Carcinogenesis Inhibition of Phenolic Derivatives in Duhat (Syzygium cumini); An in Silico Analysis
Download
Abstract
Background: Colorectal cancer (CRC) is one of the most prevalent types of cancer that is commonly treated with traditional chemotherapy; this approach, however, can be toxic, nonspecific, and occasionally ineffective thus necessitating alternative therapeutic strategies. Epidemiological studies suggest that phytochemicals, particularly phenolic compounds, possess antioxidant and antitumor properties, potentially reducing cancer risk. Given that Syzygium cumini is abundant in tropical and subtropical regions and is rich in phenolic compounds, this study explores its therapeutic potential against CRC, addressing the limitations in conventional cancer treatment methods.
Materials and Methods: Molecular docking, dynamics simulation techniques, and ADMET analysis were employed to analyze the binding potential between phenolic compounds from Syzygium cumini to key proteins involved in colorectal cancer pathways, and evaluate the potential drug-likeness and systemic bioavailability of the representative phenolic compounds.
Results: The findings revealed that Myricetin-3-Ogalactoside (-10.1), rutin (-8.8), and gallocatechin (-10.0) showed high binding affinities for essential oncogenic and tumor-suppressor proteins, such as MLH1, p53, and BRAF, with rutin showing the highest affinity across proteins targets belonging in different pathways suggesting that this compound could reduce tumor growth, suppress metastasis, and promote apoptosis. These phenolic compounds not only bind effectively to their CRC-related proteins but also have enhanced structural integrity upon ligand binding, increasing its potential as therapeutic agents against colorectal carcinogenesis.
Conclusion: The study suggests that further formulation of S. cumini phenolic compounds may be necessary due to bioavailability challenges identified in their ADMET analysis. Myricetin-3-O-rhamnoside and rutin showed limited intestinal permeability, while ellagic acid and gallocatechin showed higher absorption rates and non-toxic characteristics.
Introduction
Cancer is a widespread disease that remains one of the leading causes of mortality around the world [1]. This bodily threat is defined by the fast and uncontrolled proliferation of abnormal cells that expand beyond their normal parameters, which can sometimes possibly spread to other parts of the body in a process called metastasis [2]. The World Health Organization (WHO) have reported cancer as the cause of roughly 10 million deaths in 2020 worldwide, based from the GLOBOCAN estimates of cancer mortality determined by the International Agency for Research on Cancer Global Cancer Statistics for the year 2022 recorded almost 20 million new cancer cases, along with nearly 10 million deaths, in a total of 185 countries. The most frequent cancers include cancers of the lung, breast, colorectal, prostate, stomach, and liver [3, 4]. In 2022, lung cancer was reported to be the most commonly diagnosed cancer and the leading cause of cancer mortality, causing 2,480,301 cases and 1,817,172 deaths around the world. It is followed by breast cancer, causing 2,308,897 new cases and 665,684 deaths, placing fourth in cancer-related mortality. Lung cancer is the most common cancer to occur in males, while it is reportedly breast cancer for females [5].
Among several sites where cancer can occur in the body, the Global Cancer Observatory and WHO have declared that colorectal cancer (CRC) persists to be the third most frequently diagnosed cancer worldwide, accounting for 9.6% of all cancers with a total of 1,926,118 new cases in 20223 [6]. It is also the second primary reason for cancer-related mortality in 2022, causing 903,859 deaths globally [5]. In the Philippines, colorectal cancer is overall ranked third in terms of the number of most frequent new cases in 2022, with 20,736 cases (11%) combined for both males and females, as well as the fourth leading cause of cancer-related death locally, with a total of 10,692 cases [6].
Surgery, chemotherapy, radiotherapy, and immunotherapy are four conventional approaches to treating cancer, used singly or in combination. These forms of treatment, while traditionally used, tend to be harmful and non-specific, and they can inadvertently encourage the increase in the propagation and survival of cancer cells [7]. Patients diagnosed with colorectal cancer (CRC) are typically treated with the same traditional chemotherapy approach. However, toxicity prompted by chemotherapy, along with ineffective responses that can happen during the process, could deter patients with CRC from undergoing chemotherapy [8]. Such tendencies and instances immensely highlight the significance of exploring alternative forms of treatment that can work alongside traditional cancer treatments.
Given the limitations in conventional cancer treatment methods, which further aggravate the looming health threat that cancer poses to several countries globally, there is an increasing urgency to discover new strategies for the prevention and treatment of cancer. The diet of a person plays an enormous role in the emergence, course, and treatment of cancer. A variety of epidemiological studies have stated that consuming phytochemicals is associated with a decreased risk of cancer [9-12]. Plants have been found to contain most phytochemicals, including phenolic compounds, with known bioactive properties [12]. These bioactive properties comprise antioxidant activity, which slows down the progression of cancer through stimulating apoptosis, and antitumor activity, which explicitly promotes apoptosis, inhibits tumor cell growth, and prevents metastasis [13, 14]. Due to their safety and therapeutic potential, polyphenols are consequently receiving a lot of interest, although the absorption of such compounds, especially gastrointestinal absorption, has yet to be extensively studied. This contributes to limitations in understanding the bioavailability of these compounds [10]. Gallic acid and ellagic acid are part of the broad classification of polyphenols. While quercetin and myricetin are also polyphenols, they are more commonly classified under flavonoids, primarily plant- based polyphenols.
Syzygium cumini, also known as Java plum or duhat in the Philippines, is an evergreen tropical tree that commonly grows in several countries, particularly in the tropical parts of the world [15]. This plant has been stated to have extensive nutritional and pharmacological functions, with just about every part fruit, leaves, bark, seeds used for food and non-food purposes throughout the centuries. The fruit and seed extracts from S. cumini possess anticancer and chemopreventive potential directed at numerous types of cancer, namely, colon, breast, and cervical cancers [11]. The plant also has anti-dysentery, antiviral, anti-rheumatic, and anti-diabetic effects. Furthermore, the plant has antiproliferative bioactive phenolic compounds that can act on a wide range of cancer cell lines, including colorectal cancer. In particular,
S. cumini comprises phenolic compounds such as gallic acid, ellagic acid, quercetin, and myricetin [16]. The ability of S. cumini phenolic compounds to influence important oncogenic signaling pathways connected to the proliferation of cells and apoptosis constitutes a potential option for cancer therapy.
However, there are still gaps in knowledge about the bioactivity of these phenolic compounds, especially regarding their bioavailability and the most appropriate dosage for use in human clinical settings. Therefore, this study aims to employ in silico techniques in investigating the carcinogenesis inhibition of the phenol derivatives found in Syzygium cumini, primarily focusing on the modulation of oncogenic signaling pathways involved in cancer cell proliferation and their potential apoptosis- promoting activity against active cancer cells.
Materials and Methods
Retrieval of ligands
The ligands and known chemotherapeutic agents were obtained through NCBI PubChem via https://pubchem. ncbi.nlm.nih.gov/. The corresponding 3D structures were retrieved and saved as Structure Data File (SDF) format, which was later converted to Protein Data Bank (PDB) format through BIOVIA Discovery Studio from https:// discover.3ds.com/.
Retrieval of proteins
The proteins were sourced from the Protein Data Bank via https://www.rcsb.org/ and Uniprot via https://www. uniprot.org and downloaded in the PDB file format for molecular docking. The preparation of protein involves the removal of unwanted molecules such as water, ions, and ligands was done through BIOVIA Discovery Studio. To ensure accurate docking interactions, polar hydrogen atoms were added using AutoDockTools (ADT). Afterward, the cleaned protein was saved in PDBQT format, which was required for docking.
Molecular docking interactions and visualization
Initially, the PyRx software was obtained from its official source https://pyrx.sourceforge.io/. The prepared protein and ligand were then imported into PyRx by selecting the molecules from the respective files. The docking setup involved defining the grid box around the target binding site. Using AutoDock Vina mode, the protein was selected, and the grid box dimensions (x, y, z) were adjusted to set the active site where ligand binding was expected. The exhaustiveness parameter was set to 25 to enhance the accuracy of binding energy calculations and improve the reliability of the predicted binding affinities [17]. PyRx subsequently generated docking scores, where lower binding energy values (kcal/mol) indicated stronger ligand-protein interactions. The protein-ligand complex was generated using UCSF Chimera, a molecular visualization and analysis tool. The software was obtained from its official source (https:// www.cgl.ucsf.edu/chimera/).
Molecular dynamics
GROMACS was used to assess the stability of protein-ligand complexes. The researchers utilized a web-based analysis platform, Galaxy (usegalaxy.org), to access GROMACS as a tool. The molecular dynamics simulation began by fixing the protein-ligand complex using the SwissPDB Viewer from https://spdbv.unil.ch/. The fixed complex will be imported to the site, and the protein and ligand coordinates will be separated using the Search in Textfiles (grep) tool. Then, protein topology will be prepared using the GROMACS initial setup tool. Simultaneously, the ligand topology was generated using the Compound conversion and Generate MD topologies for small molecules tools. For the second tool in generating the topology of the ligand, the parameter set was zero (0) for the charge of the molecule, one (1) for multiplicity, gaff for the force field used in the parameterization, and bcc for the charge method. Then, both topologies were combined using the Merge GROMACS topologies tool. The simulation box was created using the GROMACS structure configuration tool, with dimensions set to one (1) nanometer and the box type defined as triclinic. Then, the system was solvated using the GROMACS solvation and adding ions tool. The GROMACS energy minimization tool was used to relieve any steric clashes or unfavorable interactions within the system, where the parameters were set at 5000 steps for MD simulation and 1000 EM tolerance. After Minimization, the system was set to equilibrate under controlled temperature and pressure conditions to ensure stability. The GROMACS simulation tool was used for the NVT and NPT equilibration, with bond constraints being all-bonds, temperature at 300 Kelvin, step length at 0.001 ps, and number of steps for simulation at 50000. After equilibration, the production MD simulation was conducted to observe the dynamic behavior of the protein-ligand complex over time, with parameters similar to NVT/NPT equilibration, except that the number of steps for this simulation was 1000000. Then, the trajectory and coordinate formats were converted to DCD and PDB files using GROMACS structure configuration and MDTraj file converter, respectively.
The RMSD Analysis tool was used to assess a docking program’s accuracy in reproducing a ligand’s experimental pose within a protein’s binding site [18]. The RMSF analysis was applied to quantify how much individual residues in proteins fluctuate from their average positions [19].
ADMET Analysis of S. cumini Phenolic Ligands
The SwissADME web server was used to assess compounds’ physicochemical and drug-likeness properties, especially in the context of drug discovery. The phenolic compounds were gathered through PubChem and were downloaded using the SMILE file format. This estimated the physicochemical characteristics by uploading the downloaded file in SwissADME (http://www.swissadme. ch/index.php). After determining the physicochemical characteristics, the phenolic compounds’ drug-likeness was evaluated following Egbuna et al. ‘s approach in 2023, which applied Lipinski’s Rule of Five. Lipinski et al. indicate that this rule assesses oral bioavailability based on the following key criteria [20].
The pharmacokinetic profile of a compound is characterized by its ADME properties, encompassing absorption, distribution, metabolism, and excretion [21]. Along with the ADME properties, toxicity assessment is vital for ensuring the safety of potential drugs [22]. Determining the compound’s absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties is essential to predict the effectiveness and safety of various compounds as drug candidates, as they significantly influence how the body processes a compound.
PkCSM, a computational tool used in drug development and safety evaluation to predict the pharmacokinetic and toxicological profiles of small molecules, was utilized to provide the ADMET profile of each ligand. The pkCSM web server employs graph-based structural signatures to generate a comprehensive ADMET profile. This approach involves inputting chemical compounds in canonical SMILES format in http://biosig.unimelb.edu.au/pkcsm/ prediction to undergo full prediction of pharmacokinetic and toxicological (ADMET) properties [20, 23].
Results and Discussion
I. Structural Modelling and Corresponding Binding Afinities
Molecular binding scores of the phenolic ligands derived from the docking simulation were used in the selection of potential therapeutic candidates; thus, non-leading ligands are all excluded for further analysis. Likewise, the 3D models of the complexes of the leading ligands and their target proteins involved in the four main pathways of colorectal cancer are visualized using Biovia Discovery Studio in Figure 1.1 and Figure 1.2, and their corresponding binding affinities are presented in Table 1.
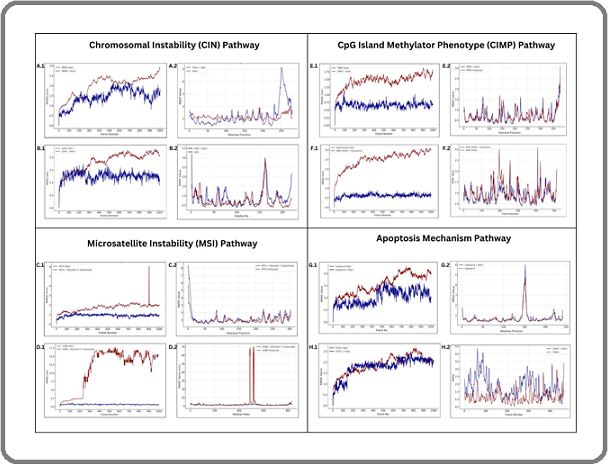
Figure 1. Root Mean Square Deviation (RMSD) and Root Mean Square Fluctuations (RMSF) plots of proteins and docked protein-ligand complexes in different colorectal cancer pathways generated through Galaxy MDS. CIN Pathway: (A.1) 7BWN and 7BWN + Rutin RMSD, (A.2) 7BWN and 7BWN + Rutin RMSF, (B.1) 1DD1 and 1DD1 + Rutin RMSD, (B.2) 1DD1 and 1DD1 + Rutin RMSF; MSI Pathway: (C.1) 4P7A and 4P7A + Myricetin 3’-Galactoside RMSD, (C.2) 4P7A and 4P7A + Myricetin 3’-Galactoside RMSF, (D.1) 208B and 208B + Myricetin 3’-Rhamnoside, (D.2) 208B and 208B + Myricetin 3’-Rhamnoside RMSF; CIMP Pathway: (E.1) TIMP3 and TIMP3 + Rutin RMSD, (E.2) TIMP3 and TIMP3 + Rutin RMSF, (F.1) BRAF-V600E and BRAF-V600E + Epicatechin RMSD, (F.2) BRAF- V600E and BRAF-V600E + Epicatechin RMSF; Apoptosis Mechanism: (G.1) Caspase-8 and Caspase-8 + Rutin RMSD, (G.2) Caspase-8 and Caspase-8 + Rutin RMSF; (H.1) CHEK2 and CHEK2 + Rutin RMSD, (H.2) CHEK2 and CHEK2 + Rutin RMSF.
| Pathway | Protein | Phenolic Compound | Binding Affinity |
| (kcal/mol) | |||
| Chromosomal Instability (CIN) Pathway | Adenomatous Polyposis coli (APC) protein | Myricetin-3-O-rhamnoside | -5.9 |
| Rutin | -5.7 | ||
| Ellagic acid | -5.4 | ||
| Chlorogenic acid | -5.3 | ||
| Catechin | -5.3 | ||
| 5-Fluorouracil | -3.3 | ||
| Temozolomide | -4.5 | ||
| Irinotecan | -6.4 | ||
| Cellular tumor antigen p53 | Rutin | -8.8 | |
| Myricetin-3-O-rhamnoside | -8.4 | ||
| Ellagic acid | -8.2 | ||
| Laricitrin-3-O-galactoside | -8.2 | ||
| Myricetin-3-O-pentoside | -8 | ||
| 5-Fluorouracil | -5.4 | ||
| Temozolomide | -6.1 | ||
| Irinotecan | -9.7 | ||
| Phosphatidylinositol 4,5-bisphosphate | Myricetin-3-O-rhamnoside | -7.7 | |
| 3-kinase catalytic subunit alpha isoform | |||
| Chlorogenic acid | -6.8 | ||
| Laricitrin-3-O-galactoside | -6.8 | ||
| Rutin | -6.7 | ||
| Epicatechin | -6.6 | ||
| 5-Fluorouracil | -4.2 | ||
| Temozolomide | -5.6 | ||
| Irinotecan | -8.9 | ||
| Mothers against decapentaplegic homolog 4 | Rutin | -8 | |
| Protein (SMAD4) | Myricetin-3-O-rhamnoside | -7.6 | |
| Myricetin-3-O-galactoside | -7.4 | ||
| Quercetin | -7.1 | ||
| Myricetin | -7 | ||
| 5-Fluorouracil | -4.8 | ||
| Temozolomide | -5.5 | ||
| Irinotecan | -8.1 | ||
| Microsatellite Instability (MSI) Pathway | DNA mismatch repair protein Mlh1 | Myricetin-3-O- galactoside | -10.1 |
| Myricetin-3-O-rhamnoside | -10.1 | ||
| Rutin | -9.7 | ||
| Laricitrin-3-O-galactoside | -9.4 | ||
| Myricetin-3-O-pentoside | -9.2 | ||
| 5-Fluorouracil | -5 | ||
| Temozolomide | -6.2 | ||
| Irinotecan | -8.8 | ||
| DNA mismatch repair protein Msh2 | Myricetin-3-O-rhamnoside | -10 | |
| Rutin | -9.4 | ||
| Ellagic acid | -8.9 | ||
| Quercetin | -8.8 | ||
| Myricetin | -8.5 | ||
| 5-Fluorouracil | -4.7 | ||
| Temozolomide | -5.6 | ||
| Irinotecan | -10.8 | ||
| Mismatch repair endonuclease PMS2 | Ellagic acid | -7.8 | |
| Myricetin-3-O-pentoside | -7.8 | ||
| Rutin | -7.8 | ||
| Myricetin-3-O-rhamnoside | -7.8 | ||
| Laricitrin-3-O-glucoside | -7.3 | ||
| 5-Fluorouracil | -5.1 | ||
| Temozolomide | -5.9 | ||
| Irinotecan | -8.4 | ||
| DNA mismatch repair protein Msh3 | Gallocatechin | -9.2 | |
| Rutin | -9 | ||
| Myricetin-3-O-rhamnoside | -8.7 | ||
| Myricetin | -8.4 | ||
| Epicatechin | -8.4 | ||
| 5-Fluorouracil | -5.5 | ||
| Temozolomide | -6 | ||
| Irinotecan | -8.8 | ||
| CpG Island Methylator Phenotype (CIMP) Pathway | Cyclin-dependent kinase inhibitor 2A | Rutin | -7.8 |
| Myricetin-3-O-glucoside | -7 | ||
| Myricetin-3-O-rhamnoside | -7.4 | ||
| Gallocatechin | -7.2 | ||
| Epigallocatechin | -7.5 | ||
| 5-Fluorouracil | -4.6 | ||
| Temozolomide | -5.3 | ||
| Irinotecan | -8.2 | ||
| Methylated-DNA-protein-cysteine | Rutin | -8.5 | |
| methyltransferase | |||
| Myricetin-3-O-pentoside | -8.1 | ||
| Ellagic Acid | -8 | ||
| Myricetin-3-O-rhamnoside | -8 | ||
| Gallocatechin | -7.9 | ||
| 5-Fluorouracil | -5.4 | ||
| Temozolomide | -6.4 | ||
| Irinotecan | -10.1 | ||
| Metalloproteinase inhibitor 3 | Rutin | -9.6 | |
| Epigallocatechin | -9.4 | ||
| Gallocatechin | -9.4 | ||
| Quercetin | -8.8 | ||
| Myricetin | -8.5 | ||
| 5-Fluorouracil | -5.8 | ||
| Temozolomide | -7.5 | ||
| Irinotecan | -10.3 | ||
| Serine/threonine-protein kinase B-raf | Gallocatechin | -10 | |
| Laricitrin-3-O-galactoside | -9.9 | ||
| Laricitrin-3-O-glucoside | -9.8 | ||
| Myricetin-3-O-glucoside | -9.8 | ||
| Rutin | -9.6 | ||
| 5-Fluorouracil | -5.7 | ||
| Temozolomide | -7 | ||
| Irinotecan | -10.5 | ||
| Apoptosis Mechanism Pathway | RAC-alpha serine/threonine-protein kinase | Rutin | -8.4 |
| Epigallocatechin | -8.2 | ||
| Gallocatechin | -8.1 | ||
| Myricetin | -8 | ||
| Quercetin | -7.9 | ||
| 5-Fluorouracil | -4.3 | ||
| Resveratrol | -5.7 | ||
| Temozolomide | -7.5 | ||
| Irinotecan | -10.3 | ||
| Apoptosis regulator BAX | Rutin | -8 | |
| Epigallocatechin | -7.8 | ||
| Gallocatechin | -7.7 | ||
| Myricetin | -7.6 | ||
| Quercetin | -7.5 | ||
| 5-Fluorouracil | -4.7 | ||
| Resveratrol | -6.8 | ||
| Temozolomide | -5.5 | ||
| Irinotecan | -9.3 | ||
| Serine/threonine-protein kinase Chk2 | Rutin | -8.5 | |
| Epigallocatechin | -8.3 | ||
| Gallocatechin | -8.2 | ||
| Myricetin | -8.1 | ||
| Quercetin | -8 | ||
| 5-Fluorouracil | -5 | ||
| Resveratrol | -7 | ||
| Temozolomide | -6.9 | ||
| Irinotecan | -10.1 | ||
| Caspase-8 | Rutin | -8.6 | |
| Epigallocatechin | -8.4 | ||
| Gallocatechin | -8.3 | ||
| Myricetin | -8.2 | ||
| Quercetin | -8.1 | ||
| 5-Fluorouracil | -5 | ||
| Resveratrol | -6.6 | ||
| Temozolomide | -5.5 | ||
| Irinotecan | -9.6 |
The protein structure for every pathway is highlighted in violet, while the docked ligand molecule is emphasized in yellow. The molecular docking results of phenolic compounds for the major pathways of colorectal cancer are shown in Table 1, ranked by binding affinity (kcal/mol). The binding affinity (kcal/mol) scores of known chemotherapy drugs against colorectal cancer are also demonstrated in Table 2. Among the tested chemotherapy drugs, Irinotecan consistently exhibited the strongest binding interactions across all target proteins. In the Chromosomal Instability Pathway (CIN), the ligand myricetin-3-O-rhamnoside demonstrated the highest binding affinity to the proteins Adenomatous Polyposis Coli (APC) protein and Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform with values reaching -5.9 kcal/mol and -7.7 kcal/mol, respectively. Similarly, rutin demonstrated the strongest interaction, among other ligands, to Cellular tumor antigen p53 and Mothers against decapentaplegic homolog 4 Protein (SMAD4) with -8.8 kcal/mol and -8.0 kcal/mol, respectively. Moreover, in the Microsatellite Instability (MSI) Pathway, the interaction of DNA mismatch repair protein M1h1 to myricetin-3-O-galactoside and myricetin- 3-O-rhamnoside both gained the highest binding affinity of -10.1 kcal/mol. DNA mismatch repair protein Msh2 to myricetin-3-O-rhamnoside interaction gained -10.0 kcal/mol, Mismatch repair endonuclease PMS2 to ligands ellagic acid, myricetin-3-O-pentoside, rutin, and myricetin-3-O-rhamnoside, all gained -7.8 kcal/mol, which are the highest values among their corresponding protein-ligand complex. Lastly, DNA mismatch repair protein Msh3 and gallocatechin had the highest binding affinity of -9.2 kcal/mol among the other protein-ligand complexes. In the CpG Island Methylator Phenotype (CIMP) Pathway, rutin showed the most favorable binding affinities with proteins Cyclin-dependent kinase inhibitor 2A, Methylated-DNA-protein-cysteine methyltransferase, and Metalloproteinase inhibitor 3, yielding values of -7.8 kcal/mol, -8.5 kcal/mol, and -9.6 kcal/mol, respectively. Meanwhile, gallocatechin demonstrated the highest binding affinity for its interaction with Serine/threonine- protein kinase B-raf, producing a binding affinity of -10.0 kcal/mol. In the Apoptosis Mechanism Pathway, rutin exhibited the strongest binding interactions with proteins RAC-alpha serine/threonine-protein kinase, Apoptosis regulator BAX, Serine/threonine-protein kinase Chk2, and Caspase-8, yielding binding affinities of -8.4 kcal/mol, -8.0 kcal/mol, -8.5 kcal/mol, and -8.6 kcal/mol, respectively.
I I. Molecular Docking Interactions of Ligands and Target Proteins
The leading ligands that showed exemplary binding scores were further analyzed to assess their interactions with their target proteins and determine the domains that were affected by the formation of the complexes. The data derived from the protein-ligand complex interaction analysis were summarized in Table 2, which includes the type of bonds formed by ligands and the protein’s reactive residues, as well as their associated domains.
| Pathway | Protein | Phenolic Compound | Amino Acids Involved | Interactions | Affected Domains | Distance |
| Chromosomal Instability (CIN) Pathway | Adenomatous polyposis coli (APC) protein | Myricetin-3-O -rhamnoside | Asn20, Gln25 | Conventional hydrogen bond | 3.81Å, 4.30Å | |
| Glu28 | π-anion, π-cation | Armadillo/beta-catenin -like repeats | 6.30Å, 4,71Å | |||
| Arg24 | π-anion, π-cation, π-alkyl | 4.16Å, 4.35, 4.81Å | ||||
| Cellular tumor antigen p53 (TP53) | Rutin | Asp197, Tyr237, Val193, Leu195, Gly241 | Conventional hydrogen bond | 4.11Å, 3.24Å, 4.24Å, 4.12Å, 2.75Å | ||
| Arg80 | Conventional hydrogen bond, Unfavorable donor-donor | P53 DNA-binding domain | 4.61Å, 3.42Å | |||
| Phe277 | Conventional hydrogen bond, π-π stacked | 3.07Å, 5.39Å | ||||
| Pro192 | π-alkyl | 6.33Å | ||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | Myricetin-3- O-rhamnoside | Ile70, Asp111, Ser6, Trp156 | Conventional hydrogen bond | 5.61Å, 4.21Å, 3.53Å, 4.24Å | ||
| Pro71 | Carbon hydrogen bond, π-donor hydrogen bond | 4.67Å, 3.91Å | ||||
| Phosphatidylinositol 3-kinase, adaptor-binding domain | ||||||
| Leu113 | Carbon hydrogen bond, π-donor hydrogen bond, π-alkyl | 4.78Å, 4.08Å | ||||
| Ser115 | Unfavorable donor-donor | 3.72Å | ||||
| Mothers against decapentaplegic homolog 4 Protein (SMAD4) | Myricetin-3- O-rhamnoside | Arg416 | Conventional hydrogen bond, π-alkyl | 4.40Å, 6.05Å | ||
| Gln446, Asp415 | Conventional hydrogen bond | Class I alpha phosphatidylinositol 3-kinases (PI3Ks) | 5.31Å, 4.95Å, 5.18Å | |||
| Tyr412 | Conventional hydrogen bond, π-sigma | 5.71Å, 4.61Å | ||||
| Pro422 | π-alkyl | 4.63Å, 4.54Å, 4.49Å | ||||
| Microsatellite Instability (MSI) Pathway | DNA mismatch repair protein Mlh1 | Myricetin-3 -rhamnoside | Leu104 | Conventional hydrogen bond, π-alkyl | 4.66Å, 5.27Å | |
| Arg100, Ser83 | Unfavorable bump, Unfavorable donor-donor | 5.25, 5.39Å, 5.19Å, 4.44Å, 3.45Å, 3.15Å, 5.62Å | ||||
| Gly67 | Conventional hydrogen bond | 2.97Å, 4.06Å | ||||
| Ile68, Ala42, | Conventional hydrogen bond | 3.42Å | ||||
| Ala103 | Histidine kinase-like ATPase domain | |||||
| Asn38 | π-alkyl | 5.50Å, 5.86Å, 4.75Å | ||||
| Lys84 | Amide-π stacked | 4.62Å | ||||
| π-cation | 5.94Å | |||||
| Microsatellite Instability (MSI) Pathway | ||||||
| Myricetin- 3-galactoside | Leu104, Asp72, Asp63, Lys84 | Conventional hydrogen bond | 3.76Å, 4.84Å, 5.48Å. 4.95Å | |||
| Asn38 | Conventional hydrogen bond, Unfavorable donor-donor | 4.00Å, 4.44Å, 5.10Å | ||||
| Val76 | Conventional hydrogen bond, π-sigma | Histidine kinase-like ATPase domain | 4.74Å, 6.21Å | |||
| Ala42 | π-alkyl | 6.62Å | ||||
| π-alkyl, π-sigma | ||||||
| Ile68 | 5.10Å, 3.86Å, 4.86Å | |||||
| Microsatellite Instability (MSI) Pathway | DNA mismatch repair protein Msh2 | Myricetin- 3-rhamnoside | Asn671, Lys675, Ser676, Thr677, Gln681 | Conventional Hydrogen Bond, | 4.69Å, 3.18Å, 3.61Å, 4.16Å, 5.39Å | |
| Tyr815, Phe650, | 4.36Å, 5.16Å | |||||
| π-π Stacked | 5.70Å, 7.34Å | |||||
| Met672 | ATP-binding cassette domain | 4.24Å, 5.06Å | ||||
| Carbon Hydrogen Bond, | ||||||
| Unfavorable donor-donor | ||||||
| Mismatch repair endonuclease PMS2 | Myricetin- 3-rhamnoside | Thr285, Ala182, Gln186, Phe290 | Conventional hydrogen bond | 3.33Å, 3.92Å, 3.68Å, 5.15Å | ||
| Ala190 | hPSM2 domain, Histidine kinase-like ATPase domain | 6.37Å | ||||
| π-alkyl | ||||||
| Rutin | Lys40, Cys297 | Conventional hydrogen bond | 5.28Å, 4.27Å | |||
| Val187, Lys183 | π-alkyl | 4.92Å, 5.19Å | ||||
| Asp298, Phe290 | Unfavorable donor-donor, Unfavorable a cceptor-acceptor | 5.35Å, 3.65Å | ||||
| Conventional hydrogen bond, Unfavorable donor-donor, Unfavorable acceptor-acceptor | ||||||
| Thr285 | 3.83Å, 4.09Å | |||||
| hPSM2 domain | ||||||
| Gln288 | Conventional hydrogen bond, Carbon hydrogen bond | 2.64Å, 6.08Å | ||||
| Myricetin- 3-pentoside | Gln186, Phe290, Asp298, Thr285 | Conventional hydrogen bond | 4.69Å, 5.06Å, 4.00Å, 3.77Å | |||
| Lys183 | 5.28Å | |||||
| Gln288 | π-alkyl | 7.09Å | ||||
| Carbon hydrogen bond | hPSM2 domain | |||||
| Cys297 | Unfavorable acceptor-acceptor | 4.40Å | ||||
| Ellagic acid | Thr155, Ser46 | Conventional hydrogen bond | 4.35Å, 3.92Å | |||
| Ala49 | π-alkyl | 4.61Å, 5.79Å | ||||
| Cys73 | π-sulfur | 5.06Å, 7.40Å | ||||
| Val75 | π-alkyl, π-sigma | Histidine kinase-like ATPase domain | 4.60Å, 5.14Å | |||
| Asn45 | Conventional hydrogen bond, π-sigma, Amide-π stacked, Unfavorable donor-donor | 4.14Å, 4.79Å, 6.90Å, 3.37Å | ||||
| DNA mismatch repair protein Msh3 | Gallocatechin | Gln681, Ala649, Ile651, Gly673 | Conventional hydrogen bond | DNA-binding domain | 3.97Å, 3.97Å, 3.98Å, 3.98Å | |
| Phe650, Tyr815 | π-donor hydrogen bond, π-alkyl | 2.75Å, 4.64Å, 4.54Å | ||||
| Asn653 | π-donor hydrogen bond | 6.47Å | ||||
| Ile648 | π-alkyl | 5.73Å | ||||
| CpG Island Methylator Phenotype (CIMP) Pathway | Cyclin-dependent kinase inhibitor 2A (CDKN2A) | Rutin | Arg112, Ile145, Arg107, Asp105 | Conventional hydrogen bond | Ankyrin repeat domain | 6.70 Å, 4.94Å, 3.76Å, 3.85Å |
| Arg144 | Conventional hydrogen bond, π-cation, π-alkyl | 5.09Å, 4.69Å, 4.09Å | ||||
| Leu113 | π-alkyl | 6.36Å | ||||
| Ala143 | Carbon hydrogen bond | 3.92Å | ||||
| Arg131 | Unfavorable donor-donor | 3.43Å | ||||
| Methylated-DNA- protein-cysteine methyltransferase (MGMT) | Rutin | Glu172, Ala170, His171 | Conventional hydrogen bond | 4.60Å | ||
| Gly109 | Conventional hydrogen bond, Carbon hydrogen bond | ATase domain | 3.58Å | |||
| Glu74 | Conventional hydrogen bond, π-Anion | 3.98Å | ||||
| Pro73 | Conventional hydrogen bond, Alkyl, π-Alkyl | 4.49Å, 4.69 | ||||
| Lys107 | Alkyl, π-Alkyl | 4.39Å | ||||
| Metalloproteinase inhibitor 3 (TIMP3) | Rutin | Gln108, Tyr390, His7, Arg100, Glu99 | Conventional hydrogen bond | 4.89Å, 6.71Å, 5.58Å, 5.62Å, 4.26Å | ||
| Val98 | Conventional hydrogen bond, Alkyl, π-Alkyl | ADAM type metalloprotease domain, | 4.04Å | |||
| Ala11 | ADAM10/ADAM17 catalytic domain | |||||
| Phe97 | Alkyl, π-Alkyl | 5.83Å | ||||
| Ser6 | π-π Stacked | 4.17Å | ||||
| Carbon hydrogen bond | 3.58Å | |||||
| Serine/threonine-protein kinase B-raf (BRAF-V600E) | Epicatechin | Ser465, Cys532 | Conventional Hydrogen Bond | 3.63Å, 2.76Å | ||
| Val471, Ile463, Ala481 | Alkyl, π-Alkyl | 4.92Å, 5.36Å, 6.80Å | ||||
| Lys483 | Protein kinase domain | |||||
| Unfavorable donor-donor | 3.58Å | |||||
| Apoptosis Mechanism Pathway | RAC-alpha serine/ threonine-protein kinase (AKT1) | Rutin | Glu17 | Pi- Anion | 4.46Å | |
| Val83 | Conventional Hydrogen Bond | 4.44Å | ||||
| Lys14 | Conventional Hydrogen Bond, Carbon Hydrogen Bond | 5.69Å, 4.30Å | ||||
| Arg25 | Conventional Hydrogen Bond | 6.02Å | ||||
| Conventional Hydrogen Bond, | Protein Kinase B, pleckstrin homology domain | |||||
| Gly16 | Carbon Hydrogen Bond | 3.75Å, 3.64Å | ||||
| Apoptosis regulator BAX (BAX) | Rutin | Asp33 | Conventional Hydrogen Bond, Carbon Hydrogen | Bcl-2 family Domain | 3.37Å | |
| Pro49 | Carbon Hydrogen Bond | 3.59Å | ||||
| Gln52 | Conventional Hydrogen Bond | 2.71Å | ||||
| Lys57 | Carbon Hydrogen Bond, π-Alkyl | 5.10Å, 4.21Å, 4.57Å | ||||
| Pi-Anion, | ||||||
| Glu61 | 5.42Å | |||||
| Ser60 | Carbon Hydrogen Bond | 3.98Å | ||||
| Serine/threonine-protein kinase Chk2 (CHK2) | Rutin | Arg148, Val109, Lys135, Asp101 | Conventional Hydrogen Bond | 5.65Å, 5.06Å, 4.21Å, 4.21Å | ||
| Gln100 | Carbon Hydrogen Bond | 4.39Å | ||||
| Asn196 | Protein Kinase Domain | |||||
| Glu149 | Unfavorable Donor-Donor | Forkhead Associated Domain (FHA) Domain | 3.65Å | |||
| Pi-Anion | 6.03Å, 4.63Å | |||||
| Apoptosis Mechanism Pathway | Caspase-8 | Rutin | Arg260 | Conventional Hydrogen Bond, | 6.84Å, 6.00Å | |
| Arg413 | π-cation Conventional Hydrogen Bond, π-Donor Hydrogen Bond | 4.13Å | ||||
| Gln358 | π-Alkyl | 6.84Å | ||||
| Cys360 | Conventional Hydrogen Bond | Peptidase C14A, caspase catalytic domain | 4.60Å | |||
| Ser316 | Conventional Hydrogen Bond, π-Donor Hydrogen Bond | 5.29Å | ||||
| His317 | Conventional Hydrogen Bond | 2.78Å | ||||
| Gly318 | Conventional Hydrogen Bond |
Likewise, 2D models of these interactions are all presented in Figure 2.1 and Figure 2.2 to visualize the interaction between the ligands and their target proteins. To elaborate, the results of the interaction analysis revealed strong interactions between the phenolic compounds and key proteins within the chromosomal instability (CIN) pathway of colorectal cancer. Thus, it is suggested that modulation of multiple oncogenic signaling pathways by phenolic compounds is possible in the progression of colorectal cancer. These findings are shown in myricetin-3-O-rhamnoside, which exhibited multiple interactions with the APC protein, including conventional hydrogen bonding with Asn20 and Gln25 (3.81Å, 4.30Å) and π-anion/π-cation interactions with Glu28 and Arg24 (distances ranging from 3.45Å to 6.30Å) with the Armadillo/beta-catenin-like repeats domain. Binding at this site is essential for regulating β-catenin in the Wnt signaling pathway, which is often disrupted in colorectal cancer [24]. Similarly, rutin is bound to p53 via conventional hydrogen bonds with Arg80 (4.61Å, 3.42Å) and Phe277 (3.07Å, 5.39Å), along with π-alkyl interactions with Pro192 (6.33Å) within the DNA-binding domain of p53, which is responsible for recognizing specific p53-responsive elements in tumor suppression and induces CRC when a frameshift mutation occurs. Disruption in these regions can affect how p53 responds effectively to DNA damage by either apoptosis or cell-cycle arrest [25]. Additional hydrogen bonds were observed with Ile70, Asp111, Ser6, and Trp156 (bond distances between 3.53Å and 6.21Å), reinforcing the ligand’s potential affinity for p53 within the p53 DNA-binding domain, suggesting restoration of p53’s tumor suppressor function, thereby enhancing DNA repair and apoptosis in CRC cells. Further interactions were detected in PIK3CA and SMAD4, suggesting significant binding potential for phenolic ligands. Myricetin-3-O- rhamnoside interacted with PIK3CA through carbon- hydrogen bonding (Pro71, Leu113) and π-alkyl/π-donor hydrogen bonds (distances ranging from 3.72Å to 4.78Å) within the phosphatidylinositol 3-kinase, adaptor-binding domain, potentially inhibiting growth and survival of CRC cells by reducing tumorigenic signalling, which may reduce its resistance to target treatments [26]. Finally,myricetin-3-O-rhamnoside is bound to MSH4 through conventional hydrogen bonding with Gln446 and Asp415 (4.95Å, 5.31Å) and π-alkyl interactions with Pro422 (4.63Å) within the Class I alpha phosphatidylinositol 3-kinases (PI3Ks). Although MSH4 is not a canonical MMR protein, its interaction with myricetin-3-O- rhamnoside suggests a potential role in suppressing tumor cells. This interaction may stabilize its inhibitory effect on tumor cell proliferation through the TGF-β signaling pathway, which is known to regulate cell growth and promote antitumor activity [27]. These findings highlight the diverse binding interactions of phenolic compounds with CIN pathway proteins, potentially influencing their structural and functional stability.
Moreover, molecular docking analysis of proteins under the Microsatellite Instability (MSI) Pathway revealed various interactions with key mismatch repair (MMR) proteins, potentially restoring their function. MLH1, targeted by myricetin-3-rhamnoside and myricetin- 3-galactoside, exhibited stable binding in its histidine kinase-like ATPase domain, forming hydrogen bonds with Leu104, Gly67, Ala103, Asn38, and Lys84, along with π-alkyl interactions involving Arg100, Ser83, Ile68, and Ala42 (2.97Å–6.62Å). MSH2, responsible for mismatch recognition, interacted with myricetin-3-rhamnoside in its ATP-binding cassette domain, establishing hydrogen bonds with Asn671, Lys675, Ser676, Thr677, and Gln681, π-π stacking with Tyr815 and Phe650, and a carbon- hydrogen bond with Met672 (3.18Å–7.34Å), suggesting stabilization of its DNA-binding activity. PMS2, essential for mismatch excision, interacted with myricetin-3- rhamnoside, rutin, and myricetin-3-pentoside in its hPSM2 domain, forming hydrogen bonds with Thr285, Gln186, Asp298, and Phe290, and π-alkyl interactions with Ala190, Lys183, Gln288, and Cys297 (2.64Å–7.09Å), suggesting reinforcement of the MLH1-PMS2 repair complex. Ellagic acid also bound PMS2’s histidine kinase-like ATPase domain, forming hydrogen bonds with Thr155 and Ser46, π-alkyl and π-sulfur interactions with Ala49, Cys73, and Val75, and amide-π stacking with Asn45 (3.37Å–7.40Å), potentially stabilizing ATP-driven repair activity. MSH3, which corrects insertion-deletion loops, exhibited strong interactions with gallocatechin in its DNA-binding domain, forming hydrogen bonds with Gln681, Ala649, Ile651, and Gly673, π-donor hydrogen bonds with Phe650 and Tyr815, and π-alkyl interactions with Asn653 and Ile648 (2.75Å–6.47Å). These interactions are elaborated in a study wherein mutations in the genes coding for MMR proteins are described as a hallmark of cancer due to the absence of DNA repair mechanisms often observed in gastrointestinal malignancies [28]. The authors continued that mutations in MLH1, MSH2, MSH3, and PMS2 are responsible for correcting in conserved regions in the genome called microsatellites, which are especially prone to frameshift mutations and mismatched base pairing. MLH1 and PMS2 form MutLalpha, while MSH2 and MSH3 form MutSbeta and MutSalpha, with MSH6 consequently. The less typical MutSbeta complex usually repairs larger errors, whereas the MutSalpha complex becomes activated through ATPase activity, which allows the complex to bind to the DNA and repair the mismatches. Once the MutSalpha complex identifies errors such as single-base mismatches and insertion-deletion loops, it forms a sliding clamp structure surrounding the DNA, triggers ATP hydrolysis, and allows the MutLalpha complex to bind and join in the detection and repair of DNA errors. These complexes coordinate with enzymes, including the DNA polymerase and exonuclease 1 (EXO1), to excise the mismatched region and resynthesize the corrected DNA strand28,29. Deficiencies or mutations in mismatch repair (MMR) proteins impair the body’s ability to correct replication errors. Properly forming MMR protein complexes is essential for recognizing and repairing abnormal DNA. When these proteins are mutated or their expression is lost, the MMR system fails to function effectively, allowing DNA replication errors to accumulate, particularly in microsatellite regions. This results in microsatellite instability (MSI), which significantly increases the risk of tumor development, especially in colorectal cancer [29]. Given the crucial role of ATPase activity in certain MMR proteins for their DNA mismatch repair function, phenolic compounds stably binding to the ATP domains of these proteins may modulate their ATPase activity, potentially enhancing repair function, which consequently reduces MSI.
Meanwhile, the docked proteins from the CIMP pathway revealed that rutin actively interacts with the Cyclin-dependent kinase inhibitor 2A (CDKN2A) via conventional hydrogen bonds with its Arg112, Ile145, Arg107, Asp105, and Arg144 residues, π-cation and π-alkyl interactions with Leu113, Ala143, and Arg144, and carbon hydrogen bonding at the Arg131 residue, with bond distances ranging from 3.43Å to 6.70Å within the ankyrin repeat domain which is responsible for regulating p16INK4a expression, suggesting that rutin may prevent CDKN2A silencing thereby restoring its tumor-suppressive functions and contributing to the inhibition of CRC cell proliferation [30]. Beyond its role in cell cycle regulation, CDKN2A is implicated in modulating the tumor immune microenvironment. High expression levels of CDKN2A have been associated with increased infiltration of immune cells, suggesting a potential role in enhancing antitumor properties. Likewise, rutin also exhibited strong interactions with Methylated- DNA-protein-cysteine methyltransferase (MGMT), specifically with Gly109, Ala170, His171, and Glu172, via conventional hydrogen bonds and carbon-hydrogen bonds (3.58Å – 4.60Å) within the ATPase domain, relatively expressing stable bonds with the mounted ligand. This could help restore MGMT activity, reducing the mutagenic effects of alkylating agents in CRC [31]. Likewise, π-Anion, π-Alkyl, and Alkyl bonds are also observed with Glu74, Pro73, and Lys107 (3.98Å- 4.69Å). Additionally, rutin also expressed adequately stable interactions with Metalloproteinase inhibitor 3 (TIMP3), forming four conventional hydrogen bonds with Gln108, Tyr390, His7, Arg100, and Glu99 in TIMP3 (4.26Å - 6.71Å). Moreover, the following hydrophobic bonds are also observed in TIMP3: Alkyl, π-Alkyl, and π-π stacked bonds with Val98, Ala11, and Phe97 (4.04Å - 5.83Å). Rutin formed strong hydrogen bonds within the ADAM-type metalloprotease domain of the TIMP3 protein, potentially inhibiting matrix metalloproteinases (MMPs). Given that MMPs contribute to ECM remodeling, cancer cell invasion, and metastasis, rutin’s binding to TIMP3 suggests potential inhibition of MMP activity, possibly limiting CRC metastasis by preserving ECM integrity [32]. Furthermore, epicatechin exhibited strong conventioznal hydrogen bonds (Ser465, Cys532) with the BRAF-V600E protein (3.63Å, 2.76Å), hydrophobic Alkyl and π-Alkyl interactions with Val471, Ile463, Ala481 residues (4.92Å - 6.80Å), and unfavorable donor-donor interaction with Lys483 (3.58Å) at the protein kinase domain. Since BRAF mutations drive CRC progression via the MAPK pathway, epicatechin binding suggests potential inhibitory effects by blocking the domain responsible for the activation of MEK/ERK protein that triggers the mentioned pathway [33].
Lastly, molecular docking of ligands to proteins responsible for the apoptotic mechanisms involved in CRC revealed multiple interactions between phenolic ligands and target proteins involved in colorectal cancer pathways. Rutin exhibited strong binding with AKT1, forming conventional hydrogen bonds with Val83 (4.44Å), Arg25 (6.02Å), and Lys14 (5.69Å, 4.30Å), alongside carbon hydrogen bonds with Lys14 and Gly16 (3.75Å, 3.64Å) within the pleckstrin homology domain potentially inhibiting excessive AKT signaling by impeding AKT1 membrane translocation and activation, thereby inhibiting its downstream pro-survival signaling cascade and ultimately disabling the resistance of cancer cells against other anticancer agents [34]. Similarly, rutin’s interaction with BAX involved multiple conventional hydrogen bonds (Asp33, Gln52, Ser60) and carbon hydrogen bonds (Pro49, Lys57, Glu61), including a π-alkyl interaction with Glu61 at 5.42Å within the Bcl-2 family domain which may enhance BAX activation and mitochondrial pore formation by stabilizing the conformational activation of BAX and facilitating the release of cytochrome c and initiating the intrinsic apoptosis pathway. Further analysis showed rutin binding effectively with CHK2 via conventional hydrogen bonding with Arg148, Val109, Lys135, and Asp101 (ranging from 4.21Å to 5.65Å), carbon hydrogen bonding with Gln100 (4.39Å), and a π-anion interaction with Glu149 (6.03Å, 4.63Å) within the protein kinase domain, which is essential for DNA damage-induced apoptosis, primarily through the phosphorylation of downstream apoptotic receptors such as p53 in these damaged cells [35]. The strongest binding interactions were observed with Caspase-8, where rutin formed conventional hydrogen bonds with Arg260, Gln358, Ser316, His317, Gly318, and Tyr365, with distances ranging from 2.78Å to 6.84Å. Additional interactions included π-cation bonding (Arg413), π-alkyl (Cys360), and π-donor hydrogen bonds (Arg413, His317), further highlighting the diverse binding modes of rutin. Rutin’s interaction with caspase-8 is within its catalytic domain, suggesting an enhanced caspase- 8-mediated apoptosis in CRC cells by mimicking the activity of the innate ligand responsible for the activation of this protease and triggering apoptosis via the extrinsic pathways that are often dysregulated in CRC30. These findings suggest that phenolic ligands establish crucial interactions with key CRC-related proteins, potentially influencing their biological activity.
III . Comparison with Chemotherapeutic Inhibitors
To further evaluate the effectiveness of the ligands against their target proteins, the binding affinities of chemotherapy drugs docked against the selected proteins were also calculated and compared to the binding scores of the leading ligands. Specifically, the binding scores of known inhibitors such as 5-fluorouracil, Irinotecan, Temozolomide, and Resveratrol are indicated in Table 3 to serve as the comparative basis for the effectiveness of the binding affinities of the phenolic ligands.
| S/N | Compound | MW | HBA | HBD | LogP | Lipinski’s Rule | Caco-2 | Intestinal Abs.(%) | BBB | CNS | CYP2D6 | CYP3A4 | Ames | Htox |
| 1 | Ellagic acid | 302.19 | 8 | 4 | 1.31 | Yes; 0 violation | 0.335 | 86. 684 | -1.272 | -3.533 | No | No | No | No |
| 2 | Epigallocatechin | 306.27 | 7 | 6 | 1.25 | Yes; 1 violation: NHorOH > 5 | -0.375 | 54.128 | -1.377 | -3.507 | No | No | No | No |
| 3 | Gallocatechin | 306.27 | 7 | 6 | 1.25 | Yes; 1 violation: NHorOH > 5 | -0.375 | 54.128 | -1.377 | -3.507 | No | No | No | No |
| 4 | Myricetin-3- O-galactoside | 480.38 | 13 | 9 | -0.83 | No: 2 violations: NorO > 10, NHorOH > 5 | -1.34 | 33.394 | -2.078 | -4.747 | No | No | No | No |
| 5 | Myricetin-3- O-pentoside | 450.35 | 12 | 8 | -0.19 | No; 2 violations: NorO >10, NHorOH > 5 | -0.973 | 42.509 | -1.789 | -4.435 | No | No | No | No |
| 6 | Myricetin-3- O-rhamnoside | 463.37 | 12 | 7 | 0.63 | No; 2 violations: NorO >10, NHorOH > 5 | 0.364 | 49.987 | -1.576 | -4.511 | No | No | No | No |
| 7 | Rutin | 610.52 | 16 | 10 | -1.69 | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 | -0.949 | 23.446 | -1.899 | -5.178 | No | No | No | No |
Among the tested drugs, Irinotecan consistently exhibited the strongest binding interactions across all target proteins, with the lowest binding affinities recorded at (-10.5 kcal/ mol) for Serine/threonine-protein kinase B-raf, (-10.3 kcal/ mol) for Metalloproteinase inhibitor 3 and RAC-alpha serine/threonine-protein kinase, and (-10.1 kcal/mol) for both Methylated-DNA-protein-cysteine methyltransferase and Serine/threonine-protein kinase Chk2. These values suggest that Irinotecan forms the most stable interactions, making it a potentially effective agent for targeting key proteins involved in colorectal cancer. Temozolomide demonstrated moderate binding affinity, with values ranging from (-5.3 kcal/mol) for Cyclin-dependent kinase inhibitor 2A to (-7.5 kcal/mol) for Metalloproteinase inhibitor 3 and RAC-alpha serine/threonine-protein kinase. Resveratrol was only tested for certain proteins and showed moderate binding, such as (-7.0 kcal/mol) for Serine/threonine-protein kinase Chk2 and (-6.8 kcal/ mol) for Apoptosis regulator BAX. Additionally, among the tested drugs, 5-Fluorouracil displayed the weakest binding interactions, with affinities ranging from (-4.3 kcal/mol) for RAC-alpha serine/threonine-protein kinase to (-5.8 kcal/mol) for Metalloproteinase inhibitor 3. These values indicate that 5-Fluorouracil may be less effective in targeting these proteins compared to Irinotecan and Temozolomide.
When comparing the molecular docking results of chemotherapy drugs with those of phenolic compounds, it is evident that phenolic compounds exhibit comparable or even stronger binding affinity for key colorectal cancer- related proteins (Table 1). For the CpG Island Methylator Phenotype (CIMP) Pathway, Irinotecan had the strongest binding affinity to Metalloproteinase inhibitor 3 (-10.3 kcal/mol), while the strongest phenolic compound, rutin, showed a slightly weaker affinity at (-9.6 kcal/mol). Similarly, Irinotecan is bound to Serine/threonine-protein kinase B-raf at (-10.5 kcal/mol), whereas the strongest phenolic compound, gallocatechin, had a binding affinity of (-10.0 kcal/mol). In the Chromosomal Instability (CIN) Pathway, phenolic compounds such as rutin (-8.8 kcal/ mol), myricetin-3-O-rhamnoside (-8.4 kcal/mol), and ellagic acid (-8.2 kcal/mol) demonstrated significantly higher binding affinities than chemotherapy drugs like 5-Fluorouracil (-5.4 kcal/mol) and Temozolomide (-6.1 kcal/mol) for p53, though Irinotecan (-9.7 kcal/mol) remained the highest binding score. Similarly, for SMAD4, rutin (-8.0 kcal/mol) exhibited high affinity, nearly comparable to Irinotecan (-8.1 kcal/mol) and outperforming 5-Fluorouracil (-4.8 kcal/mol) and Temozolomide (-5.5 kcal/mol).
Although chemotherapy drugs generally exhibited stronger binding, certain phenolic compounds, such as gallocatechin and laricitrin-3-O-galactoside (-9.9 kcal/mol), showed competitive binding efficiencies. In the Apoptosis Mechanism Pathway, Irinotecan also demonstrated the lowest binding affinity for Caspase-8 (-9.6 kcal/mol), which was stronger than rutin (-8.6 kcal/ mol) and epigallocatechin (-8.4 kcal/mol). Similarly, for Serine/threonine-protein kinase Chk2, Irinotecan had the strongest binding affinity at (-10.1 kcal/mol), surpassing rutin (-8.5 kcal/mol) and epigallocatechin (-8.3 kcal/mol). These findings indicate that while phenolic compounds exhibit strong interactions with apoptosis-related proteins, chemotherapy drugs generally show stronger and more stable binding. Moreover, in the Microsatellite Instability (MSI) pathway, myricetin-3-O-galactoside and myricetin- 3-O-rhamnoside (-10.1 kcal/mol) had higher binding affinities than Irinotecan (-8.8 kcal/mol), as well as 5-Fluorouracil (-5.0 kcal/mol) and Temozolomide (-6.2 kcal/mol) for MLH1. A similar trend was observed for MSH2, where myricetin-3-O-rhamnoside (-10.0 kcal/mol) and rutin (-9.4 kcal/mol) exhibited high affinities, closely following Irinotecan (-10.8 kcal/mol) and surpassing both 5-Fluorouracil (-4.7 kcal/mol) and Temozolomide (-5.6 kcal/mol).
The comparison of molecular docking results between chemotherapy agents and phenolic compounds shows their potential as therapeutic drugs for cancer. Chemotherapy drugs like Irinotecan have demonstrated strong and stable interactions with critical proteins, which is consistent with their established role in improving survival rates through targeted mechanisms [36]. Although these phenolic compounds may display competitive binding efficiency, they still have lower affinities compared to traditional chemotherapy drugs; hence, natural compounds may serve their purpose better by complementing cancer therapies rather than replacing them [37]. Nonetheless, these compounds’ potential to improve CRC treatment approaches still shows potential in interacting with proteins linked to apoptosis, especially when combined with well-known chemotherapeutic agents like Irinotecan.
IV. Molecular Dynamics Simulation & MM-GBSA (RMSD & RMSF)
Root Mean Square Deviation (RMSD) measures the average distance between atoms of superimposed molecules and is commonly used in bioinformatics to assess the similarity of protein complexes. In molecular dynamics simulation (MDS), the root mean square deviation is used to investigate the behavior of proteins. It is also useful for investigating the structural stability of a protein complex [38]. However, the native structure of the protein must be known in advance to serve as a reference for the stability of the structure [39]. In the CIN Pathway, the 7BWN protein (Figure 3.A.1) showed continued structural shifts up to 3.0 Å, whereas its rutin-bound complex stabilized earlier at 1.5-2.0 Å. The 1DD1 (Figure 3.B.1) exhibited the same structural shifts with values reaching <2.5 Å, while its ligand bound complex also stabilized earlier at 1.0-1.5 Å. Similarly, for MSI Pathway, 4P7A (Figure 3.C.1) displayed a major instability spike (~6.0 Å at frame 900), while its myricetin 3’-galactoside complex maintained a stable 0.5-1.0 Å RMSD. 2O8B protein (Figure 3.D.1) exhibited extreme fluctuations (7.5-17.5 Å between frames 200- 400), whereas the 2O8B + myricetin 3’-rhamnoside complex stabilized within 0.3-1.0 Å. The TIMP3 and BRAF-V600E (Figure 3.E.1 and 3.F.1] proteins, for CIMP Pathway, exhibited gradual RMSD increases, reaching 2.0 Å and 3.0 Å, respectively, with persistent fluctuations. Their ligand-bound counterparts, TIMP3 + Rutin and BRAF-V600E + Epicatechin, stabilized much earlier with RMSD values below 1.0 Å and 0.75 Å. In the Apoptosis Mechanism Pathway (Figure 3.G.1 and 3.H.1), Caspase-8’s fluctuations (1.0-2.0 Å) suggest structural rearrangement, whereas its rutin-bound complex stabilized between 0.5-1.5 Å, showing less conformational change. Lastly, CHEK2 exhibited spikes exceeding 2.5 Å, while CHEK2 + Rutin stabilized at 1.5-2.2 Å. In contrast, the protein-ligand complexes consistently show lower RMSD values and faster stabilization than the unbound proteins. Similar results have been documented in the study of Islam and Shibly, which shows that protein-ligand complexes have lower RMSD, suggesting stability and structural preservation [40]. The stabilization is due to the ligand binding to the protein, which highlights the ligand’s role in improving the protein stability and reducing conformational shifts.
Similar to RMSD, Root Mean Square Fluctuations (RMSF) is a numerical measurement of the positional differences of a residue over time that indicates its flexibility or how much a residue fluctuates over a simulation [41]. High RMSF values indicate increased dynamic and flexibility, while regions with low RMSF values are typically more rigid and structurally stable as observed with residues showing limited motion during molecular dynamic simulation. Thus, the considered acceptable fluctuation value for a small protein is less than 2Å which approximately contributes to the protein’s function to fluctuate around a stable conformation [42]. Hence, the RMSF values discussed below are the most stable protein structure observed for each pathway. Based on the RMSF values, the highest fluctuation peaks at the first residue were observed in (Figure 3.D.2) at (GLY1=44.94Å) and (Figure 3.B.2) at (ASN1= 3.49Å) which suggests terminal flexibility followed by a spike that dramatically drop at (ALA6= 3.85Å) and (GLY4= 0.58Å) respectively, a trend found similarly in (Figure 3.G.2), where an initial peak in the first residue (SER1= 1.57Å) declined before a major fluctuation at (GLY152=5.98Å). In contrast, (Figure 3.C.2) showed the highest fluctuations at (GLU831= 5.58), particularly towards the C-terminus of RMSF values, a pattern also observed in (Figure 3.A.2), where the highest peak within (GLY251=5.13Å) followed by (ARG252=4.94Å) which are both concentrated in the later part of the region, where multiple values above 4 are observed. This suggests the potential pattern that could indicate significant variability in the end terminal with increased mobility, allowing interaction with other molecules. In addition, (Figure 3.H.2) displayed different fluctuations at residues (GLU1 to TYR464), resembling the flexible regions of (Figure 3.F.2), where (PRO409=3.08Å) exhibited significant fluctuation. While, stable regions are observed in (Figure 3.G.2) from GLU160 to LYS240, which fluctuated between 0.4 and
1.2 and (Figure 3.E.2) from residues GLU116 to THR121 which has a value of less than 1 which suggests that despite the multiple fluctuations there are still relatively rigid and stable regions in structured elements, suggesting a balance between flexible and stable regions. Overall, the interplay of both rigidity and flexibility among proteins’ regions are necessary to achieve optimal performance as indicated in the protein’s ability to undergo change for ligand binding.
V. Drug-Likeness and ADMET Prediction
SwissADME was used to evaluate the molecular features specified in Lipinski’s rule of five and to determine the drug-likeness of the front runners derived from the molecular docking results. As shown in Table 3, only three compounds ellagic acid, epigallocatechin, and gallocatechin demonstrated favorable drug-likeness profiles, adhering to Lipinski’s rule of five with no more than one violation.
| Distribution | Metabolism | Excretion | Toxicity | |||||||
| Int. abs | P-gp | BBB | CNS | CYP2D6 | CYP3A4 | TC | Ames | MTD | LD50 | Htox |
| 86.684 | - | -1.272 | -3.533 | No | No | 0.537 | No | 0.476 | 2.399mol/kg | No |
| 54.128 | - | -1.377 | -3.507 | No | No | 0.328 | No | 0.506 | 2.492mol/kg | No |
| 54.128 | - | -1.377 | -3.507 | No | No | 0.328 | No | 0.506 | 2.492mol/kg | No |
| 33.394 | - | -2.078 | -4.747 | No | No | 0.413 | No | 0.499 | 2.543mol/kg | No |
| 42.509 | - | -1.789 | -4.435 | No | No | 0.303 | No | 0.454 | 2.536mol/kg | No |
| 49.987 | - | -1.576 | -4.511 | No | No | 0.395 | No | 0.443 | 2.533mol/kg | No |
| 23.446 | - | -1.899 | -5.178 | No | No | -0.369 | No | 0.452 | 2.491mol/kg | No |
Other compounds, including myricetin- 3-O-galactoside, myricetin-3-O-pentoside, myricetin- 3-O-rhamnoside, and rutin, exhibited 2 to 3 violations, primarily due to exceeding the acceptable number of hydrogen bond acceptors and donors. The results show that ellagic acid, epigallocatechin, and gallocatechin possess the appropriate physicochemical properties necessary for adequate absorption and distribution in the body, highlighting their potential as orally bioavailable drug candidates. The researchers used the study of Egbuna et al. from 2023 as their basis for interpreting the results. As mentioned in the study, the Lipinski’s rule of 5 claims that there is a greater probability of poor absorption when there are more than (i) five hydrogen-bond donors, (ii) ten hydrogen-bond acceptors, (iii) a molecular weight greater than 500, and (iv) a computed Log P (cLogP) greater than 5. Compounds with two or more violations are likely to exhibit suboptimal oral bioavailability, which can significantly reduce the compound’s ability to enter systemic circulation effectively [20].
The ADMET properties of selected phenolic compounds having the most favorable binding affinities were determined using the pkCSM platform to evaluate their potential as safe therapeutic agents. The values for each ADMET parameter are summarized in Table 4. The interpretation of the ADMET profiles is primarily based on the tabulated acceptable ranges and significance of parameters outlined in the study of Egbuna et al. [20]. The potential absorption of the selected leading phenolic compounds was mainly analyzed through the parameters of Caco-2 cell permeability and intestinal absorption. Myricetin-3-O-rhamnoside had the highest Caco-2 cell permeability value at 0.364. As for intestinal absorption, ellagic acid demonstrated the highest value at approximately 87%, while rutin ranked lowest at approximately 23%. These findings suggest that while myricetin-3-O-rhamnoside exhibits the highest Caco-2 permeability among the compounds, its value remains insufficient for predicting effective oral drug absorption in the intestinal lining. In terms of intestinal absorption, all compounds except rutin exhibited high potential. Under distribution, the compounds were evaluated using the blood-brain barrier (BBB) and central nervous system (CNS) permeability parameters. For BBB permeability, all of the compounds demonstrated values lower than -1. Similarly, CNS permeability values for all compounds were below -3. Based on these values, all compounds are likely to demonstrate limited brain distribution, and none of them would be able to penetrate the CNS effectively. The collective results for both parameters indicate that all compounds are likely to target CRC cells without significantly interacting with the BBB and CNS. Under metabolism, none of the phenolic compounds were metabolized and deactivated by CYP2D6 and CYP3A4, indicating a lower risk of drug interactions, but this does not signify that the compounds are not affected by any metabolic pathways and enzymes. The potential toxicity of the compounds was assessed using the parameters of Ames, a known mutagenicity predictor, and hepatotoxicity. All compounds showed negative results for both parameters, positively impacting their potential as therapeutic agents, as they are unlikely to cause cellular genetic changes and liver damage.
In summary, the ADMET profiles of the selected phenolic compounds showed variation in the absorption parameters of Caco-2 cell permeability and intestinal absorbance, but were largely uniform in results across distribution, metabolism, and toxicity parameters. Hence, choosing the most favorable compounds based on their ADMET profiles depends on the differences in their absorption properties. All tested compounds demonstrated low Caco-2 cell permeability values, indicating a need for further investigation to improve oral bioavailability or explore alternative routes of administration. The phenolic compounds with the greatest potential for this parameter include myricetin-3-O-rhamnoside, followed closely by ellagic acid, and epigallocatechin and gallocatechin having the exact similar values. As for intestinal absorption, ellagic acid demonstrated the highest estimated drug absorption percentage, followed by epigallocatechin and gallocatechin with similar values, and myricetin-3-O-rhamnoside. Considering data for all ADMET parameters, ellagic acid, myricetin-3-O- rhamnoside, epigallocatechin, and gallocatechin were identified as the four most promising compounds for further development as safe therapeutic agents targeting CRC pathways. The findings of this study are in line with previous findings, which reported that rutin is predicted to have low gastrointestinal absorption, and that both rutin and myricetin-3-O-rhamnoside cannot cross the BBB and inhibit metabolic CYP450 enzymes such as CYP2D6 and CYP3A4, suggesting that these compounds are nonhepatotoxic [43]. Similar findings indicated that ellagic acid has an absorption rate of 86.7%, supporting its potential as an effective therapeutic agent, especially in the context of CRC therapy [44].
In conclusions, this study demonstrates the strong inhibitory potential of Syzygium cumini phenolic derivatives against key colorectal cancer (CRC)-associated proteins through molecular docking and dynamics simulations. The collected data revealed that myricetin-3-O-galactoside, rutin, and gallocatechin exhibited high binding affinities for crucial oncogenic and tumor-suppressor proteins, such as MLH1, p53, and BRAF, suggesting their role in disrupting CRC progression. Meanwhile, molecular dynamics simulations further confirmed the structural stability of these ligand-protein complexes, indicating their potential to modulate oncogenic pathways by reducing conformational flexibility. The phenolic ligands formed strong hydrogen bonds and hydrophobic interactions with key CRC-associated residues, with myricetin derivatives reinforcing DNA mismatch repair and rutin restoring tumor-suppressor function. Computational analyses also highlighted the ability of these compounds to interfere with Wnt/β-catenin signaling, PI3K/AKT activation, and DNA repair deficiencies, suggesting their role in tumor suppression and metastasis inhibition. ADMET analysis revealed that while myricetin-3-O-rhamnoside showed limited intestinal permeability, ellagic acid and gallocatechin exhibited better absorption and non-toxic properties, making them promising drug candidates. Although these findings support the therapeutic potential of S. cumini phenolic derivatives, further in vitro, in vivo, and formulation studies are necessary to optimize their pharmacokinetic properties and clinical applicability.
Acknowledgements
Author contribution
All the authors have thoroughly discussed, analyzed, and reported the findings of this study as well as written their respective parts in this article. K.L.B: conceptualizing the methodology, designing content, writing, reviewing, and editing; N.B.D.A., A.J.A.B., R.B.M.A., A.J.P.B., M.J.D.C.B., E.A.O.B.: writing, designing content,reviewing, editing; E.A.A.C: writing, reviewing, and editing. All the authors have read and gave their consent in the publication of this article.
Funding
None to declare.
Conflict of interest
None to declare.
Ethics approval
None to declare.
References
- Modelling incidence and mortality cancer parameters with respect to GLOBOCAN 2020Age standardized world estimates Acquah J, Bosson-Amedenu S, Eyiah-Bediako F, Buabeng A, Ouerfelli N. Heliyon.2024;10(17). CrossRef
- Flavonoid-based polymeric nanoparticles: A promising approach for cancer and diabetes treatment Maity S, Acharyya A, Sankar Chakraborti A. European Polymer Journal.2022;177. CrossRef
- World Health Organization. Colorectal cancer [Internet]. World Health Organization. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer .
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- Global Cancer Observatory. Philippines: Statistics at a glance, 2022 [Internet] 2022. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/608-philippines-fact-sheet.pdf.
- Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: A narrative review Doustmihan A, Fathi M, Mazloomi M, Salemi A, Hamblin MR , Jahanban-Esfahlan R. Journal of Controlled Release: Official Journal of the Controlled Release Society.2023;363. CrossRef
- Targeting cancer stem cells as therapeutic approach in the treatment of colorectal cancer Parizadeh SM , Jafarzadeh-Esfehani R, Hassanian SM , Parizadeh SMR , Vojdani S, Ghandehari M, Ghazaghi A, et al . The International Journal of Biochemistry & Cell Biology.2019;110. CrossRef
- Medicinal Plants in the Prevention and Treatment of Colon Cancer Aiello P, Sharghi M, Mansourkhani SM , Ardekan AP , Jouybari L, Daraei N, Peiro K, et al . Oxidative Medicine and Cellular Longevity.2019;2019. CrossRef
- Plant-derived bioactive compounds in colon cancer treatment: An updated review Esmeeta A, Adhikary S, Dharshnaa V, Swarnamughi P, Ummul Maqsummiya Z, Banerjee A, Pathak S, Duttaroy AK . Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2022;153:113384. CrossRef
- Targeting cancer stem cells and signalling pathways through phytochemicals: A promising approach against colorectal cancer Liao W, Zhang L, Chen X, Xiang J, Zheng C, Chen N, Zhao M, et al . Phytomedicine: International Journal of Phytotherapy and Phytopharmacology.2023;108. CrossRef
- Medicinal plants with anti-colorectal cancer bioactive compounds: Potential game-changers in colorectal cancer management Macharia JM , Mwangi RW , Rozmann N, Zsolt K, Varjas T, Uchechukwu PO , Wagara IN , Raposa BL . Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2022;153. CrossRef
- Antiproliferative Activity and Apoptotic Efficiency of Syzygium cumini Bark Methanolic Extract against EAC Cells In Vivo Siddika A, Das PK , Asha SY , Aktar S, Tareq ARM , Siddika A, Rakib A, Islam F, Khanam JA . Anti-Cancer Agents in Medicinal Chemistry.2021;21(6). CrossRef
- Molecular Complexity of Colorectal Cancer: Pathways, Biomarkers, and Therapeutic Strategies Yang Z, Wang X, Zhou H, Jiang M, Wang J, Sui B. Cancer Management and Research.2024;16. CrossRef
- Medicinal Potential of Jamun (Syzygium cumini Linn): A Review Ahmad N, Nawab M, Kazmi MH . Journal of Drug Delivery and Therapeutics.2019;9(5). CrossRef
- Jamun (Syzygium cumini (L.) Skeels): The conventional underutilized multifunctional plant-an exotic gleam into its food and functional significance Kumar S, Sharma S, Kumar V, Sharma A, Kaur R, Saini R. Industrial Crops and Products.2023;191. CrossRef
- Speed vs Accuracy: Effect on Ligand Pose Accuracy of Varying Box Size and Exhaustiveness in AutoDock Vina Agarwal R, Smith JC . Molecular Informatics.2023;42(2). CrossRef
- Relevance of Molecular Docking Studies in Drug Designing Jakhar R, Dangi M, Khichi A, Chhillar AK . Current Bioinformatics.2020;15(4). CrossRef
- Investigating the binding affinity, molecular dynamics, and ADMET properties of curcumin-IONPs as a mucoadhesive bioavailable oral treatment for iron deficiency anemia Alsedfy MY , Ebnalwaled A. A., Moustafa M, Said AH . Scientific Reports.2024;14(1). CrossRef
- Wnt/β-catenin signaling pathway inhibitors, glycyrrhizic acid, solanine, polyphyllin I, crocin, hypericin, tubeimoside-1, diosmin, and rutin in medicinal plants have better binding affinities and anticancer properties: Molecular docking and ADMET study Egbuna C, Patrick-Iwuanyanwu KC , Onyeike EN , Uche CZ , Ogoke UP , Riaz M, Ibezim EN , et al . Food Science & Nutrition.2023;11(7). CrossRef
- In Silico ADME/T Properties of Quinine Derivatives using SwissADME and pkCSM Webservers Mvondo JGM , Matondo A, Mawete DT , Bambi SN , Mbala BM , Lohohola PO . International Journal of TROPICAL DISEASE & Health.2021. CrossRef
- In silico Pharmacokinetic and Toxicity Prediction of Compounds from Andrographis paniculata (Burm.f.) Nees Izatunnafis I, Murti YB , Sudarmanto BSA . Journal of Food and Pharmaceutical Sciences.2023;:830-38. CrossRef
- pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures Pires DEV , Blundell TL , Ascher DB . Journal of Medicinal Chemistry.2015;58(9). CrossRef
- Oncogenic Mutations in Armadillo Repeats 5 and 6 of β-catenin Reduce Binding to APC, Increasing Signaling and Transcription of Target Genes Liu P, Liang B, Liu M, Lebbink JH , Li S, Qian M, Lavrijsen M, et al . Gastroenterology.2020;158(4). CrossRef
- The importance of protein domain mutations in cancer therapy Chitluri KK , Emerson IA . Heliyon.2024;10(6). CrossRef
- PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis Alqahtani A, Ayesh HSK , Halawani H. Cancers.2019;12(1). CrossRef
- Multiple roles of mothers against decapentaplegic homolog 4 in tumorigenesis, stem cells, drug resistance, and cancer therapy Dai C, Cao Y, Huang F, Wang Y. World Journal of Stem Cells.2022;14(1). CrossRef
- Microsatellite instability and mismatch repair protein deficiency: equal predictive markers? Nádorvári ML , Lotz G, Kulka J, Kiss A, Tímár J. Pathology and Oncology Research.2024;30. CrossRef
- Microsatellite Instability: Diagnosis, Heterogeneity, Discordance, and Clinical Impact in Colorectal Cancer Evrard C, Tachon G, Randrian V, Karayan-Tapon L, Tougeron D. Cancers.2019;11(10). CrossRef
- The Regulatory Mechanisms of Tumor Suppressor P16INK4A and Relevance to Cancer Li J, Poi MJ , Tsai MD . Biochemistry.2011;50(25). CrossRef
- The Versatile Attributes of MGMT: Its Repair Mechanism, Crosstalk with Other DNA Repair Pathways, and Its Role in Cancer Fang Q. Cancers.2024;16(2). CrossRef
- Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics Raeeszadeh-Sarmazdeh M, Do LD , Hritz BG . Cells.2020;9(5). CrossRef
- BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies Wang PP , Lin C, Wang J, Margonis GA , Wu B. Cancers.2022;14(17). CrossRef
- Identification of AKT1/β-catenin mutations conferring cetuximab and chemotherapeutic drug resistance in colorectal cancer treatment Hasbal-Celikok G, Aksoy-Sagirli P, Altiparmak-Ulbegi G, Can A. Oncology Letters.2021;21(3). CrossRef
- BAX BCL2 associated X, apoptosis regulator [Homo sapiens (human)] - Gene - NCBI [Internet]. Available from: https://www.ncbi.nlm.nih.gov/gene/581 .
- Colorectal Cancer: Current Updates and Future Perspectives Marcellinaro R, Spoletini D, Grieco M, Avella P, Cappuccio M, Troiano R, Lisi G, et al . Journal of Clinical Medicine.2023;13(1). CrossRef
- Assessing the antioxidant properties of Naringin and Rutin and investigating their oxidative DNA damage effects in breast cancer Pravin B, Nanaware V, Ashwini B, Wondmie GF , Jardan YAB , Bourhia M. Scientific Reports.2024;14(1). CrossRef
- Mutation-induced change in chignolin stability from π-turn to α-turn Maruyama Y, Koroku S, Imai M, Takeuchi K, Mitsutake A. RSC advances.2020;10(38). CrossRef
- Analysis of Protein Folding Simulation with Moving Root Mean Square Deviation Maruyama Y, Igarashi R, Ushiku Y, Mitsutake A. Journal of Chemical Information and Modeling.2023;63(5). CrossRef
- Exploring Bryophyllum pinnatum compounds as potential inhibitors for Vespula vulgaris allergen proteins: A systematic computational approach Islam S, Shibly AZ . Heliyon.2024;10(15). CrossRef
- RMSD/RMSF Analysis | BioChemCoRE 2018 [Internet]. Available from: https://ctlee.github.io/BioChemCoRe-2018/rmsd-rmsf/ .
- Molecular dynamics and simulation analysis against superoxide dismutase (SOD) target of Micrococcus luteus with secondary metabolites from Bacillus licheniformis recognized by genome mining approach Bagewadi ZK , Yunus Khan T. M., Gangadharappa B, Kamalapurkar A, Mohamed Shamsudeen S, Yaraguppi DA . Saudi Journal of Biological Sciences.2023;30(9). CrossRef
- An In-Depth Study on the Metabolite Profile and Biological Properties of Primula auriculata Extracts: A Fascinating Sparkle on the Way from Nature to Functional Applications Kurt-Celep I, Zheleva-Dimitrova D, Gevrenova R, Uba AI , Zengin G, Yıldıztugay E, Picot-Allain CMN , Lorenzo JM , Mahomoodally MF , Montesano D. Antioxidants (Basel, Switzerland).2022;11(7). CrossRef
- Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study Kiani HS , Ahmad W, Nawaz S, Farah MA , Ali A. Molecules (Basel, Switzerland).2023;28(18). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details