Evaluation of Heat Shock Protein-90 as a Potential Risk Marker in Sera Women with Breast Cancer
Download
Abstract
Background: Breast cancer (BC), the second most common kind of newly diagnosed malignancy worldwide, is the most common illness among women. Heat shock protein 90 (Hsp-90) is really important in the growth and dissemination of cancer cells by preserving the stability of overexpressed signaling proteins.
Aim of study: Evaluate Hsp-90 serum levels as risk markers in breast cancer patients as compared to those in good health.
Subject matter and methodology: The research included 180 participants. Ninety females with BC (23 stage I, 36 stage II, 24 stage III, and 7 stage IV) contrasted with 90 healthy women as a control group matched with patients in age. Serum heat shock protein-90 was measured using the ELISA technique kits. Additionally, lipid profiles and liver enzyme were assessed.
Results: Serum Hsp-90 levels were significantly increased in all stages of women with BC compared to healthy women (P < 0.001) as well as between stages. There was an uptick in serum TC, ALT, ALP, TG, LDL-C, and VLDL-C levels in patients but lower serum HDL-C in those patients in comparison with healthy controls. Hsp-90 has a significant positive correlation with age, ALP, TG, TC, HDL-C, LDL-C, ALT, AST, and VLDL-C except BMI. Heat shock protein 90 showed good diagnostic efficiency in breast cancer patients, as the cut-off value of Hsp-90 (300.5 pg/mL) with sensitivity 88.9% and specificity 87.8% (AUC 0.907, 95% CI: 0.86-0.954; P < 0.001).
Introduction
Breast cancer (BC) is still a serious threat in the context of global health issues because of its complex etiology and wide range of clinical presentations, which make prevention and treatment extremely difficult [1]. Understanding the complex nature of breast cancer is essential to creating efficient strategies for treatment, as the disease’s incidence is still rising globally [2]. The most common cancer in Iraqi females is breast cancer, which accounted for 34.27% of instances that were recently reported in 2016 [3]. When it comes to health, the WHO found that patients may have a reduced death rate if they get the treatment they need quickly after a diagnosis. The low survival rates of BC patients in Iraq may be attributed mostly to inadequate treatment decisions and inadequate early diagnosis methods [4]. Being a highly conserved member of the heat shock protein family, HSP-90 is a potential target For cancer treatment since it controls several proteins and signaling pathways that are implicated in cancer. In breast cancer, there are a number of abnormal signaling pathways; among them, Hsp-90 is abundant in breast cells [5]. HSP-90, a heat shock protein of 90 kDa, helps stabilize the shape and mature the action of several proteins that promote cancer. Previous research has found over 300 HSP-90 clients, which points to Hsp90’s essential function in cancer cell fate determination [6]. In addition to being essential components of cells, lipids also play a role in metabolism and signal transmission inside cells. Studies have demonstrated that lipids have an ever-increasing role in cancer, including its genesis, development, migration, and cell death. As lipidomics technology develops and advances, this link is predicted to increase [7]. With a disproportionately high incidence among women and a catastrophic impact on people’s lives worldwide, breast cancer is a serious public health problem in today’s society. Because of this issue, researchers are investigating the link between lipids and breast cancer [8-10].
Materials and Methods
The present study is a case-control study samples were collected from the National Cancer and Hematology Institute in Najaf Al-Ashraf, Iraq during the period from December 2023 to April 2024. For the current study, 180 participants between the ages of 36 - 65 years. The study included two groups: Ninety women with BC newly diagnosed with breast cancer by a specialized physician (the women were screened for breast cancer using ultrasound, mammography, and magnetic resonance imaging as well as, the histopathological results were used to categorize the breast cancer patients) and ninety apparently healthy women of the same age, who met the exclusion criteria.
Exclusion criteria
The study excluded all women who were pregnant, suffering from autoimmune disorders, microbial infections, chronic diseases (such as hypertension, cardiovascular disease, kidney disease, and thyroid disorders), taking oral contraceptives or any hormonal medications, and undergoing surgery.
Samples Collection
Following interviews regarding the participants’ age range, a questionnaire developed by the researcher was used to collect information on their place of residence, employment, marital status, and family history of BC. Subsequently, blood samples for 5 mL were taken from each participant’s veins. Gel tubes were utilized to transport blood samples following a breast cancer diagnosis and prior to the initiation of any treatment. the blood is centrifuged at 3000xg for 10 min to separate the sera into four Eppendorf tubes, then the samples are stored at -80°C until analysis.
Immunological and Routine Assays
Serum Hsp90 was measured using enzyme-linked immunosorbent assay (ELISA) kit from Melsin, China. This company has been utilized in previous research study [11]. Serum activities of ALT, AST, and ALP were measured spectrophotometrically, while TC, TG, and HDL-C were assessed using the enzymatic colorimetric method (Biolabo, France). LDL-C values were calculated by the Friedewald formula [12].
Statistical Analysis
The Kolmogorov-smirnov test was used to examine the distribution types of the results group. To compare the parameters between two groups, we used the Student’s t-test. The analysis of variance one way (ANOVA) test was employed to assess differences in scale variable between diagnostic groups. The results were expressed as (mean±standard deviation) for normally distributed value. For correlation study, the Pearson’s correlation coefficients (r) examine association between scale variable to find out the correlation between parametric parameter and other variable. Statistical significance was determined for all hypothesis tests with p-values less than 0.05 (two-tailed). Receiver operating characteristics (ROC) curves were measured to examine the diagnostic ability of the measured biomarkers to diagnose the disease. The cut-off values of the concentrations produce the best sensitivity and specificity from the area under the curve (AUC). IBM’s Statistical Package for Social Sciences, version-27 (SPSS, Chicago, Illinois, USA), was used to compile and analyze the data.
Ethical Approval
All participants provided written informed permission in compliance with this present edition of the Helsinki Declaration, subsequent to clearance from the ethics commission (IRB) of the College of Science, University of Kufa, Iraq (order 41507 in 12/October/2023).
Results
The study included 90 breast cancer patients and 90 apparently healthy controls. The patients were divided into four groups according to the stages (stage I (23), stage II (36), stage III (24), and stage IV (7)). Compared with the control groups, the mean of HSP-90 level was significantly higher in the BC group (390.911 ± 94.528 vs. 279.333 ± 36.169 pg/mL, p ˂ 0.0001; Table 1).
| parameters | Patients group | Controls group | P- value |
| ( mean ± SD ) | ( mean ± SD ) | ||
| Number | 90 | 90 | ------- |
| Age (years) | 48.344 ± 6.657 | 49.988 ± 8.074 | 0.1378 (NS) |
| BMI (kg/m 2 ) | 28.758 ± 4.209 | 28.956 ± 6.559 | 0.8096 (NS) |
| ALT (IU/L) | 22.185 ± 7.882 | 7.566 ± 3.187 | P˂ 0.0001 |
| AST (IU/L) | 20.557 ± 4.773 | 7.683 ± 3.078 | P˂ 0.0001 |
| ALP (IU/L) | 241.233 ± 62.521 | 86.733 ± 31.102 | P˂ 0.0001 |
| TG (mg/dL) | 209.387 ± 25.511 | 196.721 ± 33.714 | P˂ 0.005 |
| TC (mg/dL) | 235.642 ± 28.936 | 214.047 ± 34.246 | P˂ 0.0001 |
| HDL-C (mg/dL) | 34.559 ± 5.067 | 48.661 ± 5.537 | P˂ 0.0001 |
| LDL-C (mg/dL) | 159.205 ± 26.159 | 126.041 ± 30.651 | P˂ 0.0001 |
| VLDL-C (mg/dL) | 41.877 ± 5.102 | 39.344 ± 6.742 | P˂ 0.0001 |
| HSP-90 (pg/mL) | 390.911 ± 94.528 | 279.333 ± 36.169 | P˂ 0.0001 |
Data represented as mean ± SD, SD: Standard Deviation, NS: non-significant, BMI: Body Mass Index, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase, TG: Triglyceride, TC: Total Cholesterol, HDL-C: High Density Lipoprotein-Cholesterol, LDL-C: Low density Lipoprotein-Cholesterol, VLDL-C: Very Low Density Lipoprotein-Cholesterol, HSP-90: Heat shock protein 90.
Laboratory data such as liver enzymes and lipid profiles were significantly increased in the BC patients except for HDL-C, which was reduced when compared with healthy individuals. There was no significant difference between the two groups (patients and healthy groups) with respect to age and body mass index (p = 0.1378, p = 0.8096, respectively). Table 2 and Figure 1 revealed that the level of HSP-90 was significantly higher for all stages.
| Variables | Patients Stages (No.= 90) | |||||
| Stage I | Stage II | Stage III | Stage IV | |||
| Number (n%) | 23 (25.555%) | 36 (40%) | 24 (26.666%) | 7 (7.777%) | ||
| Age (years) | 43.739 ± 5.941 | 49.388 ± 6.442 | 50.041 ± 5.834 | 52.285 ± 6.156 | ||
| p-value: a) 0.001 | b ) 0.001 | c ) 0.002 | d ) 0.001 | e) 0 .687 | h) 0.397 | |
| BMI (kg/m 2 ) | 27.815 ± 3.574 | 29.122 ±4.561 | 29.292 ± 4.232 | 28.154 ±4.489 | ||
| p-value : a) 0.251 | b) 0.235 | c) 0.853 | d) 0.879 | e) 0 .582 | h) 0.533 | |
| ALT (IU/L) | 14.095 ± 4.031 | 20.586 ± 4.558 | 28.379 ± 5.175 | 35.757 ± 1.912 | ||
| p- value: a) 0.0001 | b) 0.0001 | c) 0.0001 | d) 0.0001 | e) 0.0001 | h) 0.0001 | |
| AST (IU/L) | 17.982 ± 4.478 | 20.077± 4.923 | 22.937 ± 3.682 | 23.328 ± 3.552 | ||
| p-value: a) 0.079 | b) 0.0001 | c) 0.006 | d) 0.016 | e) 0.078 | h) 0.837 | |
| ALP (IU/L) | 185.913 ± 46.193 | 235.778 ± 54.168 | 296.458 ± 42.601 | 261.714±38.573 | ||
| p-value: a) 0.0001 | b) 0.0001 | c) 0.0001 | d) 0.0001 | e) 0.197 | h) 0.097 | |
| TG (mg/dL) | 211.196 ±29.001 | 201.235 ±22.372 | 217.036 ± 26.149 | 219.140±16.535 | ||
| p-value: a) 0.138 | b) 0.424 | c) 0.462 | d) 0.01 | e) 0.085 | h) 0.845 | |
| TC (mg/dL) | 231.408 ± 32.218 | 230.633 ± 28.745 | 243.430 ± 27.695 | 248.615±13.983 | ||
| p-value: a) 0.92 | b) 0.154 | c) 0.168 | d) 0.094 | e) 0.132 | h) 0.675 | |
| HDL-C (mg/dL) | 34.224 ± 4.874 | 32.335 ± 5.151 | 37.261 ± 4.011 | 37.832 ± 2.338 | ||
| p-value: a) 0.131 | b) 0.028 | c) 0.075 | d) 0.0001 | e) 0.005 | h) 0.775 | |
| LDL-C (mg/dL) | 154.944 ± 28.872 | 158.051 ± 26.590 | 162.761 ± 25.969 | 166.954±13.637 | ||
| p-value: a) 0.66 | b) 0.312 | c) 0.294 | d) 0.499 | e) 0.416 | h) 0.712 | |
| VLDL-C (mg/dL) | 42.239 ± 5.800 | 40.247 ± 4.474 | 43.407 ± 5.229 | 43.828 ± 3.307 | ||
| p-value: a) 0.138 | b) 0.424 | c) 0.462 | d) 0.01 | e) 0.085 | h) 0.845 | |
| HSP-90 (pg/mL) | 310.478 ± 36.913 | 342.055 ± 33.448 | 489.541 ± 40.302 | 568.285±7.718 | ||
| p-value: a) 0.001 | b) 0.0001 | c) 0.0001 | d) 0.0001 | e) 0.0001 | h) 0.0001 |
Data represented as mean ± SD, SD: Standard Deviation, BMI: Body Mass Index, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase, TG: Triglyceride, TC: Total Cholesterol, HDL-C: High Density Lipoprotein-Cholesterol, LDL-C: Low density Lipoprotein-Cholesterol, VLDL-C: Very Low Density Lipoprotein-Cholesterol, HSP-90: Heat shock protein 90. Significant differences between stages; a) Stage I vs. Stage II, b) Stage I vs. Stage III, c) Stage I vs. Stage IV, d) Stage II vs. Stage III, e) Stage II vs. Stage IV, h) Stage III vs. Stage IV.
Figure 1. Comparison of Serum HSP-90 Levels in Patients' stages.
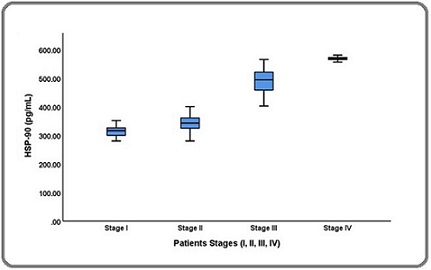
No significant difference was observed in TC, TG, LDL-C, or VLDL-C levels in the four stages. However, HDL-C showed significant variation between II vs. III and II vs. IV stages. There was no BMI difference between the four stages. The linear regression analysis was used to verify the relationship of biochemical parameters with the serum level of HSP-90. As shown in Table 3 and Figure 2 were significant positive correlation of all parameters with HSP-90.
| Parameters | r | p-value | Parameters | r | p-value |
| Age (years) | 0.354 ** | 0.001 | TG (mg/dL) | 0.322 ** | 0.002 |
| BMI (kg/m 2 ) | 0.156 | 0.142 | TC (mg/dL) | 0.321 ** | 0.002 |
| ALT (IU/L) | 0.753 ** | 0.0001 | HDL-C (mg/dL) | 0.422 ** | 0.0001 |
| AST (IU/L) | 0.308 ** | 0.003 | LDL-C (mg/dL) | 0.21 * | 0.047 |
| ALP (IU/L) | 0.523 ** | 0.0001 | VLDL-C (mg/dL) | 0.322 ** | 0.002 |
**. A significance level of 0.01 (2-tailed) indicates a correlation. *. Assuming a 2-tailed significance threshold of 0.05, the correlation is evident. r, The Pearson correlation coefficient
Figure 2. Correlation between HSP-90 Levels and A, Age; B, BMI; C, ALT; D, AST; E,ALP; F, TG; G, TC; H, HDL-C; I, LDL-C; J, VLDL-C.
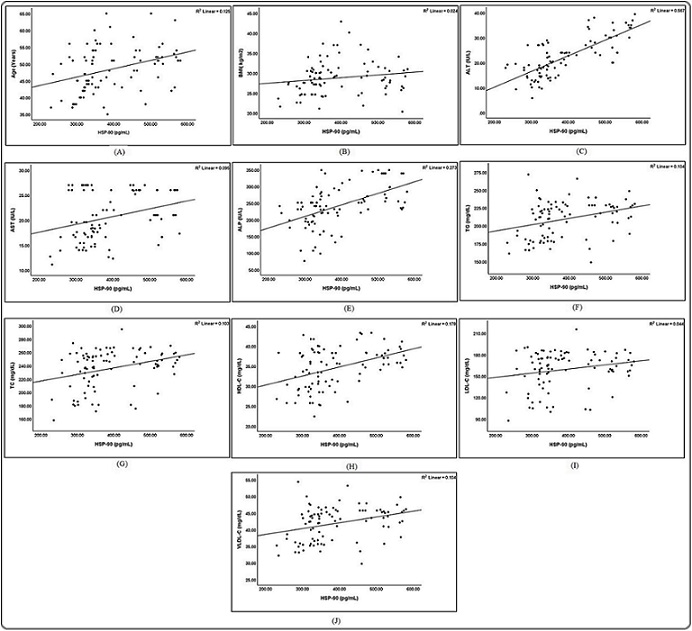
Despite the significant decrease in HDL-C levels in patients with PCOS, a positive correlation with HSP-90 was observed. This correlation arose because many samples exhibited an increase in HSP-90 that corresponded an increase in HDL-C.
However, there was no correlation between HSP-90 and BMI.
The results of the receiver operating curve (ROC) and area under the curve (AUC) analysis for HSP-90 are presented in Table 4 and Figure 3.
| Variables | HSP-90 ( pg/mL) |
| Area Under Curve (AUC) | 0.907 |
| Cut-off value | 300.5 |
| P-value | p ˂ 0.0001 |
| Specificity | 88.90% |
| Sensitivity | 87.80% |
| Confidence interval 95% | 0.86-0.954 |
Figure 3. Receiver Operating Characteristic Curve for Breast Cancer Patients vs. Healthy Control.
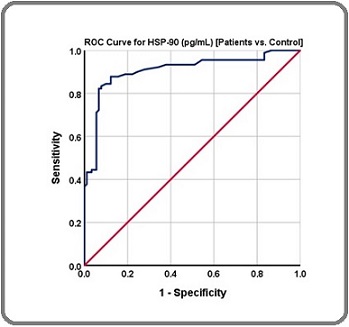
The AUC was 0.907 (CI of 0.86-0.954), with sensitivity of 87.8% and a specificity of 88.9%. The cut-off value at 300.5 (pg/mL) demonstrated the marker ability to diagnose breast cancer.
Discussion
Normal cells typically express HSP-90 at modest levels, but when under stress, its expression rises to defend biological proteins from damage or target aggregation. However, HSP-90 is often elevated in several cancer types, including breast cancer. It stabilizes and activates its targets, many of which are oncogenes, such as transcription factors, kinases, and downstream gene regulators, to enhance tumor cell adhesion, motility, and metastasis [13]. Heat shock protein 90 is a preserved molecular chaperone that aids in folding cytosolic proteins, maintain structural integrity, and control signal transduction and cell cycle [14]. This could contribute to clarifying because HSPs are essential for controlling processes in cells, such as apoptosis, metastasis, and proliferation. Furthermore, inside the tumor microenvironment, several of the crucial HSPs also control the delicate balance between the immune responses that are destructive and protective [15]. HSP-90 satellite proteins influence various stages of cancer progression, including growth, immortality, apoptosis, angiogenesis, invasion, and metastasis. The presence of a high-affinity transcript is essential for cancer cells with altered satellite proteins, as the proportion of HSP-90 in high-affinity states may impact cancer progression and therapeutic efficacy [16]. High levels of HSP-90 expression in breast cancer are linked to the disease’s recurrence and lymph node metastases. Research has demonstrated that elevated expression of HSP- 90 is a strong predictor of BC survival and a disease prognostic factor in itself of itself [17]. HSP-90 is crucial for the stability of a number of proteins involved During the course of, survival, and tumor development in breast cancer. It also plays a significant role in the stabilization of various proteins associated with cancer growth and survival [18]. Because HSP-90 is found on the surface of tumor cells and is released by them via exosomes, it is a potential and readily available biomarker. In BC, elevated levels of HSP-90 are linked to a poor prognosis [19]. The ongoing production of inflammatory signals and the elevation of intracellular HSPs as a result of enhanced cellular turnover are the causes of the increased release of HSPs into the extracellular environment [20]. Breast cancer patients in the advanced stages have high levels of HSP-90 specific autoantibodies; the HSP-90 chaperone contributes to the development of a variety of human malignancies, including breast cancers [21]. Given evidence that HSP-90 This molecular chaperone has emerged as a promising therapeutic target due to its central involvement in breast cancer biology and its capacity to inhibit the function of several receptors, kinases, and transcription factors implicated in human cancer [22]. Hsps expression has been related to tumor cell differentiation and proliferation, as well as to poor prognosis and resistance to apoptosis. HSPs are elevated in BC tissue, as evidenced by several findings, and the degree of the increase corresponds to the degree of malignancy [23].
In conclusions, the promising results regarding HSP-90 may reveal its potential as a reliable biomarker in the diagnosis of breast cancer. Elevation of HSPs in breast cancer tissue underscores a significant relationship between these proteins and malignancy. Higher levels of HSP-90 are not merely byproducts of cellular stress but are closely associated with the degree of malignancy present in tumors. HSP-90 levels could serve as a valuable biomarker for assessing tumor aggressiveness and potentially guiding treatment strategies.
Acknowledgements
We would like to thank the medical staff, patients, and staff at the National Cancer and Hematology Institute in Najaf Al-Ashraf, Iraq, as well as healthy subjects, for their commitment and continuous cooperation throughout this research.
Conflict of Interest
Author declares no conflict of interest.
References
- Risk factors for breast cancer in women: an update review Fakhri N, Chad MA , Lahkim M, Houari A, Dehbi H, Belmouden A, El Kadmiri N. Medical Oncology (Northwood, London, England).2022;39(12). CrossRef
- Breast cancer organoids derived from patients: A platform for tailored drug screening Tzeng YT , Hsiao J, Tseng L, Hou M, Li C. Biochemical Pharmacology.2023;217. CrossRef
- Demographic and clinical profiles of female patients diagnosed with breast cancer in Iraq Alwan NAS , Tawfeeq FN , Mallah NAG . Journal of Contemporary Medical Sciences.2019;5(1). CrossRef
- Promoting Clinical Breast Examination as A screening Tool for Breast Cancer in Iraq Al-Alwan N, Mualla F. Iraqi National Journal of Nursing Specialties.2014;27(1). CrossRef
- Heat shock protein 90 and ubiquitin E3 ligases (SIAHs) in breast cancer patients: a correlational study Takanlou LS , Cecener , Takanlou MS , Nazlioglu HO , Unlu HT , Isik خ, et al . J Public Heal Iran.2022;51(8):1836.
- Hsp90 chaperone facilitates E2F1/2-dependent gene transcription in human breast cancer cells Kotwal A, Suran S, Amere Subbarao S. European Journal of Cell Biology.2021;100(1). CrossRef
- The Function and Mechanism of Lipid Molecules and Their Roles in The Diagnosis and Prognosis of Breast Cancer Guo R, Chen Y, Borgard H, Jijiwa M, Nasu M, He M, Deng Y. Molecules (Basel, Switzerland).2020;25(20). CrossRef
- Association of genetic polymorphisms in MIF with breast cancer risk in Chinese women Lin S, Wang M, Liu X, Zhu W, Guo Y, Dai Z, Yang P, et al . Clinical and Experimental Medicine.2017;17(3). CrossRef
- Impact of chemotherapy on medium-term physical function and activity of older breast cancer survivors, and associated biomarkers Extermann M, Leeuwenburgh C, Samiian L, Sehovic M, Xu J, Cubitt C, Jacobsen PB , et al . Journal of Geriatric Oncology.2017;8(1). CrossRef
- Dietary Risk with Other Risk Factors of Breast Cancer Shetty V, Kundapur R, Chandramohan S, Baisil S, Saxena D. Indian Journal of Community Medicine: Official Publication of Indian Association of Preventive & Social Medicine.2021;46(3). CrossRef
- The interleukin-6/interleukin-23/T helper 17-axis as a driver of neuro-immune toxicity in the major neurocognitive psychosis or deficit schizophrenia: A precision nomothetic psychiatry analysis Al-Hakeim HK , Al-Musawi AF , Al-Mulla A, Al-Dujaili AH , Debnath M, Maes M. PloS One.2022;17(10). CrossRef
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge Friedewald W. T., Levy R. I., Fredrickson D. S.. Clinical Chemistry.1972;18(6).
- HSP90 and the chaperoning of cancer Whitesell L, Lindquist SL . Nature Reviews. Cancer.2005;5(10). CrossRef
- Heat-shock protein 90, a chaperone for folding and regulation Picard D.. Cellular and molecular life sciences: CMLS.2002;59(10). CrossRef
- Heat Shock Proteins and Breast Cancer Zhang M, Bi X. International Journal of Molecular Sciences.2024;25(2). CrossRef
- The role of heat shock proteins in bladder cancer Ischia J, So AI . Nature Reviews. Urology.2013;10(7). CrossRef
- Inhibition of HSP90 sensitizes a novel Raf/ERK dual inhibitor CY-9d in triple-negative breast cancer cells Chen Y, Wang X, Cao C, Wang X, Liang S, Peng C, Fu L, He G. Oncotarget.2017;8(61). CrossRef
- The Chaperone System in Breast Cancer: Roles and Therapeutic Prospects of the Molecular Chaperones Hsp27, Hsp60, Hsp70, and Hsp90 Alberti G, Vergilio G, Paladino L, Barone R, Cappello F, Conway de Macario E, Macario AJL , Bucchieri F, Rappa F. International Journal of Molecular Sciences.2022;23(14). CrossRef
- Extracellular heat shock proteins and cancer: New perspectives Albakova Z, Siam MKS , Sacitharan PK , Ziganshin RH , Ryazantsev DY , Sapozhnikov AM . Translational Oncology.2021;14(2). CrossRef
- Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, et al . Clinical Laboratory.2009;55(1-2). CrossRef
- Hsp90, an unlikely ally in the war on cancer Barrott JJ , Haystead TAJ . The FEBS journal.2013;280(6). CrossRef
- Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response Marcus A, Mao AJ , Lensink-Vasan M, Wang L, Vance RE , Raulet DH . Immunity.2018;49(4). CrossRef
- Heat-shock protein 90α protects NME1 against degradation and suppresses metastasis of breast cancer Zhang Y, Zhao G, Yu L, Wang X, Meng Y, Mao J, Fu Z, et al . British Journal of Cancer.2023;129(10). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details