Assessment of Serum P53, GPX, SOD and CA15-3 Levels in Breast Cancer Patients Under Treatment: A Cross-sectional Study in Basrah, Iraq
Download
Abstract
Objective: This study aimed to evaluate the relationship between tumor protein (P53), glutathione peroxidase (GPx), Super oxide dismutase (SOD), cancer antigen (CA15-3) in breast cancer, as well as the impact of variables such as age, location, duration of Illness, and kind of therapy.
Methodology: This case-control study carried out between November 2024 to April 2025, at Sadr Teaching Hospital, Oncology Department and Al-Zubair General Hospital, Basrah, Iraq. A total of 178 participants were chosen as a sample; we chose 89 breast cancer patients as cases and 89 healthy individuals as controls. We measured the levels of P53, GPx, SOD, CA15-3.
Results: The study revealed significant differences in P53 levels between patients and healthy controls (P<0.001), associated with age, geographic location, and treatment type, while GPx levels were decreased in patients. No significant differences were observed in SOD or CA15-3 levels. Factors such as geographic location, treatment type, and disease duration had no significant impact on the measured concentrations. Additionally, positive correlations were found between P53 and GPx1, as well as between GPx1 and SOD.
Conclusion: Age is a major risk factor for breast cancer. The decreased levels of P53 and GPx1 indicate the role of oxidative stress, highlighting the need to investigate biomarkers and genetic factors to improve disease understanding and management. No significant differences were observed in the other variables.
Introduction
Breast cancer is the second most common life- threatening cancer in women worldwide. It is a disease that is both clinically and molecularly diverse [1]. It accounts for around 36.1% of all reported cancer cases in Iraq, making it the most prevalent cancer among women. This indicates that breast cancer accounts for one out of every three cancer diagnoses in women [2]. The Iraqi Ministry of Health report for 2023 states that 8,849 instances of breast cancer were reported overall, with 8,708 of those cases occurring in women and 141 in men [3]. With an incidence rate of 78 infections per 100,000 people, around 2,300 new cases are recorded annually in the Basra governorate alone [4]. Numerous causes contribute to the rise in instances, but environmental contamination from oil extraction is the main culprit. There is an obvious correlation between environmental pollution and the incidence of breast cancer in high-incidence areas like Zubair [5]. Invasive ductal carcinoma (IDC) [6], ductal carcinoma in situ [7], invasive breast cancer [8], Paget’s disease of the breast [9], triple-negative breast cancer (TNBC) [10], and inflammatory breast cancer (IBC) [11] are among the several clinical types of breast cancer that are determined by symptoms and pathological characteristics. Among them is the p53 protein, referred to as the guardian of the genome, which controls a variety of cellular processes and guards against DNA damage and tumor development [12]. CA15-3, a non-invasive serum marker for breast cancer, widely used for immediate diagnosis, monitoring, and prediction of breast cancer at both early and advanced/ metastatic stages [13]. Superoxide Dismutase (SOD) is an antioxidant enzyme known for its role in neutralizing reactive oxygen species. It also acts as an anti-cancer agent and inhibits the initiation and progression stages of carcinogenesis [14]. Glutathione (GSH) represents the second line of defense against reactive oxygen species through enzymatic activity. GSH is the most abundant cellular thiol, capable of directly scavenging free radicals. Studies have shown a reduction in antioxidant levels in various cancers, including breast cancer [15]. The current study intends to investigate the association between these biochemical markers and breast cancer, as well as evaluating the influence of factors like age, disease duration, location, and treatment type. Thus, to recognize their potential as diagnostic or prognosticators, and examine the biomarkers to serve as a part of a detection tool for early detection and enhance therapeutic strategies. In addition, this research not only increases the scientific literature on cancer, but also has a significant practical value that could directly affect the health of women in Iraq.
Material and Methods
Study population
This case-control research carried out between November 2024 and April 2025, at Sadr Teaching Hospital, Oncology Department and Al-Zubair General Hospital, Basrah, Iraq. The study population consisted of breast cancer patients diagnosed with invasive ductal carcinoma IDC, which represented the most prevalent histological subtype among the selected cases (For patients under treatment). It included 89 healthy people as controls and 89 breast cancer patients as cases out of a total of 178 participants. Patients with breast cancer commonly visited the oncology department at Sadr Teaching Hospital for routine checkups or consultations. Blood samples were taken from the patients after they agreed to participate in the research, which was approved by the Basrah Health Department. On June 11, 2024, the Ethics Committee authorized protocol number 705, and we followed the Declaration of Helsinki while conducting this research.
Exclusion Criteria
Individuals with thyroid, heart, or kidney problems, and patients younger than 20 and older than 60 years were not included. Breast cancer patients who had undergone mastectomy for one or both breasts, and patients with early screening were also excluded. Pregnancy, polycystic ovary disease, and chronic diseases were also excluded from the control group. A questionnaire containing information including age, place of residence, duration of illness and type of treatment was completed by both patients and the control group in the morning at the hospital.
Sample Collection
5 ml of blood was drawn from the subjects via a forearm vein. The venous blood samples were placed in semi-gel tubes. After clotting, the serum was centrifuged at 3,000 rpm for 10 minutes. The serum was then stored in a deep freezer at -40°C until analysis.
Laboratory Tests
The levels of p53 (catalog number: E1711Hu) (batch number: 202501011), superoxide dismutase (SOD) (catalog number: E0918Hu) (batch number: 202501011), glutathione peroxidase (GPx) (catalog number: E3921Hu) (batch number: 202501011), and human cancer antigen 15-3 (CA 15-3) (catalog number: E7534Hu) (batch number: 202501011) were measured using ELISA kits specifically designed for humans from Bioassay Technology Laboratory Co., Ltd. (BT LAB), located at 501 Chan sheng South Road, Nanhu District, Jinxing, China. These tests were performed at the Central General Laboratory, ELISA Unit, Department of Chemistry, College of Education for Pure Sciences, University of Basrah. The absorbance was measured at a wavelength of 450 nm. Biotech (USA) 800TS microplate reader and plotted a standard curve of optical density versus levels based on the dilutions indicated on each package. Concentrations were obtained based on the standard curve, which had an analyte-specific detection range.
Statistical Analysis
SPSS version 25 (IBM, Armonk, New York) was used. Results were extracted using descriptive statistics such as mean and standard deviation (SD), and differences between quantitative data for the two sets of parameters were tested using techniques such as the Mann-Whitney test and the Kruskal-Wallis test. Spearman correlation coefficient was used to obtain the correlation coefficient (r value). P-values less than 0.05 were considered statistically significant.
Ethical Approval
The Ethical Committee of the Department of Chemistry, College of Science, University of Basrah, and the Training and Human Development Center at the Basrah Health Directorate approved the research protocol on June 11, 2024, number 705. This study was conducted in accordance with the Declaration of Helsinki.
Results
The concentration levels of the studied variables are shown in Table 1 for both participants, indicating statistically significant differences between the patients and the control group in the decrease concentration of p53 and GPx at the significance level (p< 0.001) in the patients. No statistically significant difference was observed in SOD and CA15-3.
| Parameters | Patients (89) | Control (89) | |||
| Mean±SD | Min-Max | Mean±SD | Min-Max | P-value | |
| P53 | 429.70±211.15 | 71.50-1306.50 | 935.288±485.806 | 101.50-3203.16 | 0 |
| (150-2400ng/L) | |||||
| GPX1 | 127.55±65.65 | 35.32-346.87 | 174.64±101.53 | 9.32-714.37 | 0 |
| (30-480 μU/ml) | |||||
| SOD | 162.62±64.57 | 46.34-337.55 | 171.58±81.30 | 64.27-616.00 | NS |
| (30-480 U/L) | |||||
| CA15-3 | 61.17± 35.92 | 14.85- 327.75 | 54.29± 22.78 | 5.59- 164.23 | NS |
| (15-240 U/mL) |
Mann-Whitney test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001), no significant (NS)
To investigate the effect of age on serum variables, participants were divided into two age groups as shown in Table 2.
| Parameters | Patient ≤44 | Control≤44 | P-value | Patient≥45 | Control≥45 | P-value |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| P53 | 398.26±183.25 | 815.59±421.50 | 0 | 457.79±231.63 | 987.41±505.61 | 0 |
| (150-2400ng/L) | ||||||
| GPX1 | 138.31±73.24 | 155.01±63.96 | 0.009 | 117.93±57.13 | 183.19±113.51 | 0.011 |
| (30-480 μU/ml) | ||||||
| SOD | 173.62±66.95 | 144.58±49.57 | NS | 152.80±61.41 | 183.33±89.58 | NS |
| (30-480 U/L) | ||||||
| CA15-3 | 56.93± 52.50 | 53.67± 17.92 | NS | 64.96± 43.10 | 55.69± 31.62 | NS |
| (15-240 U/mL) |
Mann-Whitney test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001), no significant (NS)
The first group was aged ≤44 years and included 42 patients and 44 control subjects. The second group was female (≥45 years) and included 47 patients and 45 controls. There were significant differences in p53, with P<0.001, GPX, P<0.01, respectively. There were no significant differences in SOD and CA15-3 in the first group. In the second group, there were significant differences in p53 p<0.001, GPX, P<0.01, while there were no significant differences in SOD and CA15-3.
Table 3, showed no significant differences were found between the chemical variables in breast cancer patients.
| Parameters | Patient ≤44 | Patient ≥45 | P-value |
| Mean ± SD | Mean ± SD | ||
| P53 | 398.26±183.25 | 457.79±231.63 | NS |
| (150-2400ng/L) | |||
| GPX1 | 138.31±73.24 | 117.93±57.13 | NS |
| (30-480 μU/ml) | |||
| SOD | 173.62±66.95 | 152.80±61.41 | NS |
| (30-480 U/L) | |||
| CA15-3 | 56.93± 52.50 | 64.96± 43.10 | NS |
| (15-240 U/mL) |
Mann-Whitney test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001), no significant (NS)
In Table 4, the patients were divided into two groups according to their place of residence (city center group and extremity group). A significant difference was observed at P53 (P<0.05), and no significant differences were found in the other variables.
| The Center (49) | Extremities (40) | ||
| Parameters | Mean ± SD | Mean ± SD | P-value |
| P53 | 364.37±181.56 | 461.28±218.50 | 0.039 |
| (150-2400ng/L) | |||
| GPX1 | 124.45±63.99 | 129.04±66.92 | NS |
| (30-480 μU/ml) | |||
| SOD | 151.02±61.54 | 168.23±65.74 | NS |
| (30-480 U/L) | |||
| CA15-3 | 57.18±51.69 | 63.10±41.11 | NS |
| (15-240 U/mL) |
Mann-Whitney test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001) , no significant (NS)
There was a significant difference in P53 (P <0.05) and no significant variations in the other variables when the patient group was split into three groups based on the kind of treatment (hormone therapy, radiation and chemotherapy), Table 5.
| Chemical | Radiation | Hormonal | ||
| Parameters | Mean ± SD | Mean ± SD | Mean ± SD | P-value |
| P53 | 426.12±193.46 | 308.84 | 463.00±228.41 | 0.047 |
| (150-2400ng/L) | ||||
| GPX1 | 123.16±61.91 | 121.38±42.27 | 131.88±73.57 | NS |
| (30-480 μU/ml) | ||||
| SOD | 161.18±67.24 | 198.94±60.46 | 153.94±62.11 | NS |
| (30-480 U/L) | ||||
| CA15-3 | 67.05±25.73 | 61.40±20.63 | 58.07±43.43 | NS |
| (15-240 U/mL) |
Kruskal-Wallis Test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001) , no significant (NS)
Patients are grouped in Table 6, based on the length of their illness.
| Less than 5 years | More than 5years | ||
| Parameters | Mean ± SD | Mean ± SD | P. value |
| P53 | 424.05 ±198.61 | 445.93 ±247.84 | NS |
| (150-2400ng/L) | |||
| GPX1 | 131.23 ±68.11 | 116.97 ±58.12 | NS |
| (30-480 μU/ml) | |||
| SOD | 159.92 ±56.32 | 170.36 ±84.96 | NS |
| (30-480 U/L) | |||
| CA15-3 | 60.55 ± 39.39 | 62.95±23.94 | NS |
| (15-240 U/mL) |
Mann-Whitney test, significant (p <0.05) high significant (p ≤0.01) very high significant (p≤ 0.001), no significant (NS)
The patients were therefore split into two groups: those who had been there for less than five years and those who had been there for five years or more. No variable showed statistically significant differences between the two groups.
Table 7, illustrates the correlation coefficients (r) between p53 and other biochemical variables in patients.
| Correlations | r | P. value |
| P53 vs. GPX1 | 0 .324** | 0 |
| P53 vs. SOD | -0.002 | NS |
| P53 vs. CA15-3 | -0.075 | NS |
| GPX1 vs. SOD | 0 .385** | 0 |
| CA15-3 vs. GPX1 | -0.063 | NS |
| CA15-3 vs. SOD | -0.012 | NS |
Spearman's correlations test, r = strength of correlation or correlation coefficient, (-) inversely correlation, (+) proportional correlation., significant (*p < 0.05), high significant (**p ≤ 0.001), very high significant (***p ≤ 0.0001).
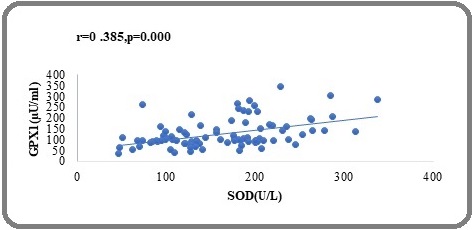
In breast cancer patients, P53 was considerably favourably connected with GPX (r=0.324, P<0.000), GPX1 was strongly positively correlated with SOD (r=0.385, P<0.000), Figure 1 and 2, showed a positive relationship in the biochemical variables.
Figure 1. Correlation between p53 and GPx in Brest Cancer patients .
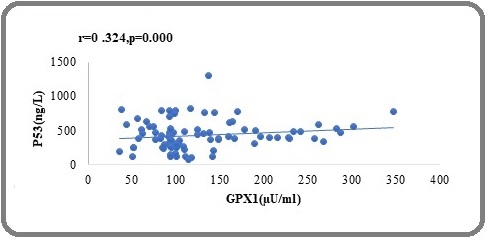
Figure 2. Correlation between GPx and SOD in Brest Cancer Patients .
Discussion
Scientific evidence demonstrates a clear association between increasing age and a higher risk of breast cancer, with age recognized as one of the most significant risk factors for this disease. Data from 2016 reported elevated incidence rates among women over forty years old, with prevalence rates of 99.3% and 71.2% in the age groups of 40 years and above 60 years, respectively, in the United States [16]. In the present study, the sample included breast cancer patients undergoing treatment, excluding those in fully metastatic stages or very early stages not requiring immediate therapy, which precluded classification of the disease according to traditional staging systems.
The findings revealed a significant decrease in P53 levels in patients compared to healthy controls, consistent with a local study conducted in Basrah in 2022 [17]. This aligns with other reports indicating that missense mutations in the P53 gene often result in reduced protein levels or the production of aberrant, unstable forms that undergo rapid intracellular degradation, particularly in cancer contexts [18]. Additionally, the present study observed more pronounced differences between patients and controls in individuals under 45 years old, supporting previous findings that breast cancer in younger women frequently exhibits more aggressive molecular patterns [19].
The current study also demonstrated higher mean P53 levels among patients residing in peripheral regions compared to those from the city center (p < 0.039), which may reflect environmental influences, delayed diagnosis, or variations in mutation patterns across different geographical areas [20]. Moreover, P53 levels exhibited significant differences according to treatment modalities, suggesting that different therapies may influence protein stability or treatment responses, warranting further investigations into the relationship between P53 status and treatment types [21].
Regarding the enzyme GPx, a study revealed a significant decrease in its levels among patients compared to healthy controls (p < 0.001) [22]. Another study linked low GPx levels with higher tumor spread, suggesting that decreased GPx could serve as a marker of disease severity or poor prognosis [23].
The current findings are in line with another study indicating that reduced GPX1 activity in breast cancer tissues may be associated with lower gene expression of the enzyme, leading to diminished antioxidant defenses, elevated oxidative stress levels, and potential impacts on tumor progression and treatment response [24]. Although scientific literature emphasizes the influence of environmental factors such as air pollution, heavy metals (cadmium, lead), and pesticides on GPx levels and oxidative stress [25], the current study did not observe significant differences in GPx levels based on geographic location, treatment type, or disease duration. This finding agrees with local study conducted at the University of Basrah, which similarly found no significant differences in oxidative stress markers between patients from the city center and peripheral regions [26].
Regarding the tumor marker CA15-3, the study observed higher mean levels among patients compared to healthy controls; however, this increase did not reach statistical significance in some analyses. These results are consistent with statistical analyses conducted at the University of Basrah in 2024, indicating that CA15- 3 levels in breast cancer patients were higher than in healthy controls but without statistical significance [27]. This finding aligns with previous studies highlighting that CA15-3 is more sensitive for disease monitoring rather than early diagnosis, often rising in advanced stages or during recurrence [28]. Recent studies have shown that the glycosylated variant Neu5Gc CA15-3 exhibits higher sensitivity (AUC ~ 0.886) than the conventional assay, underscoring the importance of developing more precise diagnostic tools for early breast cancer detection [29].
While the results showed an increase in SOD in healthy individuals compared to patients, it did not reach the level of significant differences, while other studies recorded clear significant differences in breast cancer patients [15,30]. Perhaps the absence of significant differences in SOD does not necessarily mean the absence of a biological change, but rather the reasons may be due to: differences in disease and treatment stages between patients, compensation with other enzymes, great variation between individuals, and the type and methods of measurement used.
The current study identified a significant positive correlation between P53 and GPX1 levels, consistent with prior findings suggesting that oxidative stress activates P53, and that cells with high P53 expression demonstrate relatively elevated levels of antioxidant enzymes such as GPX1 as a defensive mechanism against oxidative damage [31].
Moreover, the study revealed a strong, significant positive correlation between GPX1 and SOD, reflecting their functional interplay in mitigating oxidative stress [32]. Nevertheless, other studies have reported that GPX1 levels may decrease in certain cancer types while SOD levels remain unchanged, suggesting that the relationship between these enzymes is not invariably consistent [33]. As for tumor markers such as CA15-3, most studies have found no clear association between these markers and antioxidant enzymes like GPX1 or SOD, which is consistent with the findings of the present study [34]. Sample size calculations showed that for P53 and GPx1, our sample size (n=89 per group) provided sufficient power (>80%) to detect significant differences. However, for variables with small effect sizes such as SOD and CA15-3, much larger sample sizes (>300 per group) would be required, potentially explaining the lack of statistical significance observed in these markers.
Overall, these results underscore the importance of investigating biomarkers alongside environmental and genetic factors to better understand the dynamics of breast cancer, predict disease progression, and enhance treatment responsiveness. Such insights are crucial for developing more effective diagnostic and therapeutic strategies.
In conclusion, age is a major risk factor for breast cancer, particularly above 40 years. A significant decrease in P53 and GPx levels was observed in patients compared to healthy controls, reflecting the role of oxidative stress in cancer development. P53 showed a positive correlation with GPx1, whereas CA15-3 exhibited a weaker association with antioxidant enzymes. No significant differences in GPx levels were observed with respect to geographic location, type of treatment, or disease duration. These findings underscore the importance of investigating biomarkers, environmental factors, and genetic determinants in breast cancer to improve disease understanding and management.
Acknowledgments
This research was supported by the Department of Chemistry, College of Sciences, University of Basrah, Basrah, Iraq. The authors are thankful to Mrs Ayat Amer Hassan for her kind guidance in the ELISA experiments. It is a part of the Doctoral graduation requirements.
Funding
Funding The study was funded by Department of
Chemistry, College of Sciences, University of Basrah.
Author contributions
Conceptualization, AF and DS: Methodology; AF: Software; AF: Validation, AF and DS: Formal Analysis; AF: Investigation; DS and AAAA: Supervision, project administration and data Curation; AF and DS: Writing original draft preparation; AAAA: Review & Editing the manuscript; AF: Funding Acquisition. All authors were approved the final version of manuscript.
Conflicts of interest
The authors deny there are any financial conflicts of interest.
Data availability upon reasonable request
References
- Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments Testa U, Castelli G, Pelosi E. Medical Sciences (Basel, Switzerland).2020;8(1). CrossRef
- Cancer statistics, 2025 Siegel RL , Kratzer TB , Giaquinto AN , Sung H, Jemal A. CA: a cancer journal for clinicians.2025;75(1). CrossRef
- Clinical Profile of Female Patients Presented with Breast Diseases: An Institutional Analysis in Baghdad, Iraq Wahhab RASA , Mohammed LK , Abdulhassan BA . Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 ).2025;8(1). CrossRef
- Epidemiology of Different Types of Cancers Reported in Basra, Iraq Abood RA , Abdahmed KA , Mazyed SS . Sultan Qaboos University Medical Journal.2020;20(3). CrossRef
- Breast Cancer in Basra Oncology Center: A Clinico- Epidemiological Analysis Abood RA . Asian Pacific journal of cancer prevention: APJCP.2018;19(10). CrossRef
- Breast cancer: pathogenesis and treatments Xiong X, Zheng L, Ding Y, Chen Y, Cai Y, Wang L, Huang L, Liu C, Shao Z, Yu K. Signal Transduction and Targeted Therapy.2025;10(1). CrossRef
- Update on the management of ductal carcinoma in situ of the breast: current approach and future perspectives Kanbayashi C, Iwata H. Japanese Journal of Clinical Oncology.2025;55(1). CrossRef
- Reporting on invasive lobular breast cancer in clinical trials: a systematic review Van Baelen K, Van Cauwenberge J, Maetens M, Beck G, Camden A, Chase M, Fraser V, et al . NPJ breast cancer.2024;10(1). CrossRef
- Mammary Paget's Disease Mimicking Benign and Malignant Dermatological Conditions: Clinical Challenges and Diagnostic Considerations Scott-Emuakpor R, Reza-Soltani S, Altaf S, Nr K, Kołodziej F, Sil-Zavaleta S, Nalla M, et al . Cureus.2024;16(7). CrossRef
- Advanced and Metastatic Triple Negative Breast Cancer-Potential New Treatment Pajewska M, Partyka O, Czerw A, Deptała A, Sygit K, Gąska I, Porada S, et al . Cancers.2025;17(7). CrossRef
- Characterization of mammographic markers of inflammatory breast cancer (IBC) Barkana BD , Ahmad B, Essodegui F, Lembarki G, Pfeiffer R, Soliman AS , Roubidoux MA . Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB).2025;129. CrossRef
- Biomarkers in Breast Cancer: An Old Story with a New End Neves Rebello Alves L, Dummer Meira D, Poppe Merigueti L, Correia Casotti M, Prado Ventorim D, Ferreira Figueiredo Almeida J, Pereira de Sousa V, et al . Genes.2023;14(7). CrossRef
- Evaluation of new tumor marker CA 27-29 as a diagnostic biomarker for breast cancer in comparison to the standard CA 15-13 HadI SF , Al.Ibraheem SAH , Abbas HJ . Anaesthesia, Pain & Intensive Care.2025;29(1). CrossRef
- Glutathione peroxidase (GPx) in Breast Carcinoma Verma P. Research & Reviews: A Journal of Life Sciences.2018;8(3). CrossRef
- Superoxide dismutase activity in breast cancer patients treated with anastrozole Hasan HA , Al-Ani AW . Onkologia i Radioterapia.2023;17(11). CrossRef
- Risk Factors and Preventions of Breast Cancer Sun Y, Zhao Z, Yang Z, Xu F, Lu H, Zhu Z, Shi W, et al . International Journal of Biological Sciences.2017;13(11). CrossRef
- Assessment of CA 15-3 and P53 biomarkers in diagnosis of breast cancer Khadhum HS , Ameen AA , Thuwaini MM . 2022;140(1):1343-4292.
- Blinded by the Light: The Growing Complexity of p53 Vousden KH , Prives C. Cell.2009;137(3). CrossRef
- Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, Wei W, Zhang Y, Sun S. Oncotarget.2017;8(17). CrossRef
- Environmental factors in relation to breast cancer characterized by p53 protein expression Furberg H, Millikan R. C., Geradts J., Gammon M. D., Dressler L. G., Ambrosone C. B., Newman B.. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2002;11(9).
- Targeting Mutant p53 for Cancer Treatment: Moving Closer to Clinical Use? Duffy MJ , Tang M, Rajaram S, O'Grady S, Crown J. Cancers.2022;14(18). CrossRef
- Association between Oxidative Stress Parameters and Hematological Indices in Breast Cancer Patients Danesh H, Ziamajidi N, Mesbah-Namin SA , Nafisi N, Abbasalipourkabir R. International Journal of Breast Cancer.2022;2022. CrossRef
- Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients - A review Cecerska-Heryć E, Surowska O, Heryć R, Serwin N, Napiontek-Balińska S, Dołęgowska B. Clinical Biochemistry.2021;93. CrossRef
- The ESR1 and GPX1 gene expression level in human malignant and non-malignant breast tissues Król MB , Galicki M, Grešner P, Wieczorek E, Jabłońska E, Reszka E, Morawiec Z, Wąsowicz W, Gromadzińska J. Acta Biochimica Polonica.2018;65(1). CrossRef
- Assessing oxidative stress resulting from environmental exposure to metals (Oids) in a middle Eastern population Rafiee A, Delgado-Saborit JM , Aquilina NJ , Amiri H, Hoseini M. Environmental Geochemistry and Health.2022;44(8). CrossRef
- Evaluation of oxidative stress level and glutathione system in patients with psoriasis in Basrah Governorate, Iraq Hassan AA , Sayyah SG . 2025.
- Evaluation Of Some Biochemical Parameters In Patients With Breast Cancer In Basrah Governorate Alheriz ANY , Ali DS . Journal of Kufa for Chemical Sciences.2024;3(2). CrossRef
- Prognostic Impact of Elevation of Cancer Antigen 15-3 (CA15-3) in Patients With Early Breast Cancer With Normal Serum CA15-3 Level Ryu JM , Kang D, Cho J, Lee JE , Kim SW , Nam SJ , Lee SK , et al . Journal of Breast Cancer.2023;26(2). CrossRef
- Improved breast cancer diagnosis using a CA15-3 capture antibody-lectin sandwich assay Nikseresht S., Shewell L. K., Day C. J., Jennings M. P., Chittoory H., McCart Reed A. E., Simpson P. T., et al . Breast Cancer Research and Treatment.2025;211(3). CrossRef
- Long-Term Yogic Intervention Improves Symptomatic Scale and Quality of Life by Reducing Inflammatory Cytokines and Oxidative Stress in Breast Cancer Patients Undergoing Chemotherapy and/or Radiotherapy: A Randomized Control Study Jain M, Mishra A, Yadav V, Shyam H, Kumar S, Mishra SK , Ramakant P. Cureus.2023;15(1). CrossRef
- Effect of oxidative stress on tumor suppressor protein p53 and some biochemical markers in breast cancer patients in Erbil governorate Ahmad AA , Abdoulrahman K. Zanco J Pure Appl Sci.2020;32(6):60-71. CrossRef
- Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study Gusti AMT , Qusti SY , Alshammari EM , Toraih EA , Fawzy MS . Antioxidants (Basel, Switzerland).2021;10(4). CrossRef
- Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer Zhao Y, Wang H, Zhou J, Shao Q. Cancers.2022;14(10). CrossRef
- Assessment of lipid peroxidation and total antioxidant capacity in patients with breast cancer Babakr AT , Nour Eldein MM . Exploration of Targeted Anti-Tumor Therapy.2025;6. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details