Global Liver Cancer Incidence and Mortality Rates, the Role of Human Development Index
Download
Abstract
Introduction: Liver cancer (LC) is one of the most common malignant tumors worldwide which have been a major public health challenge worldwide. This study aimed to identify the global effect of HDI in the incidence and mortality rates of liver LC.
Material and Methods: Data about the incidence and mortality rate of LC for the year 2012 was obtained from the global cancer project for 172 countries. Data about the HDI and other indices were obtained for 169 countries from the United Nations Development Programme database in 2012. Linear regression models were used for assessment of the HDI effect on LC occurrence rates. Inequality in the age-specific incidence and mortality rates (ASR) of LC according to the HDI were assessed by using the concentration index.
Results: Linear regression model showed that increasing of HDI had a negative effect on the increase in both incidence (B=-12.2, P=0.03) and mortality (B=-12.7, P=0.015) rates of LC. The mean of life expectancy at birth, mean years of schooling, GNI per capita, percent of urbanization, and age-standardized obesity had also a negative effect on increasing in both incidence and mortality rates.
Conclusion: incidence and mortality rate of LC are significantly concentrated in regions with medium and low HDI. The negative relationship between LC incidence and mortality with HDI and its component can be considered as targets for prevention and treatment intervention or tracking geographic disparities.
Introduction
Liver cancer (LC) is one of the most common malignant tumors worldwide which has been a major public health problem in the world [1]. According to GLOBOCAN 2012, it was estimated that 782,000 people were diagnosed with LC and that 746,000 people died of this disease, accounting for 5.6% of all new cancer cases and 9.1% of all cancer deaths worldwide, making liver cancer the sixth most common type of cancer and the second leading cause of cancer-related death [2]. LC has a high mortality rate; the geographic distribution of mortality is similar to that of incidence. Developing countries have the most burden of liver cancer, where almost 85% of the cases occur [3].
The major risk factors for LC have been identified as three categories: 1) the established factors are infection with hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic cirrhosis, dietary aflatoxins, and tobacco smoking; 2) the likely factors are diabetes mellitus, inherited metabolic disorders-alpha antitrypsin deficiency, hemochromatosis, porphyria cutanea tarda, cirrhosis of any etiology; and 3) the possible factors are decreased consumption of vegetables, oral contraceptives, high parity, ionizing radiation, and organic trichloroethylene solvent [4]. The family history of LC, genetic susceptibility or genetic polymorphisms, economic status, and even negative life events are also potential factors for the LC, especially in the high-risk area. These factors, however, may not account for the occurrence of all LC, and also may not be necessary for any cases of the disease. Furthermore, the differences in cancer incidence and mortality among different ethnic groups may also illustrate distinctions in risk factors for liver cancer in various countries or in different ethnic groups [5]. Several studies showed that the incidence and mortality disparities between countries can be attributed to some factors such as differences in life expectancy, education level, income level and access to health care [6 7, ]. Human Development Index (HDI) as a key socioeconomic determinant of health is composed of three components including education, life expectancy and gross national income [8]. The relation of different cancers and levels of HDI at national and sub-national level is studied and a possible inverse association is found [9, 10, 11].
It is necessary to get information on epidemiology and inequalities related to the incidence and mortality of cancer to use for planning and further research. This study aimed to identify the role of human development in the incidence and mortality rates of LC worldwide.
Materials and Methods
This ecological study was performed on the relation of the age-specific incidence and mortality rate (ASR) of LC and HDI. HDI has several main components including life expectancy at birth, mean years of schooling, and gross national income (GNI) per capita; and also some ancillary indexes including percent of urbanization, and age-standardized obesity (defined as BMI> 30) in adults (The weighted average of the age-specific obesity rate among adults ages 20 and older). ASR is a summary measure of the rate that having a standard age structure, a population distribution would have since age has a powerful influence on the risk of cancer, standardization is necessary when comparing several populations that differ with respect to age.
Data about the incidence and mortality rate of LC for the year 2012 was obtained from the global cancer project for 172 countries [2]. Data about the HDI and other indices were obtained for 169 countries from the United Nations Development Programme (UNDP) database [8].
Data analysis was restricted to 169 countries that both the epidemiologic data from the GLOBOCAN database and the HDI were available. These countries categorized into four categories including 1) Very High Human Development (27 countries); 2) High Human Development (37 countries); 3) Medium Human Development (89 countries) and 4) Low Human Development (16 countries). In this study, we used the correlation bivariate method for assessment of the correlation between the incidence and mortality rate of LC and the HDI. We also used linear regression models for assessment of the HDI effect on LC occurrence rates.
We defined inequality in the age-specific incidence and mortality rates (ASR) of LC according to the HDI by using the concentration index. The value of the concentration index is ranged from -1 to +1; the negative value is indicating that the health variable is more concentrated in the poor population and the positive value indicates in rich population.
The significance level of 0.05 was considered. Distributive Analysis Stata Package (DASP) for estimating concentration index. Data were analyzed by Stata computer software version 12 (StataCorp, College Station, TX, USA).
Results
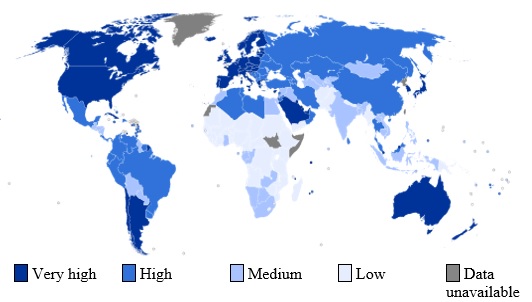
In 2012, LC was estimated to have affected a total of 782,000 individuals (crude rate: 25.9per 100,000 individuals) and caused 746,000 deaths worldwide (crude rate: 22.5 per 100,000 individuals). The highest HDI values belonged to privileged countries such as USA, Canada, Australia, and Western Europe and lowest was in Africa countries (Fig. 1).
Figure 1 :World map indicating the categories of HDI by countries (based on data 2013)
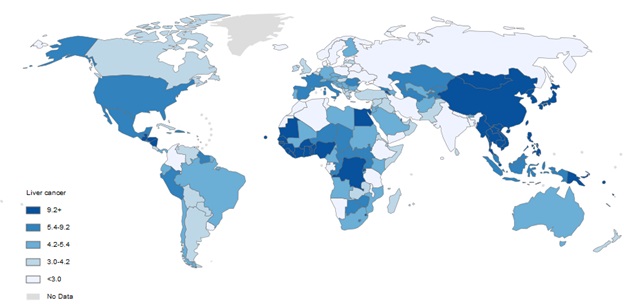
As shown in ( Fig 2), High incidence region was Eastern and South-Eastern Asia and lower rate were in Northern Europe.
Figure 2 :Incidence Age-Standardized Rates per 100,000 of LC in both sexes in the world in 2012
According to Tabl 1, the highest age-standardized incidence of LC was seen in medium and low developed regions, also the highest age-standardized mortality of LC belonged to medium and low developed countries. More specifically, in high or very high developed regions of the world, the highest value of LEB, MYS, GNI, and total HDI was estimated to be 80.2, 11.7, 40, and 0.89; respectively.
| Region | LC Incidence | LC Mortality | HDI Component | |||||
| CR | ASR | CR | ASR | LEB | MYS | GNI | HDI | |
| Very High Human Development | 5.3 | 3.6 | 4.1 | 3 | 80.2 | 11.7 | 40,046 | 0.89 |
| High Human Development | 10.4 | 5.9 | 8.1 | 4.2 | 74.5 | 8.1 | 13,231 | 0.74 |
| Medium Human Development | 10.6 | 14.3 | 9.9 | 13.5 | 67.9 | 5.5 | 5,960 | 0.61 |
| Low Human Development | 3.9 | 7.1 | 3.7 | 6.7 | 59.4 | 4.2 | 2,904 | 0.49 |
| P -value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | - |
a) LC: Lung cancer, CR: Crude Rate, ASR: Age-Standardized Rates per 100,000, HDI: Human Development Index, LEB: Life Expectancy at Birth, MYS: Mean years of schooling, GNI: Gross national income per capita
The negative value of concentration index in Tabl 2 for incidence and death from LC indicated that this cancer in more concentrated in low HDI countries.
| Cancer type | New cases diagnosed in 2012 (1,000s) | Death form LC in 2012 (1,000s) | Incidence concentration index (95%CI) | Mortality concentration index (95%CI) |
| Liver Cancer | 782 | 746 | -0.1 (-0.17, -0.027) | -0.1 (-0.17, -0.03) |
Linear regression model showed that increasing of HDI had a negative effect on the increase in both incidence (B=-12.2, P=0.03) and mortality (B=-12.7, P=0.015) rates of LC. The mean of life expectancy at birth, mean years of schooling, GNI per capita, percent of urbanization, and age-standardized obesity had also a negative effect on increasing in both incidence and mortality rates (Tabl 3).
| Variable | Liver cancer incidence | Liver cancer mortality | ||||
| β | CI | P-value | β | CI | P-value | |
| Life expectancy at birth | -0.16 | (-0.03, 0.03) | 0.1 | -0.16 | (-0.3, 0.01) | 0.07 |
| Mean years of schooling | -0.65 | (-1.2, -0.1) | 0.02 | -0.67 | (-1.2, -0.2) | 0.01 |
| Gross national income per 1000 capita | -0.01 | (-0.02, 0) | 0.06 | -0.01 | (-0.02, 0) | 0.03 |
| HDI | -12.2 | (-23, 1.2) | 0.03 | -12.7 | (-23, -2.5) | 0.015 |
| Urbanization level (%) | -0.07 | (-0.15, -0.1) | 0.047 | -0.08 | (-0.15, -0.1) | 0.024 |
| Age standardized obesity in adults | -0.25 | (-0.42, -0.1) | 0.002 | -0.24 | (-0.39, -0.1) | 0.002 |
Discussion
Our results showed that in 2012, 782,000 cases of LC worldwide has been identified that was associated with 746,000 deaths from this cancer. Therefore, it is the fifth most common cancer in men (7.5% of the total) and the ninth in women (3.4% of total). The results showed that the incidence of LC and its mortality rate are significantly concentrated in regions with medium and low HDI. According to the results of this study, there was a significant negative correlation between the HDI with the incidence and mortality of LC. So that an increase in this index, incidence and mortality rate of LC were significantly decreased. This significant negative relationship is also true for each of the main components of HDI and ancillary indexes including; the level of urbanization and age-standardized obesity in adults, but this negative relationship between life expectancy and LC mortality was not significant. This finding is consistent with results of other studies that have examined the relationship between HDI and LC [9 12, ]. The most ASR for incidence and mortality of LC occurred in countries with medium HDI, countries that fall into this category are in transition towards industrialization; this transition affects all aspects of people's lives including health-related aspects such as lifestyle, diet, smoking, alcohol consumption and sexual behavior, on the other hand, LC is one of the infection-related cancers [13] and this despite the fact that all of these factors are involved in causing cancer [14].
Forasmuch as HDI composed of main components that include: life expectancy at birth, mean years of schooling, and gross national income (GNI) per capita, the role of each of these components can be examined in this findings. Life expectancy, as one of the most important indicators of health, differs widely between countries and regions [15]. Regional differences in life expectancy and other health indicators are explained not only by individual and behavioral factors but also by the social and environmental characteristics of communities [16], including levels of social capital and cohesion, access to health services, access to green-blue spaces and environmental pollutants. However, many of these factors are effective in reducing the risk of the incidence and mortality of LC.
Increasing mean years of schooling will also increase the level of health literacy; in previous studies, the role of health literacy has been proven in reducing high-risk behaviors such as smoking, alcohol consumption, sexual behavior, physical inactivity, and poor lifestyle, which cause cancer. On the other hand, illiterate people pay more attention to health messages and will follow cancer prevention and control programs [17].
Income is another factor that affecting the change in lifestyle, particularly nutritional status. Results of other studies have shown that people with low incomes consume more tobacco and alcohol, and eat fewer fruits and vegetables than those with higher incomes [18]. Poor nutritional status and low intake of fresh fruits and vegetables will lead to malnutrition. The malnutrition accounts possible factors for LC development [4].
According to our results, increasing the level of urbanization is associated with reducing the incidence and mortality of LC. People who live in urban areas have better living conditions and socio-economic status; as a result, they have greater access to infrastructure and health services such as hygienic water, varied and healthy food [19]. Urbanization may also lead to increased levels of education and income that the role of these factors in reducing the incidence and mortality of LC was mentioned.
The role of obesity as a risk factor in cancer has been shown in several studies [20 21, ] that are not consistent with the findings of our study. Given that obesity is associated with urbanization [22] that is unavoidable in regions with high HDI, so in this study, obesity considers as a confounding factor in conjunction with the HDI.
One limitation of this study is impossible to generalize the results to small communities; it is recommended that similar studies be done at the national level and smaller communities in different countries. It also recommended that in future studies, other aspects related to EC to be surveyed.in Conclusion incidence and mortality rate of LC are significantly concentrated in regions with medium and low HDI. The negative relationship between LC incidence and mortality with HDI and its component can be considered as targets for prevention and treatment intervention or tracking geographic disparities.
Acknowledgments
This paper used data from the GLOBOCAN and the United Nations Development Programme (UNDP). The authors declare that there is no conflict of interests to report for this work.
References
[1]. Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21(1):59-69.
[2]. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86.
[3]. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-73 e1.
[4]. Mazzanti R, Arena U, Tassi R.Hepatocellular carcinoma: Where are we?.World J Exp Med. 2016; 6(1): 21–36.
[5]. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24(1):1-17.
[6]. Yovel Y, Franz MO, Stilz P, Schnitzler HU. Plant classification from bat-like echolocation signals. PLoS Comput Biol. 2008;4(3):e1000032.
[7]. Wiegrebe L. An autocorrelation model of bat sonar. Biol Cybern. 2008;98(6):587-95.
[8]. Leaf-nosed bat. Encyclopædia Britannica: Encyclopædia Britannica Online; 2009.
[9]. Greenhall AM. House bat management. Jamestown, ND: Northern Prairie Wildlife Research Center Online; 1982..
[10]. Jen PHS, Wu CH. Echo duration selectivity of the bat varies with pulse-echo amplitude difference. Neuroreport. 2008;19(3):373-7.
[11]. Brinklov S, Kalko EKV, Surlykke A. Intense echolocation calls from two ‘whispering’ bats, Artibeus jamaicensis and Macrophyllum macrophyllum (Phyllostomidae). J Exp Biol. 2009;212(1):11-20.
[12]. Holland RA, Kirschvink JL, Doak TG, Wikelski M. Bats use magnetite to detect the earth’s magnetic field. PLoS One. 2008;3(2):e1676, 1-6.
[13]. Pakzad R, Mohammadian-Hafshejani A, Khosravi B, Soltani S, Pakzad I, Mohammadian M, Salehiniya H, Momenimovahed Z. The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann Transl Med 2016;4(2):29.
[14]. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians 2015; 65(1): 5-29.
[15]. Grothe B, Park TJ. Structure and function of the bat superior olivary complex. Microsc Res Tech. 2000;51(4):382-402.
[16]. Chiu C, Xian W, Moss CF. Flying in silence: Echolocating bats cease vocalizing to avoid sonar jamming. Proc Natl Acad Sci U S A. 2008;105(35):13116-21.
[17]. Teeling EC, Springer MS, Madsen O, Bates P, O’Brien SJ, Murphy WJ. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307(5709):580-4.
[18]. Smith T, Rana RS, Missiaen P, Rose KD, Sahni A, Singh H, et al. High bat (Chiroptera) diversity in the Early Eocene of India. Naturwissenschaften. 2007;94:1003-9.
[19]. Moss CF, Sinha SR. Neurobiology of echolocation in bats. Curr Opin Neurobiol. 2003;13(6):751-8.
[20]. Mazzanti R, Arena U, Tassi R.Hepatocellular carcinoma: Where are we?.World J Exp Med. 2016; 6(1): 21–36.
[21]. DeLong CM, Bragg R, Simmons JA. Evidence for spatial representation of object shape by echolocating bats (Eptesicus fuscus). J Acoust Soc Am. 2008;123(6):4582-98.
[22]. Avila-Flores R, Medellin RA. Ecological, taxonomic, and physiological correlates of cave use by mexican bats. J Mammal. 2004;85(4):675-87.
[23]. EUROBATS Secretariat. EUROBATS: The Agreement on the Conservation of Populations of European Bats: EUROBATS; 2004 [Available from: http://www.eurobats. org/index.htm.
[24]. Chiu C, Moss CF. The role of the external ear in vertical sound localization in the free flying bat, Eptesicus fuscus. J Acoust Soc Am. 2007;121(4).
License
Copyright
© ,
Author Details