Relationship Between Red Cell Distribution Width (RDW) and Histopathological Grading and Metastasis in Breast Cancer Patients at Wahidin Sudirohusodo Hospital, Makassar, Indonesia
Download
Abstract
Background: Breast cancer (BC) is a significant global health concern with an increasing incidence. Identifying simple and affordable prognostic indicators for early detection and treatment guidance is crucial. Red Cell Distribution Width (RDW), a hematologic parameter reflecting erythrocyte volume variation, has the potential to indicate systemic inflammation and cancer progression. This study aimed to explore the role of RDW as a prognostic factor in BC patients.
Methods: This cross-sectional study involved secondary data from 517 BC patients. The data included age, RDW values, histopathological grade, and metastasis status. Statistical analysis utilized independent T-tests, ANOVA, linear regression, correlation analysis, and Receiver Operating Characteristic (ROC) curves, with a significance level set at ρ<0.05.
Results: The mean RDW was 15.4 ± 9.3%. Significant differences in RDW were observed across histopathological grades (Grade 1: 13.29 ± 1.62; Grade 2: 14.53 ± 6.90; Grade 3: 18.28 ± 14.03; ρ<0.001) and between patients with metastasis (16.49 ± 10.0) and without metastasis (15.02 ± 9.01; ρ=0.001). A 1% increase in RDW was associated with a 0.012 increase in histopathological grade (95% CI: 0.006-0.017; ρ=0.001) and a 1.016 times increased odds of metastasis (OR: 1.016; 95% CI: 1.001-1.039; ρ=0.001). The Area Under the Curve (AUC) of the ROC curve for the highest histopathological grade was 0.841 (sensitivity 83.8%, specificity 78.1%), and for metastasis prediction, the AUC was 0.624 (sensitivity 80.6%, specificity 28.7%).
Conclusion: RDW demonstrates a significant positive correlation with histopathological grade and metastasis in BC patients, suggesting its potential as an accessible prognostic indicator.
Introduction
Breast cancer (BC) is a malignancy of the breast tissue originating from the ductal or lobular epithelium [1]. In 2020, BC became the most commonly diagnosed cancer worldwide, surpassing lung and prostate cancer [2, 3]. In Indonesia, the estimated incidence of BC is
40.3 per 100,000 women, with approximately 48,998 new cases annually [1]. Consequently, Indonesia is among the countries facing a double burden, simultaneously dealing with high morbidity rates from both infectious and non-infectious diseases [4].
Currently, several diagnostic biomarkers are being developed for early screening of BC; however, their clinical use remains limited due to uncertain roles and high costs [5]. Therefore, it is crucial to identify a simple, convenient, and sensitive prognostic indicator for early tumor detection and as a guide in treatment decision-making.
Long-term inflammation is associated with the tumor microenvironment, which is closely linked to tumor aggressiveness and progression. Therefore, hematological parameters can serve as markers of systemic inflammatory response. One such parameter measured in a complete blood count is Red Cell Distribution Width (RDW). RDW is a simple and relatively inexpensive parameter that assesses the heterogeneity of red blood cell volume and is associated with anemia [6].
In recent years, RDW has been used to assess various non-hematologic diseases, including cancer. An increase in RDW may indicate systemic inflammation and the progression of several types of cancer, such as esophageal cancer, hepatocellular carcinoma, colorectal carcinoma, and BC [5].
This study aims to explore alternative screening methods and/or prognostic factors for BC, with the hope of assisting developing countries in enhancing breast tumor screening programs and early detection efforts in the future.
Materials and Methods
This study is an analytical observational study with a cross-sectional approach. Sample data were collected from the medical records of Wahidin Sudirohusodo Hospital over a three-year period (2018–2020), The sampling technique is probability sampling using the total sampling method. Demographic and clinical data were collected: age, RDW, histopathological grading, metastasis, and metastasis location.
Inclusion criteria: 1) Complete medical record data of BC patients, 2) Patients with metastatic BC as evidenced by radiological examination (Thorax x-ray, USG, CT scan or MRI), 3) Women with BC aged 20–70 years, 4) BC patients who have had RDW levels checked before surgery. Exclusion criteria: 1) Patients with anemia such as iron deficiency anemia, folic acid deficiency anemia, and thalassemia, 2) Suffering from other types of cancer (e.g. ovarian, liver, lung cancer), and 3) patients with chronic inflammation or nutritional deficiencies.
Statistical analysis was performed using SPSS version 26. T-tests, ANOVA, and ROC curve analysis were used to assess the relationship between the variables. A result was considered statistically significant if p<0.05.
Red cell distribution width (RDW)
RDW, in this case the RDW-CV, is a quantitative measurement of the size variation of circulating erythrocytes, as a mathematical expression of the variation in the volume distribution of the erythrocyte population expressed in percent and indicates the variation in erythrocyte size in the measured population (anisocytosis).
Histopathologic grading
Histopathologic grading of BC is divided into 3 groups of low, moderate and high grade (based on anatomical pathology examination) determined from mitotic index, tubular formation and nuclear pleomorphism (based on Scarff-Bloom-Richardson modification), divided into three groups: low, moderate, high.
Metastasis
BC that has metastasized, which means the cancer has spread beyond its original site, leads to symptoms that correspond to the location of the metastasis. The most common sites of BC metastasis are the bones, liver, lungs, and brain.
Results
This study involved 517 patients with BC, and their baseline characteristics are presented in Table 1.
| Characteristics | Total (n=517) |
| Age, mean ± SD (years) | 49.6 ± 8.8 |
| Histopathological grading, n (%) | |
| 1 | 55 (10.6) |
| 2 | 320 (61.6) |
| 3 | 142 (27.5) |
| RDW (mean ± SD) | 15.4 ± 9.3 |
| Metastasis, n (%) | 144 (27.9) |
| Metastasis location, n (%) | |
| Lung | 35 (6.8) |
| Liver | 31 (6.0) |
| Bone | 25 (4.8) |
| Lung and liver | 2 (0.4) |
| Liver and bone | 9 (1.7) |
| Lung and bone | 14 (2.7) |
| Lung, liver, and bone | 3 (0.6) |
| Brain | 12 (2.3) |
| Brain and bone | 2 (0.4) |
| Lung and brain | 1 (0.2) |
| Lung and intestinal | 1 (0.2) |
| Lymph node | 5 (1.0) |
| Lung and lymph node | 1 (0.2) |
| Spinal (vertebral) | 1 (0.2) |
Note; SD, mean ± standard deviation.
The results showed that 55 patients (10.6%) had histopathological grade 1, 320 patients (61.9%) had grade 2, and 142 patients (27.5%) had grade 3. The overall mean RDW was 15.4. Additionally, the most common sites of metastasis among BC patients were the lungs (35 patients, 6.8%), followed by the liver (31 patients, 6.0%), and the bones (25 patients, 4.8%).
In Table 2, the RDW values for each histopathological grade were as follows: 13.29±1.62 for grade 1, 14.53±6.90 for grade 2, and 18.28±14.03 for grade 3. Bivariate analysis showed a significant difference in RDW values among histopathological grades 1, 2, and 3 in patients with BC.
| Characteristics | Grade 1 | Grade 2 | Grade 3 | p-value |
| (n=55) | (n=320) | (n=142) | ||
| Mean + SD | Mean + SD | Mean + SD | ||
| RDW value | 13.29 ± 1.62 | 14.53 ± 6.90 | 18.28 ± 14.03 | 0.001* |
Note, * Significant if p<0.05, ANOVA Test
In Table 3, the RDW value in the metastasis group was 16.49±10.0, while in the non-metastasis group, it was 15.02±9.01. Bivariate analysis showed a significant difference in RDW values between the metastasis and non-metastasis groups in BC patients.
| Characteristics | Metastasis | p-value | |
| Yes | No | ||
| (n=144) | (n=373) | ||
| Mean + SD | Mean + SD | ||
| RDW value | 16.49 ± 10.0 | 15.02 ± 9.01 | 0.001 |
Note, * Significant if p<0.05, T-Test.
In Table 4, the relationship between RDW values and histopathological grading shows that for every 1% increase in RDW, there is an associated 0.012 increase in histopathological grading in BC patients.
| Characteristics | Histopathological grading | p-value | |
| β | CI | ||
| RDW value | 0.012 | (0.006-0.017) | 0.001 |
Note, * Significant if p<0.05, OR = Odds Ratio, CI = Convidence Interval
In Table 5, the relationship between RDW values and metastasis shows that for every 1% increase in RDW, there is a 1.016 times increase in the likelihood of metastasis in BC patients.
| Characteristics | Metastasis | p-value* | |
| OR | CI | ||
| RDW value | 1.016 | (1.001-1.039) | 0.001 |
Note, * Significant if p<0.05, Linear Regresion, OR = Odds Ratio, CI = Convidence Interval
In Table 6, the correlation coefficient (R) for histopathological grading was 0.183 with a p-value of 0.001, while for metastasis, the correlation coefficient (R) was 0.171 with a p-value of 0.001.
| Characteristics | R* | p-value** |
| Histopathological grading | 0.183 | 0.001 |
| Metastasis | 0.171 | 0.001 |
Note, * Correlation coefficient, ** Significant if p<0.05
The positive R values indicate that an increase in RDW is correlated with both higher histopathological grading and the presence of metastasis, although the strength of the correlation is considered weak. Statistically, the p-value < 0.05 for both variables suggests that this relationship is significant.
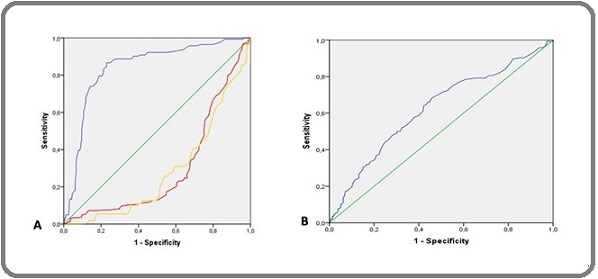
In this study, a Receiver Operating Characteristic (ROC) curve analysis was conducted to evaluate the ability of RDW to differentiate BC patients based on histopathological grading (1, 2, and 3). The results showed that in Figure 1A, Histopathological Grade 1 as “Yellow line” the AUC value was 0.286 with a 95% confidence interval (CI) of 0.221 – 0.350, a sensitivity of 65.5%, and a specificity of 15.2%. Histopathological Grade 2 as “Red Line” the AUC value was 0.298 with a 95% CI of 0.249 – 0.347, a sensitivity of 69.1%, and a specificity of 18.3%. Meanwhile, Histopathological Grade 3 as “Blue line”, the AUC value was 0.841 with a 95% CI of 0.802 – 0.880, a sensitivity of 83.8%, and a specificity of 78.1%. Furthermore, RDW demonstrated moderate predictive ability for metastasis the AUC value was 0.624 with a 95% confidence interval (CI) of 0.569 – 0.679, a sensitivity of 80.6%, and a specificity of 28.7% (Figure 1B).
Figure 1. ROC Curve: A) Showing the relationship between RDW values and histopathological grading in BC patients, Yellow: Histopathological Grade 1, Red: Histopathological Grade 2, Blue: Histopathological Grade 3. B) Showing the relationship between RDW values and BC metastasis.
Discussion
This study found that most patients had histopathological grade 2 (61.9%), with grade 1 and grade 3 accounting for 10.6% and 27.5%, respectively. This is consistent with previous studies indicating that grade 2 (moderate differentiation) is the most common in BC, suggesting intermediate malignancy [7]. Metastasis analysis revealed the lungs were the most frequent site (6.8%), followed by the liver (6.0%) and bones (4.8%), which aligns with typical BC metastatic patterns. Lung metastasis often signifies more aggressive tumors, while bone metastasis indicates advanced disease [8, 9].
Correlation analysis revealed a significant association between RDW levels and histopathological grading in BC patients. RDW was higher in grade 3 (18.28±14.03) compared to grades 1 and 2 (13.29±1.62 and 14.53±6.90, respectively; ρ = 0.001). The increasing RDW trend with higher histopathological grading suggests that RDW may reflect tumor malignancy. Elevated RDW in higher-grade tumors could be linked to chronic inflammation and oxidative stress, which contribute to increased RDW levels observed in BC patients [6, 10]. Chronic inflammation disrupts erythropoiesis, leading to red blood cell size heterogeneity, reflected in RDW values [8, 10, 11]. This process may create an immunosuppressive environment, promoting tumor growth and metastasis through the secretion of pro-tumor cytokines [12, 13].
A study in the South Indian population [14] and in Chhattisgarh, India [15] found that RDW increased with higher histopathological grades, indicating its potential as a prognostic marker in BC. These findings support previous studies [6, 16] suggesting RDW as a prognostic biomarker, with higher RDW levels often associated with more aggressive tumor malignancy. Our study also shows that elevated preoperative RDW is linked to poor prognosis and worse survival outcomes in BC patients.
This study also demonstrated a significant difference in RDW levels between patients with and without metastasis. Among the 144 metastatic BC patients, the mean RDW was 16.49±10.0, compared to 15.02±9.01 in 373 non-metastatic patients (ρ = 0.001). Elevated RDW in metastatic patients may be due to more intense systemic inflammation, oxidative stress, and erythropoiesis dysfunction, all of which contribute to increased red blood cell size heterogeneity [17]. Additionally, cancer- related anemia, resulting from tumor effects, inflammatory responses, or chemotherapy, may further increase RDW levels [18]. A meta-analysis has shown that patients with elevated RDW have larger tumors, more advanced stages, and higher rates of lymph node metastasis [19]. Another meta-analysis confirmed that high pretreatment RDW levels are linked to poor survival outcomes across various cancers, including BC, underscoring RDW’s prognostic significance [20]. These findings confirm that higher RDW levels are associated with poor prognosis and more severe disease.
Our study showed that each 1% increase in RDW was associated with a 0.012 increase in histopathological grading, suggesting that factors like microangiopathy, inflammatory anemia, and erythropoiesis dysregulation may exacerbate RDW elevation in higher-grade cancers [21]. These findings align with previous research indicating RDW as a parameter reflecting tumor aggressiveness [8].
Correlation analysis between RDW levels and metastasis revealed that each 1% increase in RDW was associated with a 1.016-fold increase in metastasis risk. This can be explained by chronic inflammation and oxidative stress, which play a crucial role in facilitating metastasis [17]. Systemic inflammation leads to erythropoiesis dysregulation and an imbalance in erythrocyte production, resulting in higher RDW levels in metastatic patients [22]. Metastatic patients also often experience more severe anemia, further exacerbating RDW elevation [19].
The correlation between RDW and histopathological grading showed an R-value of 0.183 with a p-value of 0.001, confirming a positive correlation between RDW and higher tumor grade. ROC curve analysis supported this, demonstrating that RDW has good discriminative ability in differentiating BC patients by histopathological grade. This supports previous research suggesting RDW as an indicator of cancer aggressiveness [16]. The progressive increase in RDW with higher histopathological grading highlights its potential prognostic value [21]. To improve clinical accuracy, RDW should be combined with other markers such as the neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR) [23].
Correlation analysis between RDW and metastasis revealed an R-value of 0.171 with a p-value of 0.001, indicating a statistically significant positive correlation. However, ROC curve analysis showed an AUC of 0.624, indicating weak to moderate discriminative ability in distinguishing patients with and without metastasis. This suggests that RDW, as a standalone biomarker, has limitations in predicting metastasis accurately. RDW’s non-specific nature, influenced by factors such as inflammation and anemia, likely explains its limited predictive ability for metastasis [8, 24]. Therefore, the clinical applicability of RDW as a standalone biomarker should be approached with caution, and further research is needed to explore its role in combination with other inflammatory markers like NLR and PLR for better prognostic accuracy.
Study Limitations
This study’s cross-sectional design limits the ability to establish causal relationships between RDW levels and BC progression. The data represent a single point in time, preventing conclusions about whether elevated RDW precedes or results from higher tumor grade and metastasis. To confirm RDW’s role as a prognostic factor, longitudinal or prospective cohort studies are required.
In conclusion, this study demonstrates a significant relationship between RDW and both histopathological grading and metastasis in BC patients. An increase in RDW is associated with higher tumor grade and an elevated risk of metastasis. These findings highlight the potential of RDW as a simple, accessible prognostic biomarker that may aid in the clinical assessment and management of BC patients, particularly in resource-limited settings.
Acknowledgments
None.
Competing interests
No competing interests were reported.
Funding
Self-funding
Ethics approval
Given the retrospective nature of this study and the use of de-identified secondary data from medical records, the Ethics Committee of the Faculty of Medicine, Hasanuddin University – Wahidin Sudirohusodo Hospital, Makassar (Approval No. 585A/UN4.6.4.5.31/PP36/2024) granted a waiver of informed consent from individual patients.
Data availability statement
Data is accessible upon justifiable request.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- Cancer Incidence and Mortality in a Tertiary Hospital in Indonesia: An 18-Year Data Review Prihantono n, Rusli R, Christeven R, Faruk M. Ethiopian Journal of Health Sciences.2023;33(3). CrossRef
- Survivability Rates Based on Molecular Subtype, Stage and Metastasis of 36 months cohort in Breast Cancer Patients Wijayanto A, Pieter JSLA , Prihantono P., Syamsu SA , Thaufix NS , Abdi A. Nusantara Medical Science Journal.2022. CrossRef
- Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier Kumar V, Abbas A, Aster JC . 2021.
- Is there a threshold for red cell distribution width to predict malignancy in breast masses? Akturk O. M., Yildirim D., Cakir M., Vardar Y. M., Erozgen F., Akinci M.. Nigerian Journal of Clinical Practice.2022;25(3). CrossRef
- Relationship between red cell distribution width and prognosis in patients with breast cancer after operation: a retrospective cohort study Yao D, Wang Z, Cai H, Li Y, Li B. Bioscience Reports.2019;39(7). CrossRef
- An update on the pathological classification of breast cancer Rakha EA , Tse GM , Quinn CM . Histopathology.2023;82(1). CrossRef
- Red cell distribution width and cancer Montagnana M, Danese E. Annals of Translational Medicine.2016;4(20). CrossRef
- Molecular principles of metastasis: a hallmark of cancer revisited Fares J, Fares MY , Khachfe HH , Salhab HA , Fares Y. Signal Transduction and Targeted Therapy.2020;5(1). CrossRef
- In-vitro and in-silico evidence for oxidative stress as drivers for RDW Joosse H, Oirschot BA , Kooijmans SAA , Hoefer IE , Wijk RAH , Huisman A, Solinge WW , Haitjema S. Scientific Reports.2023;13(1). CrossRef
- The value of red cell distribution width in patients with ovarian cancer Qin Y, Wang P, Huang Z, Huang G, Tang J, Guo Y, Huang P, Lai Z, Lin F. Medicine.2017;96(17). CrossRef
- Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth De Simone V., Franzè E., Ronchetti G., Colantoni A., Fantini M. C., Di Fusco D., Sica G. S., et al . Oncogene.2015;34(27). CrossRef
- Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis Chen X, Zhou S. Drug Design, Development and Therapy.2015;9. CrossRef
- Association of Hematological Parameters with Nottingham Histologic Grades of Breast Carcinoma in South Indian Population Chander USK , Vanishree M., Sulochana S, Rowland M. Journal of Pharmaceutical Research International.2022. CrossRef
- Preoperative evaluation of red blood cell distribution width as a promising biomarker for discriminating between benign and malignant breast tumors and assessing breast cancer activity Thakur As , Indoria C , Sahu R , Kujur P , Gahine R . Indian journal of pathology & microbiology.2024;67(2). CrossRef
- Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer Koma Y , Onishi A , Matsuoka H , Oda N , Yokota N , Matsumoto Y , Koyama M , et al . PloS one.2013;8(11). CrossRef
- Oxidative stress, inflammation, and cancer: how are they linked? Reuter S, Gupta SC , Chaturvedi MM , Aggarwal BB . Free Radical Biology & Medicine.2010;49(11). CrossRef
- Cancer Related Anemia: An Integrated Multitarget Approach and Lifestyle Interventions Natalucci V, Virgili E, Calcagnoli F, Valli G, Agostini D, Zeppa SD , Barbieri E, Emili R. Nutrients.2021;13(2). CrossRef
- Is red cell distribution width a prognostic factor in patients with breast cancer? A meta-analysis Yin J, Zhu K, Guo Z, Yi W, He Y, Du G. Frontiers in Surgery.2023;10. CrossRef
- Prognostic role of pretreatment red blood cell distribution width in patients with cancer: A meta-analysis of 49 studies Wang P, Song S, Guo H, Wang T, Liu N, Yan C. Journal of Cancer.2019;10(18). CrossRef
- High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis Cheng K, Lin Y, Liu C, Wu K, Lee K. Cancers.2022;14(4). CrossRef
- IL-6 in inflammation, autoimmunity and cancer Hirano T. International Immunology.2021;33(3). CrossRef
- Diagnostic accuracy of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and neutrophil-lymphocyte-to-platelet ratio biomarkers in predicting bacteremia and sepsis in immunosuppressive patients with cancer: literature review Martinez M , Espírito Santo A, Ramada D, Fontes F, Medeiros R. Porto Biomedical Journal.2024;9(3). CrossRef
- Mechanisms of breast cancer metastasis Nathanson SD , Detmar M, Padera TP , Yates LR , Welch DR , Beadnell TC , Scheid AD , Wrenn ED , Cheung K. Clinical & Experimental Metastasis.2022;39(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details