microstructural changes in Alzheimer’s disease Abbaszadeh S, Dehaghi GR , Lalaklou ZG , Verdi HB , Emami D, Dalvandi B. Neurology Letters.2024.
- Quantitative approaches for decoding the specificity of the human T cell repertoire Ghoreyshi ZS , George JT . Frontiers in Immunology.2023;14. CrossRef
- Global Health Challenges Rahmani E, Farrokhi M, Nemati P, Riahi S, Esmaielzade Rostami M, Shemshadi Golafzani R, Gheibi F, et al . Kindle.2023;4(1):1-218. Retrieved from https://preferpub.org/index.php/kindle/article/view/Book47.
- Mental Model-Based Designs: The Study in Privacy Policy Landscape Atashpanjeh H, Paudel R, Al-Ameen MN . International Journal of Human–Computer Interaction.2024. CrossRef
- A Multistage Stochastic Optimization Model for Resilient Pharmaceutical Supply Chain in COVID-19 Pandemic Based on Patient Group Priority. 2024 Systems and Information Engineering Design Symposium (SIEDS) (pp. 382-387) Mahdavimanshadi M, Anaraki MG , Mowlai M, Ahmadirad Z. IEEE.2024. CrossRef
- The effect of synbiotics on liver enzymes, obesity indices, blood pressure, lipid profile, and inflammation in patients with non-alcoholic fatty liver: A systematic review and meta-analysis of randomized controlled trials Musazadeh V, Assadian K, Rajabi F, Faghfouri AH , Soleymani Y, Kavyani Z, Najafiyan B. Pharmacological Research.2024;208. CrossRef
- The Dark Side of Digitalization: A Visual Journey of Research through Digital Game Addiction and Mental Health Nawaser K, Jafarkhani F, Khamoushi S, Yazdi A, Mohsenifard H, Gharleghi B. ResearchGate.2025. CrossRef
- Downregulation of LPAR1 Promotes Invasive Behavior in Papillary Thyroid Carcinoma Cells Bokaii Hosseini Z, Rajabi F, Morovatshoar R, Ashrafpour M, Behboodi P, Zareie D, Natami M. Cancer Informatics.2024;23. CrossRef
- Epidemiology of ovarian cancer Gaona-Luviano P, Medina-Gaona LA , Magaña-Pérez K. Chinese Clinical Oncology.2020;9(4). CrossRef
- Emerging diagnostic, prognostic and therapeutic biomarkers for ovarian cancer El Bairi K, Kandhro AJ , Gouri A, Mahfoud W, Louanjli N, Saadani B, Afqir S, Amrani M. Cellular Oncology (Dordrecht, Netherlands).2017;40(2). CrossRef
- Diagnostic and Therapeutic Pathway of Advanced Ovarian Cancer with Peritoneal Metastases Ghirardi V, Fagotti A, Ansaloni L, Valle M, Roviello F, Sorrentino L, Accarpio F, et al . Cancers.2023;15(2). CrossRef
- Ovarian cancer: Current status and strategies for improving therapeutic outcomes Chandra A, Pius C, Nabeel M, Nair M, Vishwanatha JK , Ahmad S, Basha R. Cancer Medicine.2019;8(16). CrossRef
- Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS , Casado A. European Journal of Cancer (Oxford, England: 1990).2011;47 Suppl 3. CrossRef
- Prognostic factors in advanced epithelial ovarian cancer. (Gruppo Interregionale Cooperativo di Oncologia Ginecologica (GICOG)) Marsoni S., Torri V., Valsecchi M. G., Belloni C., Bianchi U., Bolis G., Bonazzi C., et al . British Journal of Cancer.1990;62(3). CrossRef
- Paclitaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer Spencer C. M., Faulds D.. Drugs.1994;48(5). CrossRef
- Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103 Trimble E. L., Adams J. D., Vena D., Hawkins M. J., Friedman M. A., Fisherman J. S., Christian M. C., et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1993;11(12). CrossRef
- Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer Fennelly D., Aghajanian C., Shapiro F., O'Flaherty C., McKenzie M., O'Connor C., Tong W., Norton L., Spriggs D.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1997;15(1). CrossRef
- Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, et al . Lancet (London, England).2009;374(9698). CrossRef
- Nanotechnology and Microtechnology in Drug Delivery Systems Sun J, Yang Z, Teng L. Dose-Response: A Publication of International Hormesis Society.2020;18(2). CrossRef
- Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications Chen S, Hanning S, Falconer J, Locke M, Wen J. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V.2019;144. CrossRef
- Preparation and Evaluation of Paclitaxel-Loaded PEGylated Niosomes Composed of Sorbitan Esters Hosokawa M, Ito S, Noda K, Kono Y, Ogawara K. Biological & pharmaceutical bulletin.2023;46(10):1479-1483. CrossRef
- Self-degrading niosomes for encapsulation of hydrophilic and hydrophobic drugs: An efficient carrier for cancer multi-drug delivery Sharma V, Anandhakumar S, Sasidharan M. Materials Science & Engineering. C, Materials for Biological Applications.2015;56. CrossRef
- Fabrication of PEGylated SPIONs-Loaded Niosome for Codelivery of Paclitaxel and Trastuzumab for Breast Cancer Treatment: In Vivo Study Masoumi Godgaz S, Asefnejad A, Bahrami S. ACS applied bio materials.2024;7(5). CrossRef
- Niosomes encapsulating paclitaxel for oral bioavailability enhancement: preparation, characterization, pharmacokinetics and biodistribution Sezgin-Bayindir Z, Onay-Besikci A, Vural N, Yuksel N. Journal of Microencapsulation.2013;30(8). CrossRef
- Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Biomaterials.2015;37. CrossRef
- Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer Kim J, Park Y. International Journal of Nanomedicine.2017;12. CrossRef
- Targeting Ovarian Cancer Cells Overexpressing CD44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel Khayrani AC , Mahmud H, Oo AKK , Zahra MH , Oze M, Du J, Alam MJ , Afify SM , Quora HAA , Shigehiro T, Calle AS , Okada N, Seno A, Fujita K, Hamada H, Seno Y, Mandai T, Seno M. International Journal of Molecular Sciences.2019;20(5). CrossRef
- Estrone-Conjugated PEGylated Liposome Co-Loaded Paclitaxel and Carboplatin Improve Anti-Tumor Efficacy in Ovarian Cancer and Reduce Acute Toxicity of Chemo-Drugs Tang H, Xie Y, Zhu M, Jia J, Liu R, Shen Y, Zheng Y, Guo X, Miao D, Pei J. International Journal of Nanomedicine.2022;17. CrossRef
- Theranostic niosomes for direct intratumoral injection: marked enhancement in tumor retention and anticancer efficacy Nowroozi F, Dadashzadeh S, Soleimanjahi H, Haeri A, Shahhosseini S, Javidi J, Karimi H. Nanomedicine (London, England).2018;13(17). CrossRef
- Preparation, Optimization and In-Vitro Evaluation of Curcumin-Loaded Niosome@calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment Akbarzadeh I, Shayan M, Bourbour M, Moghtaderi M, Noorbazargan H, Eshrati Yeganeh F, Saffar S, Tahriri M. Biology.2021;10(3). CrossRef
- Delivery of Adriamycin Loaded Niosomes for Liver Cancer Treatment ResearchGate.2024. CrossRef
- The Optimized Formulation of Tamoxifen-Loaded Niosomes Efficiently Induced Apoptosis and Cell Cycle Arrest in Breast Cancer Cells Akbarzadeh I, Farid M, Javidfar M, Zabet N, Shokoohian B, Arki MK , Shpichka A, Noorbazargan H, Aghdaei HA , Hossein-Khannazer N, Timashev P, Makvandi P, Vosough M. AAPS PharmSciTech.2022;23(1). CrossRef
- Modeling and optimization of the niosome nanovesicles using response surface methodology for delivery of insulin Hakamivala A, Moghassemi S, Omidfar K. Biomedical Physics & Engineering Express.2019;5(4). CrossRef
- Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery Bayindir ZS , Yuksel N. Journal of Pharmaceutical Sciences.2010;99(4). CrossRef
- Spray Dried Lactose Based Proniosomes As Stable Provesicular Drug Delivery Carriers: Screening, Formulation, And Physicochemical Characterization Nasr A, Qushawy MK , Swidan SA . International Journal of Applied Pharmaceutics.2018.
- Stability studies on piroxicam encapsulated niosomes Ertekin ZC , Bayindir ZS , Yuksel N. Current Drug Delivery.2015;12(2). CrossRef
- Harmaline exerts potentially anti-cancer effects on U-87 human malignant glioblastoma cells in vitro Vahedi MM , Shahini A, Mottahedi M, Garousi S, Shariat Razavi SA , Pouyamanesh G, Afshari AR , et al . Molecular Biology Reports.2023;50(5):4357-4366. CrossRef
- Utilizing Niosome Nanoparticles for the Combined Treatment of Curcumin and Cisplatin in Oral Cancer Rezaei F, Fesharakinia T, Gavanaroudi SB , Rezaeianjam M, Goodarzi MK , Abdollahi M, Akaberi K. Asian Pacific Journal of Cancer Biology.2024;9(4):569-577.
- Dark kinase annotation, mining, and visualization using the Protein Kinase Ontology Soleymani S, Gravel N, Huang LC , Yeung W, Bozorgi E, Bendzunas NG , Kochut KJ , Kannan N. PeerJ.2023;11:e16087. CrossRef
- Comparison of pregnancy outcomes in amniocentesis recipients with normal and abnormal maternal serum analytes Shoarishoar SS , Milani F F, Adineh S S, Sorouri ZR , Attari SM . Cellular and Molecular Biology.2024;70(11):109-114. CrossRef
- pH-Responsive Microneedle Actuator Array for Precise Wound Healing: Design, Actuation, Light Filtering, and Evaluation. In 2024 IEEE 17th Dallas Circuits and Systems Conference (DCAS) (pp. 1-4) Pour MR , Tan JY , Saha R, Kim A, Kim J. IEEE.2024. CrossRef
- Analgesic effect of ketorolac added to lidocaine in surgery of traumatic arm injuries: A double-blind, randomized clinical trial Amini A, Farbod A, Eghbal MH , Ghadimi M, Shahriyari E. European Journal of Translational Myology.2022;32(4):10836. CrossRef
- Magnesium sulfate administration in difficult laryngoscopy: An effective and safe method Iravani K, Salari M, Doostkam A, Mehrabi F, Ghadimi M. American Journal of Otolaryngology.2022;43(4):103479. CrossRef
- Deep learning-based retinal abnormality detection from OCT images with limited data Talebzadeh M, Sodagartojgi A, Moslemi Z, Sedighi S, Kazemi B, Akbari F. World Journal of Advanced Research and Reviews.2024;21(3):609-698.
- Single-cell mechanical analysis and tension quantification via electrodeformation relaxation Moazzeni S, Demiryurek Y, Yu M, Shreiber DI , Zahn JD , Shan JW , Foty RA , et al . Physical review. E.2021;103(3-1):032409. CrossRef
- Enhancement of corrosion, biocompatibility and drug delivery properties of nitinol implants surface by Al-Zn-LDH nanohybrids Khakbiz M, Chagami M, Sheibani S, Amiri E, Moazzeni S, Shakibania S, et al . Colloids and Surfaces A: Physicochemical and Engineering Aspects.2025;704:135524. CrossRef
- Cadherin-based adhesion regulates mechanical polarization in the actin cortex through Rac1 Lin H, Moazzeni S, Kyker-Snowman K, Cohen R, Wang H, Li R, Shreiber D, Zahn J, Shi Z. 2023. CrossRef
- A pencil-drawn electronic tongue for environmental applications Kirsanov D, Mukherjee S, Pal S, Ghosh K, Bhattacharyya N, Bandyopadhyay R, Jendrlin M, et al . Sensors.2021;21:4471.
- Interleukin-4 mRNA Expression in Laryngeal Squamous Cell Carcinoma Patients with or without Lymph Node Involvement Amirazodi E, Razmkhah M, Jaberipour M, Hosseini A, Khademi B. Galen Medical Journal.2014;3(1):20-23.
- Changes in Expression of miRNA-320a and miRNA-497-5p in Early Stage of Breast Cancer Nashtahosseini Z, Sadeghi F, Aghamaali MR . Iranian Red Crescent Medical Journal (IRCMJ).2021;23(6):0-0. https://sid.ir/paper/695031/en.
- Circulating status of microRNAs 660-5p and 210-3p in breast cancer patients Nashtahosseini Z, Aghamaali MR , Sadeghi F, Heydari N, Parsian H. The Journal of Gene Medicine.2021;23(4):e3320. CrossRef
- "Therapeutic Potential of ZnO-Nanoparticles Synthesized Using Portulaca oleracea in Cancer Treatment: A Comprehensive Narrative Review." Amirazodi E, Zaman M, Khanchoupan M, Mortazavi Moghadam F, Faravani F, Khadem Abolfazl A, Jafarianmoghadam N. Research in Biotechnology and Environmental Science 3.2024;(4):46-53.
- Nanoliposomes Meet Folic Acid: A Precision Delivery System for Bleomycin in Cancer Treatment Shakiba D, Shabestari AM , Mokhtari T, Goodarzi MK , Saeed S, Zinatbakhsh Z, et al . Asian Pac J Cancer Biology.2024;9(4):561-8. CrossRef
- Enhancing the cytotoxic effects of Carboplatin and Cisplatin on liposomes in oral Cancer cells with Curcumin Saeidi N, Ansarikojouri M, Mardani M, Rezazadeh R, Goodarzi MK , Amiri F. Asian Pac J Cancer Biology.2024;9(4):579-87. CrossRef
- Prediction and classification of obesity risk based on a hybrid metaheuristic machine learning approach Helforoush Z, Sayyad H. Frontiers in big Data.2024;7:1469981.
- Investigating health risk behavior disparities in the United States with finite mixture modeling Jaferian S, Farhadian L. Discover Public Health.2024;21(1):81.
- J Point and ST Elevation Resembling Brugada: A Marker of Mortality in Methanol Toxicity Nikoo MH , Estedal A, Khatami K, Pakfetrat M, Arjangzadeh A, Boogar SS , Heydari ST . Cardiology Research and Practice..2021;2021(1):5541385.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details