Prognostic and Predictive Role of Inflammatory Blood Markers in Early and Locally Advanced Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: An Egyptian Single-Center Experience
Download
Abstract
Background: Inflammatory blood biomarkers (IBMs), including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), lymphocyte-to-monocyte ratio (LMR), systemic inflammation response index (SIRI), pan-immune-inflammation value (PIV), and systemic inflammation index (SII), have been proposed as prognostic and predictive markers in cancer. This study evaluated their predictive value for pathological complete response (pCR), disease-free survival (DFS), overall survival (OS), and neoadjuvant chemotherapy (NACT)-related toxicities in early and locally advanced breast cancer (BC).
Methods: A retrospective analysis was conducted on 284 BC patients receiving NACT. Associations between IBMs, treatment response, survival outcomes, and chemotherapy-related toxicities were analyzed.
Results: -IBMs were significantly associated with chemotherapy-related toxicities. Neutrophils, lymphocytes, monocytes, NLR, SII, SIRI, and PIV (all p < 0.001) strongly predicted febrile neutropenia, along with doublet anti-HER2 therapy (p = 0.032). Predictors of neutropenia included neutrophil, monocyte, NLR, MLR, LMR, SII, SIRI, PIV (p < 0.05), HER2-positive status, and doublet anti-HER2 therapy. -Subgroup analyses showed IBM predictive performance varied by subtype. NLR predicted DFS in HER2+ patients (AUC = 0.839, p = 0.010); neutrophil count was linked to peripheral neuropathy in HR+/HER2− patients (p = 0.042). PLR and LMR showed excellent discrimination for febrile neutropenia in TNBC (AUCs > 0.92). In TNBC, MLR, SIRI, and PIV showed moderate-to-high discrimination for OS (AUCs 0.71–0.74). Neutrophil (p = 0.0058) and lymphocyte (p = 0.0248) levels were associated with pCR in HER2+ patients. HR+ subtypes showed limited IBM predictive value.
Conclusion: IBMs demonstrated strong predictive value for chemotherapy-related toxicities and showed subtype-specific relevance for survival and treatment response. These findings support the integration of molecular stratification to enhance the predictive utility of IBMs in breast cancer, highlighting their clinical potential in anticipating and managing treatment-related adverse events and guiding personalized supportive care strategies.
Introduction
The great majority of female cancer diagnoses and deaths occur from breast cancer (BC) [1].
It is predicted to cause over 46,000 new cases by 2050 and has an age-standardized prevalence of 48.8 within 100,000 in Egypt, resulting in it becoming the most prevalent cancer among females [2]. While BC incidence has increased, mortality has declined in recent decades due to improved early diagnosis and neoadjuvant chemotherapy (NACT). NACT is being used more and more in early BC, especially in HER2-positive and triple-negative cases, to assess tumor chemosensitivity in vivo and to facilitate breast-conserving surgery (BCS) [3]. Since comparable prognoses can occur across different TNM stages or subtypes, the predictive accuracy of tumor size, TNM staging, Ki67 expression, receptor status (ER, PR, HER2), and molecular subtypes (luminal, HER2-positive, triple-negative) in relation to BC prognosis is inadequate [3].
Additional immunological and histological markers are prognostic but are limited by high costs and time requirements, whereas routine peripheral blood tests offer a simple, cost-effective alternative [4].
Inflammation promotes tumor growth, blood vessel formation, metastasis, and resistance to chemotherapy; It is the seventh hallmark. The number of white blood cells indicate systemic or localized inflammation, and inflammatory blood markers (IBMs), including lymphocyte (L), neutrophil (N), monocyte (M), and platelet (P) counts, as well as ratios such as platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), lymphocyte-to- monocyte ratio (LMR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and Pan-Immune-Inflammation-Value (PIV), are potential prognostic and predictive factors in BC and other cancers [5]. Investigating IBMs as potential markers of pathological complete response (pCR) and survival patients suffering from early-stage or locally advanced BC who were treated with NACT has yielded contradictory findings [6-8].
This study aimed to evaluate whether IBMs predict pCR and are prognostic for disease-free survival (DFS) and overall survival (OS) in patients with early and locally advanced BC undergoing NACT, and also to assess their predictive value for NACT-related toxicities.
Materials and Methods
Patients
This retrospective study included patients with early or locally advanced breast cancer who received neoadjuvant chemotherapy at our institution between January 2017 and December 2021. Participation was restricted to women whose core needle biopsy confirmed the presence of non-metastatic BC, who had finished neoadjuvant chemotherapy, and who had full blood count data available at baseline. Patients were excluded if baseline blood analyses were unavailable or if they had systemic inflammatory or autoimmune diseases, chronic conditions, secondary malignancies, pregnancy, or long-term use of steroids, NSAIDs, or immunomodulators. Consecutive sampling was employed, whereby all eligible patients who met the inclusion criteria during the predefined study period were included.
Treatments
All patients received neoadjuvant chemotherapy regimens that included either anthracycline or taxane or both sequentially. For 12 months, patients who tested positive for HER2 were given trastuzumab (± pertuzumab) together with taxane, and then they were given adjuvant trastuzumab (± pertuzumab) or TDM1. Patients who tested positive for hormone receptors were given adjuvant endocrine treatment for a minimum of five years. Adjuvant radiotherapy adhered to ESMO guidelines [9].
Endpoints
The lack of invasive cancer in the breast and axillary nodes, with or without in situ ductal carcinoma (ypT0/ is ypN0), was considered a pCR. [10]. We calculated DFS as the time it took from biopsy to either a local, loco-regional, distant, or progression-related relapse or death. Time from biopsy to death, regardless of cause, was referred to as OS [11]. Toxicities (neutropenia, febrile neutropenia, peripheral neuropathy) were assessed using CTCAE version 5.0 [12]. Lacking standard IBM cutoffs, thresholds were tested using ROC curves and medians. IBMs included: NLR: neutrophil count / lymphocyte count [13]. PLR: platelet count / lymphocyte count [13]. MLR: monocyte count / lymphocyte count [13]. LMR: lymphocyte count / monocyte count [13]. SII: N × P / L [14]. Systemic inflammation response index (SIRI): N × M / L [15]. PIV: N × M × P / L [13].
Statistical Analysis
The data was analyzed using SPSS version 27. coding, and analysis. For the continuous variables, means were utilized as descriptive statistics, medians, and ranges, while the categorical variables were described using percentages and frequencies. The Mann-Whitney U test or Student’s t-test were used for continuous variables in the baseline characteristics comparison, while for categorical variables, Fisher’s exact or chi-square test were employed. For Kaplan-Meier methods estimated DFS and OS, with log-rank tests comparing groups. ROC curves determined optimal IBM thresholds. A 95% confidence interval and 5% error margin were applied, with significance at P ≤ 0.05.
Ethical Considerations
The study was reviewed and approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University. Given its retrospective, non-interventional design based on routinely collected clinical data, the requirement for informed consent was waived. All patient data were anonymized to ensure confidentiality, and data handling procedures complied with institutional privacy policies and ethical standards.
Results
Patients’ Characteristics
A total of 284 patients were included. At diagnosis, the median age was 48 years, and 41.2% of the patients were postmenopausal. Clinical stage cT2 was the most common (47.2%), and clinical lymph node invasion was observed in 83.1% of cases. Histological grade two illness was observed in 78.2% of patients, while invasive ductal carcinoma was present in 95.4% of patients. Hormone receptor-positive/HER2-negative BC was the most common subtype (54.23%). Since no major comorbidities were reported, all patients were fit to undergo neoadjuvant chemotherapy. Prior to beginning treatment median values for lymphocyte, neutrophil, platelet, and monocyte counts, as well as NLR, PLR, MLR, LMR, SII, SIRI, and PIV, are summarized in Table 1.
| N= 284 | ||
| Age (years) | 48 (25-77) | |
| Menopausal status (%) | Pre | 167 (58.8) |
| Post | 117 (41.2) | |
| Pathology | IDC | 271 (95.42) |
| type (%) | ||
| ILC | 10 (3.52) | |
| Other | 3 (1.06) | |
| Grade (%) | 1 | 4 (1.41) |
| 2 | 222 (78.17) | |
| 3 | 58 (20.42) | |
| Hormonal | Negative | 45 (15.85) |
| status (%) | ||
| Positive | 239 (84.15) | |
| Her2 | Negative | 175 (61.62) |
| status (%) | ||
| Positive | 109 (38.38) | |
| Molecular subtype (%) | HR + ve, her2 -ve | 154 (54.23) |
| HR + ve, her2 + ve | 86 (30.28) | |
| her +ve | 23 (8.1) | |
| triple -ve | 21 (7.39) | |
| cT (%) | T1 | 3 (1.06) |
| T2 | 134 (47.18) | |
| T3 | 58 (20.42) | |
| T4 | 89 (31.34) | |
| Inflammatory breast cancer (IBC) (%) | Negative | 267 (94.01) |
| Positive | 17 (5.99) | |
| cN (%) | N0 | 48 (16.9) |
| N1 | 205 (72.18) | |
| N2 | 19 (6.69) | |
| N3 | 12 (4.23) | |
| Nodal status (%) | Negative | 48 (16.9) |
| Positive | 236 (83.1) | |
| Baseline biology | Neutrophil (10³/ul) | 4 (1.19-13) |
| Lymphocyte (10³/ul) | 2.31 (0.73-6.2) | |
| Monocyte (10³/ul) | 0.53 (0.07-2) | |
| Platelet (10³/ul) | 300 (122-664) | |
| NLR | 1.74 (0.37-10) | |
| PLR | 131.67 (42.1-457.5) | |
| MLR | 0.23 (0.02-1) | |
| LMR | 4.34 (1-45) | |
| SII | 509.13 (128.15-2999.38) | |
| SIRI | 0.94 (0.08-13) | |
| PIV | 278.1 (17.18-3536) |
Data is presented as median (IQR) or frequency (%). IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma, other: invasive medullary carcinoma, invasive mucinous carcinoma, and poorly differentiated carcinoma, HR: hormone receptor, cT: clinical tumor size, cN: clinical node involvement, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, MLR: monocyte to lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio, SII: systemic immune-inflammation index, SIRI: systemic inflammation response index, PIV: pan-immune-inflammation-value.
Predictive Factors for pCR
Sixty-three patients (22.18%) reached pCR. Chemotherapy regimens (P = 0.006), anti her2 regimens (especially with doublet anti her2) (P <0.001), her2 status (P < 0.001) and molecular subtype (her2 +ve) (P < 0.001) were correlated with pCR. When analyzing IBM using the ROC curve and median of variables, none of the IBM showed significant correlation with pCR. Also, no significant correlation with other variables were found such as age, tumor pathology and grade, clinical T stage, nodal status, hormonal status, and inflammatory breast cancer (IBC).
Prognostic Factors for DFS
DFS was assessed across the entire study cohort, with a median DFS of 40 months (range: 9–89 months). At final follow-up, 72 patients (25.35%) had experienced disease relapse, including 18 (6.35%) with locoregional recurrence and 54 (19%) with distant metastases.
Type of pathology (invasive lobular carcinoma (ILC), invasive mucinous carcinoma, poorly differentiated carcinoma, and invasive medullary cancer (p=0.01). Molecular subtype (triple -ve and her2 +ve) (p =0.024), higher cT stage (p= 0.018) and not reaching pCR (p <0.001) were all correlated with a higher risk of relapse. The ROC curve and median of the variables showed no significant correlation between any of the IBM and DFS. Also, no significant correlation with other variables were found such as age, tumor grade, her 2 status, nodal status, IBC, chemotherapy and anti her2 regimens.
Prognostic Factors for OS
An assessment of OS was conducted for the entire study population. The median OS was 43 months (range 13 – 89 months). At the final follow-up assessment, 50 patients (17.61%) had died. Of these, 39 patients (13.7%) died due to BC, 4 patients (1.41%) due to intercurrent illnesses, and the cause of death was unknown for 7 patients (2.5%).
Higher c T stage (p= 0.037) and not reaching pCR (p <0.001) were all correlated with a poor survival. There was no statistically significant relationship between any of the IBM and OS when ROC curve and median of variables were used. Also, no significant correlation with other variables were found such as age, tumor pathology and grade, her 2 status, nodal status, IBC, molecular subtypes, chemotherapy and anti her2 regimens.
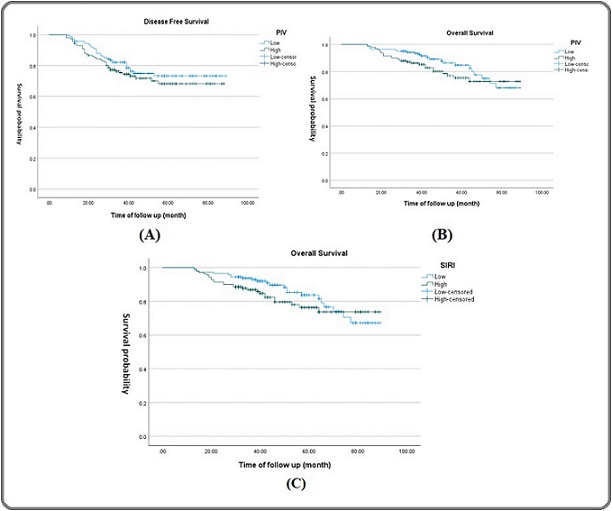
As demonstrated by our Kaplan-Mire curve (Figure 1), there is a tendency for low PIV to slightly predict better survival outcomes (DFS (p=0.317) and OS (p=0.195) and low SIRI to slightly predict better OS (p=0.283), but these tendencies are not statistically significant.
Figure 1. Kaplan-Meier Survival Curves Showing Correlation between Prognostic Indices and Survival Outcomes.(A) PIV and Disease-Free Survival (DFS), (B) PIV and Overall Survival (OS), and (C) SIRI and Overall Survival (OS).Each plot includes clearly labeled axes (Time of follow up (months) on x-axis and Survival Probability on y-axis), distinct curve labels (e.g., High vs. Low index groups).
Predictive Factors for Febrile Neutropenia
Twenty-seven (27) patients (9.51%) developed febrile neutropenia during neo-adjuvant chemotherapy. Neutrophil (AUC=0.88, 95% CI=0.836 to 0.915, p<0.001), lymphocyte (AUC=0.679, 95% CI=0.621 to 0.733, p=0.001), monocyte (AUC=0.71, 95% CI=0.653 to 0.762, p<0.001), NLR (AUC=0.746, 95% CI=0.691 to 0.796, p<0.001), SII (AUC=0.765, 95% CI=0.712 to 0.813, P<0.001), SIRI (AUC=0.82, 95% CI=0.770 to 0.863, P<0.001), PIV (AUC=0.812, 95% CI=0.762 to 0.856, P<0.001) were found as predictive factors for febrile neutropenia using ROC curve. Cut off for Neutrophils (≤2.31), lymphocyte (≤1.98), monocyte (≤0.46), NLR (≤1.31), SII (≤293.75), SIRI (≤0.71), PIV (≤215.77) using ROC curve to divide patients to 2 groups high and low that revealed patients in low groups, these inflammatory markers were predictive for febrile neutropenia. Also, by using median of variables, NLR (p<0.001), SII (P<0.001), SIRI (P<0.001), PIV (P<0.001) were predictive for febrile neutropenia. Anti her2 regimen (especially with doublet antiher2) (p= 0.032) was predictive for febrile neutropenia. However, no other variables were significant predictive for febrile neutropenia (Table 2 and 4).
| Febrile neutropenia (%) | P | ||||
| No | Yes | ||||
| Pathology type | IDC | 245 (90.41) | 26 (9.59) | 1 | |
| ILC | 9 (90) | 1 (10) | |||
| Other | 3 (100) | 0 (0) | |||
| Grade | 1 | 3 (75) | 1 (25) | 0.26 | |
| 2 | 203 (91.44) | 19 (8.56) | |||
| 3 | 51 (87.93) | 7 (12.07) | |||
| Her2 status | Negative | 160 (91.95) | 14 (8.05) | 0.291 | |
| Positive | 97 (88.18) | 13 (11.82) | |||
| Molecular subtype | HR + ve , her2 -ve | 142 (92.21) | 12 (7.79) | 0.632 | |
| HR + ve , her2 + ve | 76 (88.37) | 10 (11.63) | |||
| her +ve | 20 (86.96) | 3 (13.04) | |||
| triple -ve | 19 (90.48) | 2 (9.52) | |||
| cT | T1 | 3 (100) | 0 (0) | 0.526 | |
| T2 | 118 (88.06) | 16 (11.94) | |||
| T3 | 55 (94.83) | 3 (5.17) | |||
| T4 | 81 (91.01) | 8 (8.99) | |||
| IBC | Negative | 241 (90.26) | 26 (9.74) | 1 | |
| Positive | 16 (94.12) | 1 (5.88) | |||
| cN | N0 | 43 (89.58) | 5 (10.42) | 0.952 | |
| N1 | 185 (90.24) | 20 (9.76) | |||
| N2 | 18 (94.74) | 1 (5.26) | |||
| N3 | 11 (91.67) | 1 (8.33) | |||
| Chemotherapy | Anthracycline based | 3 (100) | 0 (0) | 0.779 | |
| Taxanes based | 17 (89.47) | 2 (10.53) | |||
| Anthracycline + taxanes based | 237 (90.46) | 25 (9.54) | |||
| Antiher2 | No | 193 (93.24) | 14 (6.76) | 0.032* | |
| Trastuzumab (single) | 52 (83.87) | 10 (16.13) | |||
| Trastuzumab + pertuzumab (doublet) | 12 (80) | 3 (20) | |||
| IBM by median | |||||
| NLR | ≤1.74 | 120 (84.5) | 22 (15.5) | <0.001* | |
| >1.74 | 137 (96.5) | 5 (3.5) | |||
| PLR | ≤131.67 | 133 (93.66) | 9 (6.34) | 0.117 | |
| >131.67 | 124 (87.32) | 18 (12.68) | |||
| MLR | ≤0.23 | 125 (88) | 17 (12) | 0.189 | |
| >0.23 | 132 (93) | 10 (7) | |||
| LMR | ≤4.34 | 132 (93) | 10 (7) | 0.189 | |
| >4.34 | 125 (88) | 17 (12) | |||
| SII | ≤509.13 | 121 (85.2) | 21 (14.8) | <0.001* | |
| >509.13 | 136 (95.8) | 6 (4.2) | |||
| SIRI | ≤0.94 | 118 (83) | 24 (17) | <0.001* | |
| >0.94 | 139 (98) | 3 (2) | |||
| PIV | ≤278.1 | 118 (83) | 24 (17) | <0.001* | |
| >278.1 | 139 (98) | 3 (2) | |||
| IBM by ROC | |||||
| Neutrophil | ≤2.31 | 10 (31.25) | 22 (68.75) | <0.001* | |
| >2.31 | 247 (98) | 5 (2) | |||
| Lymphocyte | ≤1.98 | 72 (82) | 16 (18) | 0.001* | |
| >1.98 | 185 (94.4) | 11 (5.6) | |||
| Monocyte | ≤0.46 | 86 (82) | 19 (18) | <0.001* | |
| >0.46 | 171 (95.5) | 8 (4.5) | |||
| NLR | ≤1.31 | 56 (76.7) | 17 (23.3) | <0.001* | |
| >1.31 | 201 (95.3) | 10 (4.7) | |||
| IBM by ROC | |||||
| SII | ≤293.75 | 27 (74.3) | 15 (35.7) | <0.001* | |
| >293.75 | 230 (95) | 12 (5) | |||
| SIRI | ≤0.71 | 76 (76.77) | 23 (23.23) | <0.001* | |
| >0.71 | 181 (97.8) | 4 (2.2) | |||
| PIV | ≤215.77 | 87 (79.1) | 23 (20.9) | <0.001* | |
| >215.77 | 170 (97.7) | 4 (2.3) |
Data is presented as frequency (%). * Significant P value <0.05. IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma, other: invasive medullary carcinoma, invasive mucinous carcinoma, and poorly differentiated carcinoma, HR: hormone receptor, cT: clinical tumor size, cN: clinical node involvement, IBC: Inflammatory breast cancer, IBM: inflammatory blood markers, ROC: receiver operating characteristic curves, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, MLR: monocyte to lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio, SII: systemic immune-inflammation index, SIRI: systemic inflammation response index, PIV: pan-immune-inflammation-value.
Predictive Factors for neutropenia
One hundred forty-five patients (51.06%) developed neutropenia during neo-adjuvant chemotherapy. The most common neutropenia grades are grades 2 and 3 (55 patients (19.37%) for each grade), then grade 4 (26 patients (9.15%), and last grade 1(9 patients (3.17%).
Neutrophil (AUC=0.638, 95% CI=0.579 to 0.694, p<0.001), monocyte (AUC=0.599, 95% CI =0.540 to 0.657, p=0.003), NLR (AUC=0.601, 95% CI=0.542 to 0.659, p=0.002), MLR (AUC=0.570, 95% CI=0.510 to 0.628, P=0.04), LMR (AUC=0.570, 95% CI=0.510 to 0.629, P=0.04), SII (AUC=0.602, 95% CI=0.543 to 0.660, P=0.002), SIRI (AUC=0.638, 95% CI= 0.579 to 0.694, P<0.001), PIV (AUC=0.625, 95% CI=0.566 to 0.682, P<0.001) were found as predictive factors for neutropenia using ROC curve. Cut off for Neutrophil (≤3.34), monocyte (≤0.47), NLR (≤2.29), MLR (≤0.28), LMR (>3.55), SII (≤468.13), SIRI (≤0.87), PIV (≤230.49) using ROC curve to divide patients to 2 groups high and low that revealed patients in low groups except for LMR the high group, those inflammatory markers were predictive for neutropenia. Also, by using median of variables, NLR (p=0.003), MLR (P=0.041), LMR (P=0.041), SII (P=0.003), SIRI (P<0.001), PIV (P<0.001) were predictive
for neutropenia. Her status (her2 +ve) (p=0.017), molecular subtype (her2+ ve) (p=0.018), anti her2 regimen (especially with double anti her2) (p= 0.007) were predictive for neutropenia. But no other variables were significant predictive for neutropenia (Table 3 and 4).
| Neutropenia (%) | P | ||||
| No | Yes | ||||
| Pathology type | IDC | 135 (49.82) | 136 (50.18) | 0.161 | |
| ILC | 2 (20) | 8 (80) | |||
| Other | 2 (66.67) | 1 (33.33) | |||
| Grade | 1 | 2 (50) | 2 (50) | 0.956 | |
| 2 | 108 (48.65) | 114 (51.35) | |||
| 3 | 29 (50) | 29 (50) | |||
| Her2 status | Negative | 95 (54.6) | 79 (45.4) | 0.017* | |
| Positive | 44 (40) | 66 (60) | |||
| Molecular subtype | HR + ve , her2 -ve | 84 (54.55) | 70 (45.45) | 0.018* | |
| HR + ve , her2+ve | 38 (44.19) | 48 (55.81) | |||
| her +ve | 5 (21.74) | 18 (78.26) | |||
| triple -ve | 12 (57.14) | 9 (42.86) | |||
| cT | T1 | 2 (66.67) | 1 (33.33) | 0.135 | |
| T2 | 67 (50) | 67 (50) | |||
| T3 | 34 (58.62) | 24 (41.38) | |||
| T4 | 36 (40.45) | 53 (59.55) | |||
| IBC | Negative | 132 (49.44) | 135 (50.56) | 0.509 | |
| Positive | 7 (41.18) | 10 (58.82) | |||
| cN | N0 | 21 (43.75) | 27 (56.25) | 0.56 | |
| N1 | 100 (48.78) | 105 (51.22) | |||
| N2 | 12 (63.16) | 7 (36.84) | |||
| N3 | 6 (50) | 6 (50) | |||
| Chemotherapy | Anthracycline based | 2 (66.67) | 1 (33.33) | 0.142 | |
| Taxanes based | 13 (68.42) | 6 (31.58) | |||
| Anthracycline + taxanes based | 124 (47.33) | 138 (52.67) | |||
| Antiher2 | No | 112 (54.11) | 95 (45.89) | 0.007* | |
| Trastuzumab (single) | 24 (38.71) | 38 (61.29) | |||
| Trastuzumab + pertuzumab (doublet) | 3 (20) | 12 (80) | |||
| IBM by median | |||||
| NLR | ≤1.74 | 62 (43.66) | 80 (56.34) | 0.003* | |
| >1.74 | 77 (54.2) | 65 (45.8) | |||
| PLR | ≤131.67 | 73 (51.4) | 69 (48.6) | 0.699 | |
| >131.67 | 66 (46.5) | 76 (53.5) | |||
| MLR | ≤0.23 | 65 (45.8) | 77 (54.2) | 0.041* | |
| >0.23 | 74 (52.1) | 68 (47.9) | |||
| LMR | ≤4.34 | 74 (52.1) | 68 (47.9) | 0.041* | |
| >4.34 | 65 (45.8) | 77 (54.2) | |||
| SII | ≤509.13 | 62 (43.66) | 80 (56.34) | 0.003* | |
| >509.13 | 77 (54.2) | 65 (45.8) | |||
| SIRI | ≤0.94 | 55 (38.7) | 87 (61.3) | <0.001* | |
| >0.94 | 84 (59.2) | 58 (40.8) | |||
| PIV | ≤278.1 | 56 (39.4) | 86 (60.6) | <0.001* | |
| >278.1 | 83 (58.5) | 59 (41.5) | |||
| IBM by ROC | |||||
| Neutrophil | ≤3.34 | 24 (28.24) | 61 (71.76) | <0.001* | |
| >3.34 | 115 (57.8) | 84 (42.2) | |||
| Monocyte | ≤0.47 | 43 (38.4) | 69 (61.6) | 0.003* | |
| >0.47 | 96 (55.8) | 76 (44.2) | |||
| NLR | ≤2.29 | 92 (43.6) | 119 (56.4) | 0.0028* | |
| >2.29 | 47 (64.4) | 26 (35.6) | |||
| MLR | ≤0.28 | 86 (43.9) | 110 (56.1) | 0.04* | |
| >0.28 | 53 (60.2) | 35 (39.8) | |||
| IBM by ROC | |||||
| LMR | ≤3.55 | 51 (59.3) | 35 (40.7) | 0.04* | |
| >3.55 | 88 (44.4) | 110 (55.6) | |||
| SII | ≤468.13 | 46 (38.3) | 74 (61.7) | 0.002* | |
| >468.13 | 93 (56.7) | 71 (43.3) | |||
| SIRI | ≤0.87 | 42 (33.9) | 82 (66.1) | <0.001* | |
| >0.87 | 97 (60.6) | 63 (39.4) | |||
| PIV | ≤230.49 | 39 (34.5) | 74 (65.5) | <0.001* | |
| >230.49 | 100 (58.5) | 71 (41.5) |
Data is presented as frequency (%). * Significant P value <0.05. IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma, other: invasive medullary carcinoma, invasive mucinous carcinoma, and poorly differentiated carcinoma, HR: hormone receptor, cT: clinical tumor size, cN: clinical node involvement, IBC: Inflammatory breast cancer, IBM: inflammatory blood markers, ROC: receiver operating characteristic curves, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, MLR: monocyte to lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio, SII: systemic immune-inflammation index, SIRI: systemic inflammation response index, PIV: pan-immune-inflammation-value.
| Using ROC | ||||||
| Febrile neutropenia | Neutropenia | |||||
| Neutrophil | 2.31 | Neutrophil | 3.34 | |||
| Lymphocyte | 1.98 | Monocyte | 0.47 | |||
| Monocyte | 0.46 | NLR | 2.29 | |||
| NLR | 1.31 | MLR | 0.28 | |||
| SII | 293.75 | LMR | 3.55 | |||
| SIRI | 0.71 | SII | 468.13 | |||
| PIV | 215.77 | SIRI | 0.87 | |||
| PIV | 230.49 | |||||
| Using median | ||||||
| MLR | 0.23 | |||||
| LMR | 4.34 | |||||
| NLR | 1.74 | |||||
| SII | 509.13 | |||||
| SIRI | 0.94 | |||||
| PIV | 278.1 |
ROC-derived cut-offs and Median values were used for toxicity prediction analyses. IBMs: inflammatory blood markers, ROC: receiver operating characteristic curves, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, MLR: monocyte to lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio, SII: systemic immune-inflammation index, SIRI: systemic inflammation response index, PIV: pan-immune- inflammation-value.
Predictive Factors for peripheral neuropathy
One hundred seventeen patients (41.2%) developed peripheral neuropathy during neo-adjuvant chemotherapy. The most common neuropathy grade was grade 2 (48 patients (16.9%), then grade 1 (47 patients (16.55%), and last grade 3 (22 patients (7.75%). DM (p=0.009), pathology type (IDC) (p=0.03) and anti her2 regimen (especially with single anti her2) (p= 0.032) were predictive for peripheral neuropathy. None of the IBM were predictive for peripheral neuropathy using ROC curve and median of variables. Also, no other variables were significant predictive for peripheral neuropathy such as age, tumor grade, her 2 status, molecular subtypes, nodal status, clinical T stage, IBC, molecular subtypes chemotherapy regimens.
Subgroup Analysis
To evaluate whether the associations between IBMs and clinical endpoints varied by molecular subtype, we conducted subgroup analyses across HR+/HER2−, HR+/HER2+, HER2+, and triple-negative breast cancer (TNBC) groups.
pCR
Statistically significant differences were observed only in the HER2+ subgroup, where neutrophil (p = 0.0058) and lymphocyte (p = 0.0248) levels were associated with pCR status. However, ROC curve analysis revealed limited discriminative ability for both markers (AUC = 0.154 for neutrophil and AUC = 0.219 for lymphocyte), indicating that while these markers differ significantly between pCR groups, their standalone predictive performance is limited. No other markers reached statistical significance in the remaining subtypes.
DFS
In HER2+ patients, NLR was the most predictive marker (AUC = 0.839, p = 0.010), while SII showed good sensitivity and specificity, though without statistical significance. In TNBC, monocyte count, and PIV demonstrated moderate discrimination (AUCs = 0.695 and 0.673, respectively), but were not statistically significant. In HR+ subtypes, most markers yielded modest AUCs with no significant associations.
OS
Among HR+/HER2− patients, neutrophil count showed a trend toward significance (p = 0.079) and, along with SIRI and PIV, demonstrated moderate predictive performance (AUC ≈ 0.60–0.61), though none reached statistical significance. TNBC patients exhibited the strongest IBM-based discrimination, with MLR, SIRI, and PIV achieving AUCs between 0.71 and 0.74, reflecting balanced sensitivity, specificity, and positive predictive value, yet without statistical significance. In HER2+ patients, NLR, SIRI, and PIV also showed favorable AUCs (≈ 0.64–0.74) and high negative predictive value. Conversely, HR+/HER2+ patients demonstrated limited IBM discrimination (AUCs < 0.55), with no meaningful associations.
Febrile Neutropenia
In the HR+/HER2− group, neutrophil, NLR, SII, SIRI, and PIV showed statistically significant differences (p < 0.001), although their AUCs (ranging from 0.084 to 0.202) indicated poor discrimination, limiting their clinical utility. In HR+/HER2+ patients, lymphocyte (p = 0.005), monocyte (p < 0.001), and NLR (p = 0.034) were significant, but their AUCs (0.141 to 0.292) also reflected limited predictive value. PLR and LMR demonstrated moderate discrimination (AUCs = 0.625 and 0.580, respectively), though not statistically significant. In HER2+ patients, monocyte (AUC = 0.833, p = 0.075) and platelet (AUC = 0.800, p = 0.115) showed strong discrimination but did not reach statistical significance. TNBC patients showed the most promising results, with PLR (AUC = 0.974, p = 0.019) and LMR (AUC = 0.921, p = 0.057) demonstrating excellent predictive performance, with high sensitivity and negative predictive value.
Neutropenia
In HR+/HER2− patients, neutrophil and monocyte counts, along with SIRI and PIV, were significantly associated with neutropenia (p < 0.01), though their discriminative ability was limited (AUCs < 0.40). In HR+/HER2+ patients, neutrophil and monocyte levels remained significant (p < 0.05), albeit with similarly low AUCs. LMR showed the highest AUC (0.604) without statistical significance, suggesting moderate predictive value. Among HER2+ patients, NLR and neutrophil counts yielded the strongest AUCs (> 0.56), but none reached significance. In TNBC, platelet count and LMR exhibited the highest predictive accuracy (AUCs= 0.708 and 0.648, respectively), with balanced sensitivity and specificity, yet without statistical significance.
Peripheral Neuropathy
In the HR+/HER2− group, neutrophil count was significantly higher in affected patients (p = 0.042) and showed moderate predictive accuracy (AUC = 0.597). In TNBC, platelet count demonstrated the strongest performance (AUC = 0.750, p = 0.060), suggesting potential clinical relevance. Although no markers reached statistical significance in HR+/HER2+ or HER2+ subtypes, PLR and SII exhibited relatively higher AUCs (> 0.68), indicating subtype-specific trends.
Discussion
Neoadjuvant systemic therapy for breast cancer has evolved significantly, with expanding indications creating an urgent need to identify patients most likely to benefit. The immune-inflammatory system plays a critical role in tumor progression, and peripheral IBMs offer a practical means of assessing this balance. Neutrophils promote tumor invasion through mediators like MMP-9, neutrophil elastase, and IL-8 [16], while monocytes contribute to angiogenesis and immune evasion [17]. Platelets support tumor growth and dissemination [18], whereas lymphocytes are central to anti-tumor immunity [19].
IBMs derived from these cell types reflect the interplay between inflammation and host immunity. However, conflicting results across studies and within individual indices have created uncertainty about the most reliable marker [20-24]. Our cohort, representative of typical NACT candidates mostly node-positive, cT2, or advanced- stage cases included all molecular subtypes to allow a comprehensive evaluation. While some studies support the predictive value of IBMs for pCR, survival, and toxicity, others do not, reflecting the ongoing debate in the field.
In the overall cohort, IBMs did not show statistically significant associations with pCR or survival outcomes. However, subgroup analyses revealed distinct patterns of IBM performance across molecular subtypes, suggesting that their prognostic and predictive utility may be context- dependent and influenced by underlying tumour biology. NLR has been extensively investigated as a potential predictor of treatment response and survival in breast cancer patients undergoing neoadjuvant NACT. Although some studies have reported a positive association between lower preoperative levels and higher pCR rates [25], others have failed to confirm this relationship [22, 23]. Zhou et al.’s meta-analysis found that lower NLR was significantly associated with improved DFS, OS, and pCR [26], while Xue et al. reported that although higher NLR predicted worse pCR, it had no significant impact on DFS or OS [27]. In our study, NLR did not correlate significantly with either pCR or survival in the full cohort, but significantly predicted DFS in HER2+ patients, highlighting its subtype-specific relevance.
PLR has emerged as another commonly studied marker, though findings remain inconsistent. While high PLR has been associated with poorer survival and lower pCR rates in several large-scale studies [28, 29], other research has reported contradictory findings. For example, Jin et al. observed a higher pCR rate in patients with elevated PLR [30], and Ma et al. noted lower PLR in the pCR group, though without a significant link to OS [31, 32]. Our results align with those of Jiang et al., who found no significant association between PLR and pCR, DFS, or OS in NACT-treated breast cancer patients in the overall cohort [33]. However, PLR demonstrated excellent discrimination for febrile neutropenia in TNBC patients, suggesting strong predictive utility in this subgroup.
LMR has been proposed as a marker of host immune competence. Ma et al. reported that lower LMR was associated with worse DFS despite higher pCR rates [22], while Dong et al. found that high LMR was linked to better pCR outcomes, though this was not confirmed in multivariate analysis [15]. In our cohort, LMR did not show a significant correlation with pCR or survival outcomes overall, but demonstrated excellent predictive accuracy for febrile neutropenia in TNBC, reinforcing its potential utility in toxicity risk stratification.
MLR has gained attention for its role in reflecting immune suppression and tumor promoting inflammation. Some studies have linked low MLR to improved pCR and longer OS [32], and others have associated high MLR with better distant metastasis-free survival (DMFS) [8]. In our study, MLR did not show significant associations in the full cohort, but demonstrated moderate to high discrimination for OS in TNBC, though not statistically significant.
SII offers a composite measure of systemic inflammation. The meta-analysis of 1,555 patients found no significant correlation between SII and pCR, with high heterogeneity across studies [6]. In contrast, Gu et al. reported lower SII levels in patients achieving pCR, and higher SII was linked to increased risk of metastasis and recurrence after five years [34]. Similarly, patients with low SII showed longer DFS and OS in other studies [35]. Our analysis did not find any significant association between SII and pCR or survival in the full cohort, though SII showed predictive value for febrile neutropenia and neutropenia across subtypes.
SIRI has recently gained traction as a prognostic indicator. A meta-analysis of 2,997 breast cancer patients linked high SIRI to poorer OS but not DFS [36], while Chen et al. reported better OS and DFS in patients with low SIRI [37]. Dong et al. also observed higher pCR rates in the low SIRI group [15]. However, a separate meta-analysis found the association with pCR was not statistically significant due to high heterogeneity [6]. Zhou et al. further associated high SIRI with shorter OS, DFS, and PFS in solid tumors [38]. In our study, SIRI did not correlate with pCR or survival in the overall cohort, but showed moderate discrimination for OS in TNBC and predictive value for hematologic toxicities.
PIV represents a comprehensive index incorporating multiple immune cell types. A retrospective study of 743 NACT-treated patients found that low PIV was associated with better chemotherapy response and significantly longer DFS and OS [39].Similarly, patients with low PIV were more likely to achieve pCR [32]. In our study, PIV showed no significant association with pCR or survival outcomes in the overall cohort, but demonstrated predictive value for febrile neutropenia and neutropenia, with statistically significant associations in the overall cohort and selected subtypes. It also showed moderate discrimination for OS in TNBC.
Several studies have compared IBMs to identify the most reliable prognostic and predictive markers. Truffi et al. found that higher MLR was linked to better 5-year DMFS but not to NLR, PLR, or PIV, and no correlation was observed between MLR and pCR [8].Bagherian et al. reported that elevated SIRI and SII were linked to lower pCR rates and higher recurrence, though not OS, while PLR and HER2 status were associated with DFS [40]. PIV was found to outperform NLR, MLR, and PLR in predicting pCR and survival [21, 32], and higher levels of NLR, PLR, SII, and PIV were linked to worse OS and DFS [41]. In HER2-positive patients, low SIRI predicted better pCR, DFS, and OS, while no associations were found for ALC, NLR, PLR, or LMR and pCR [42]. Panigoro et al. reported that while NLR was associated with 1-year mortality, neither NLR, LMR, nor PLR were linked to NACT response [43], whereas Wang et al. found that elevated NLR and PLR were associated with higher pCR rates[44]. Another study identified NLR but not PLR or LMR as an independent predictor of pCR [24]. In contrast, Karhan et al. found no predictive value for SII or PIV in TNBC, and median values of NLR, PLR, and LMR were similar regardless of pCR status [45].
Finding pCR following NACT is an important part of creating a personalized treatment plan, and it has been associated with better DFS and OS in other studies [46, 47], which is in line with what we found. Neutrophil and lymphocyte counts were significantly associated with pCR in HER2+ patients, although ROC analysis revealed limited predictive ability.
Beyond their proposed prognostic role, our findings highlight the potential clinical utility of IBMs in predicting chemotherapy-related toxicities. Specifically, NLR, SII, SIRI, and PIV were significantly associated with febrile neutropenia, while NLR, MLR, LMR, SII, SIRI, and PIV were predictive of neutropenia. These associations suggest that IBMs may serve as accessible, cost-effective tools, particularly in resource-limited settings, for early identification of patients at increased risk of hematologic toxicity. This could enable timely implementation of supportive care strategies such as dose modifications, prophylactic G-CSF administration, or closer monitoring. We further examined the relationship between IBMs and chemotherapy-induced peripheral neuropathy. No significant associations were observed in the overall cohort, which is consistent with findings by Yamanouchi et al., who reported no predictive value for NLR, PLR, or MLR in relation to peripheral neuropathy [48]. However, in our subgroup analysis neutrophil count was significantly associated with neuropathy in HR+/HER2− patients, suggesting subtype-specific relevance. Our results regarding febrile neutropenia align with those of Corbeau et al., who identified NLR but not PLR as a predictor of febrile neutropenia [5].Other studies have reported inconsistent findings, with some failing to demonstrate significant associations between various IBMs and chemotherapy-related toxicities [5, 48-50]. Notably, our cut-off values for predicting febrile neutropenia and neutropenia were higher than those reported by Corbeau et al. [5] and Ray-Coquard et al. [51], likely due to differences in methodology, laboratory standards, and population-specific inflammatory responses, as observed in studies from Asian cohorts [14]. These differences contribute to variability in cut-off values and limit the generalizability and clinical applicability of IBMs.
In this study, we evaluated seven IBMs to identify potential prognostic and predictive indicators in breast cancer. However, no statistically significant associations were observed between these biomarkers and pCR or survival outcomes in the overall cohort. Several factors may account for this lack of correlation. The limited sample size, combined with the intrinsic heterogeneity of breast cancer which encompasses diverse molecular subtypes and subtype-specific therapeutic regimens likely contributed to the observed variability. Additionally, inconsistencies in biomarker cut-off definitions, whether derived from ROC curves, median-based thresholds, or previously published criteria, complicate cross-study comparisons. Variations in laboratory reference ranges and ethnic diversity further complicate interpretation and generalizability. Although most studies measured IBMs within a similar timeframe, subtle differences in timing relative to diagnosis or treatment initiation may influence biomarker expression levels. Moreover, differences in treatment protocols and supportive care practices across institutions may impact clinical outcomes. These methodological and clinical discrepancies likely explain the divergence between our findings and those reported in prior meta-analyses.
Subgroup analyses, however, revealed that the predictive performance of IBMs varied substantially by molecular subtype, suggesting that their prognostic and predictive utility may be context-dependent and influenced by underlying tumour biology. To our knowledge, few studies have systematically explored IBM predictive performance across molecular subtypes in breast cancer, highlighting the importance of molecular stratification in enhancing the interpretability and clinical relevance of IBM-based models. Although several associations did not reach statistical significance, observed trends warrant further investigation in subtype-specific contexts.
This study is limited by its retrospective, single-centre design, which may introduce selection bias and limit control over confounding variables. The small sample size, disease heterogeneity, and variation in subtype-specific treatments may introduce additional heterogeneity within subgroups, reducing statistical power and complicating the interpretation of IBM associations. Additionally, variability in baseline CBC testing could affect the consistency of IBM measurements. Despite these limitations, IBMs demonstrated promising predictive value for chemotherapy-related toxicities, particularly neutropenia and febrile neutropenia. These findings may inform risk-adapted supportive care strategies in clinical practice, underscoring the need for standardized methodologies and larger, stratified cohorts to validate IBM utility across diverse clinical settings.
In conclusion, to the best of our knowledge, this is the first comprehensive Egyptian study to investigate the association between multiple IBMs and diverse clinical outcomes in breast cancer patients receiving neoadjuvant chemotherapy, using a unified analytical framework. By offering region-specific insights, it contributes novel data to the global understanding of immune-inflammatory dynamics in breast cancer. These markers reflect the intricate interplay between systemic inflammation and host immunity, both increasingly recognized as key modulators of tumor progression and therapeutic response.
Although IBMs did not demonstrate prognostic significance for OS, DFS, or predictive value for pCR or chemotherapy-induced peripheral neuropathy in the overall cohort, several markers showed promise in predicting hematologic toxicities. Specifically, NLR, SII, SIRI, and PIV were significantly associated with febrile neutropenia, while NLR, MLR, LMR, SII, SIRI, and PIV were predictive of neutropenia.
Importantly, subgroup analyses revealed notable variation in the predictive performance of IBMs across molecular subtypes. NLR significantly predicted DFS in HER2+ patients, while PLR and LMR demonstrated excellent discrimination for febrile neutropenia in TNBC. Neutrophil and lymphocyte counts were associated with pCR in HER2+ patients, and neutrophil count was linked to peripheral neuropathy in HR+/HER2− patients. In contrast, HR+ subtypes exhibited limited predictive value across endpoints. These findings underscore the subtype-specific relevance of IBMs and support the development of molecularly stratified risk models to enhance predictive precision and clinical applicability.
Achieving pCR was strongly correlated with improved DFS and OS, reinforcing its role as a robust prognostic indicator.
The ability to identify patients at increased risk for hematologic toxicity using accessible, cost-effective blood- based markers may enhance clinical decision-making, particularly in resource-limited settings. These findings highlight the potential utility of IBMs as adjunctive tools for toxicity risk stratification and personalized treatment planning in breast cancer. However, validation through future prospective, multicenter studies with larger and more homogeneous cohorts is essential to confirm these associations. Integrating IBMs with clinical and molecular predictors may further improve individualized risk assessment and support their incorporation into routine oncologic practice.
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
Conflict of interest
The authors declare no conflict of interest.
Financial Declarations
None.
Consent for publication
Each of the authors has granted their permission for this manuscript to be published in its current form.
Authors’ contributions
Tawfik H. T. Abdelmalak led the data collection process, ensured data quality, verified the accuracy of clinical information, contributed to the manuscript drafting, conducted statistical analyses, prepared figures, and ensured the overall coherence of the manuscript. Nivine.M.A.Gado reviewed the manuscript for intellectual content and provided methodological guidance. Mohamed Kelaney contributed in developing the idea for the study, helped with interpreting the results, and provided valuable feedback on the final product. Khaled Abdelaziz Kamal assembled the tables, structured the data presentation, and wrote the paper. Before the final text was submitted, all writers reviewed it and gave their approval.
Competing interests
The authors affirm that they are not involved in any conflicts of interest.
References
- Cancer Statistics, 2021 Siegel RL , Miller KD , Fuchs HE , Jemal A. CA: a cancer journal for clinicians.2021;71(1). CrossRef
- Cancer incidence in egypt: results of the national population-based cancer registry program Ibrahim AS , Khaled HM , Mikhail NN , Baraka H, Kamel H. Journal of Cancer Epidemiology.2014;2014. CrossRef
- Prognostic Relevance of Neutrophil to Lymphocyte Ratio (NLR) in Luminal Breast Cancer: A Retrospective Analysis in the Neoadjuvant Setting Grassadonia A, Graziano V, Iezzi L, Vici P, Barba M, Pizzuti L, Cicero G, et al . Cells.2021;10(7). CrossRef
- Correlation analysis of lymphocyte-monocyte ratio with pathological complete response and clinical prognosis of neoadjuvant chemotherapy in patients with breast cancer Meng X, Wang X, Jiang C, Zhang S, Cheng S. Translational Oncology.2022;18. CrossRef
- Inflammatory Blood Markers as Prognostic and Predictive Factors in Early Breast Cancer Patients Receiving Neoadjuvant Chemotherapy Corbeau I, Thezenas S, Maran-Gonzalez A, Colombo P, Jacot W, Guiu S. Cancers.2020;12(9). CrossRef
- Predictive value of pretreatment circulating inflammatory response markers in the neoadjuvant treatment of breast cancer: meta-analysis Dowling GP , Daly GR , Hegarty A, Hembrecht S, Bracken A, Toomey S, Hennessy BT , Hill ADK . The British Journal of Surgery.2024;111(5). CrossRef
- Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: meta-analysis Savioli F, Morrow ES , Dolan RD , Romics L, Lannigan A, Edwards J, McMillan DC . The British Journal of Surgery.2022;109(12). CrossRef
- Prognostic Potential of Immune Inflammatory Biomarkers in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy Truffi M, Sottotetti F, Gafni N, Albasini S, Piccotti F, Morasso C, Tibollo V, et al . Cancers.2022;14(21). CrossRef
- [Radiotherapy of breast cancer] Hennequin C., Barillot I., Azria D., Belkacémi Y., Bollet M., Chauvet B., Cowen D., et al . Cancer Radiotherapie: Journal De La Societe Francaise De Radiotherapie Oncologique.2016;20 Suppl. CrossRef
- U.S. Food and Drug Administration. Pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: Use as an endpoint to support accelerated approval. Guidance for Industry. 2012. Available from: https://www.fda.gov/media/83507/download .
- Disease-free survival as a surrogate for overall survival in patients with HER2-positive, early breast cancer in trials of adjuvant trastuzumab for up to 1 year: a systematic review and meta-analysis Saad ED , Squifflet P, Burzykowski T, Quinaux E, Delaloge S, Mavroudis D, Perez E, et al . The Lancet. Oncology.2019;20(3). CrossRef
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. National Cancer Institute. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf .
- The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab Ligorio F, Fucà G, Zattarin E, Lobefaro R, Zambelli L, Leporati R, Rea C, et al . Cancers.2021;13(8). CrossRef
- Systemic Immune-Inflammation Index Is Superior to Neutrophil to Lymphocyte Ratio in Prognostic Assessment of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy Jiang C, Lu Y, Zhang S, Huang Y. BioMed Research International.2020;2020. CrossRef
- Pretreatment systemic inflammation response index is predictive of pathological complete response in patients with breast cancer receiving neoadjuvant chemotherapy Dong J, Sun Q, Pan Y, Lu N, Han X, Zhou Q. BMC cancer.2021;21(1). CrossRef
- Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment Schimek V, Strasser K, Beer A, Göber S, Walterskirchen N, Brostjan C, Müller C, et al . Cell Death & Disease.2022;13(2). CrossRef
- Monocytes in the Tumor Microenvironment Ugel S, Canè S, De Sanctis F, Bronte V. Annual Review of Pathology.2021;16. CrossRef
- Role of platelets and platelet receptors in cancer metastasis Schlesinger M. Journal of Hematology & Oncology.2018;11(1). CrossRef
- Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape Mohme M, Riethdorf S, Pantel K. Nature Reviews. Clinical Oncology.2017;14(3). CrossRef
- Predictive Significance of Systemic Immune-Inflammation Index in Patients with Breast Cancer: A Retrospective Cohort Study Zhou Y, Guo X, Shen L, Liu K, Sun Q, Wang Y, Wang H, et al . OncoTargets and Therapy.2023;16. CrossRef
- Two Hematological Markers Predicting the Efficacy and Prognosis of Neoadjuvant Chemotherapy Using Lobaplatin Against Triple-Negative Breast Cancer Wang C, Shi Q, Zhang G, Wu X, Yan W, Wan A, Xiong S, et al . The Oncologist.2024;29(5). CrossRef
- Lymphocyte-to-Monocyte Ratio is Associated with the Poor Prognosis of Breast Cancer Patients Receiving Neoadjuvant Chemotherapy Ma Y, Zhang J, Chen X. Cancer Management and Research.2021;13. CrossRef
- Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal B-like (HER2−) breast cancer Hu Y, Wang S, Ding N, Li N, Huang J, Xiao Z. Clin Breast Cancer.2020;20(4). CrossRef
- Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients Eren T, Karacin C, Ucar G, Ergun Y, Yazici O, İmamoglu GI , Ozdemir N. Medicine.2020;99(22). CrossRef
- Association of clinical biomarkers and response to neoadjuvant therapy in breast cancer Feeney G, Waldron R, Miller N, Malone C, Sweeney K, McLaughlin R, Lowery A, Barry K, Kerin M. Irish Journal of Medical Science.2024;193(2). CrossRef
- Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with breast cancer receiving neoadjuvant chemotherapy: a meta-analysis Zhou Q, Dong J, Sun Q, Lu N, Pan Y, Han X. BMJ open.2021;11(9). CrossRef
- Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: Meta-analysis Xue LB , Liu YH , Zhang B, Yang YF , Yang D, Zhang LW , Jin J, Li J. Medicine.2019;98(1). CrossRef
- High Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Breast Cancer: A Meta-Analysis Zhang M, Huang X, Song Y, Gao P, Sun J, Wang Z. BioMed Research International.2017;2017. CrossRef
- Prognostic significance of platelet-to-lymphocyte ratio (PLR) in patients with breast cancer treated with neoadjuvant chemotherapy: a meta-analysis Qi X, Chen J, Wei S, Ni J, Song L, Jin C, Yang L, Zhang X. BMJ open.2023;13(11). CrossRef
- Prognostic implications of the peripheral platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in predicting pathologic complete response after neoadjuvant chemotherapy in breast cancer patients Jin X, Wang K, Shao X, Huang J. Gland Surg.2022;11(6). CrossRef
- A nomogram based on platelet-to-lymphocyte ratio for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy Ma R, Wei W, Ye H, Dang C, Li K, Yuan D. BMC cancer.2023;23(1). CrossRef
- Association of immune inflammatory biomarkers with pathological complete response and clinical prognosis in young breast cancer patients undergoing neoadjuvant chemotherapy Li F, Wang Y, Dou H, Chen X, Wang J, Xiao M. Frontiers in Oncology.2024;14. CrossRef
- The Pretreatment Systemic Inflammation Response Index as a Useful Prognostic Factor is Better Than Lymphocyte to Monocyte Ratio in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy Jiang C, Zhang S, Qiao K, Xiu Y, Yu X, Huang Y. Clinical Breast Cancer.2022;22(5). CrossRef
- Association between the systemic immune-inflammation index and the efficacy of neoadjuvant chemotherapy, prognosis in HER2 positive breast cancer-a retrospective cohort study Gu Q, Zhao J, Liu Y, Chen H, Wang L. Gland Surgery.2023;12(5). CrossRef
- The Systemic Immune-Inflammation Index is an Independent Predictor of Survival in Breast Cancer Patients Zhu M, Chen L, Kong X, Wang X, Li X, Fang Y, Wang J. Cancer Management and Research.2022;14. CrossRef
- Prognostic and clinicopathological value of systemic inflammation response index (SIRI) in patients with breast cancer: a meta-analysis Zhang S, Cheng T. Annals of Medicine.2024;56(1). CrossRef
- Pretreatment Systemic Inflammation Response Index in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy as a Useful Prognostic Indicator Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Cancer Management and Research.2020;12. CrossRef
- Systemic Inflammation Response Index as a Prognostic Marker in Cancer Patients: A Systematic Review and Meta-Analysis of 38 Cohorts Zhou Q, Su S, You W, Wang T, Ren T, Zhu L. Dose-Response: A Publication of International Hormesis Society.2021;19(4). CrossRef
- Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy Şahin AB , Cubukcu E, Ocak B, Deligonul A, Oyucu Orhan S, Tolunay S, Gokgoz MS , et al . Scientific Reports.2021;11(1). CrossRef
- 139P Assessing the predictive value of systemic inflammation response index (SIRI) and systemic immune-inflammation index (SII) for breast cancer patients undergoing neoadjuvant chemotherapy Bagherian M., Shiraji ST , Biglari M., Noori M., Moosavi A.. ESMO Open.2023;8(1). CrossRef
- Prognostic role of the systemic immune-inflammation index and pan-immune inflammation value for outcomes of breast cancer: a systematic review and meta-analysis Cheng H.-W., Wang T., Yu G.-C., Xie L.-Y., Shi B.. European Review for Medical and Pharmacological Sciences.2024;28(1). CrossRef
- Preoperative systemic inflammation response index: Clinicopathologic predictor of pathological complete response in HER2-positive breast cancer patients receiving neoadjuvant systemic therapy Wu H, Lin C, Tzeng Y, Hung C, Liu S, Yin C, Chen J, Chen Y, Yang J. Journal of the Chinese Medical Association: JCMA.2024;87(2). CrossRef
- Inflammatory indicators as a prognostic factor of clinical response in locally advanced breast cancer (LABC) patients receiving neoadjuvant chemotherapy (NAC) Panigoro SS , Patrianagara A, Sukartini N, Pakasi TA , Haryono SJ . Revista de Senologia y Patologia Mamaria.2024;37(1). CrossRef
- The predictive value of systemic immune-inflammatory markers before and after treatment for pathological complete response in patients undergoing neoadjuvant therapy for breast cancer: a retrospective study of 1994 patients Wang H, Huang Z, Xu B, Zhang J, He P, Gao F, Zhang R, Huang X, Shan M. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico.2024;26(6). CrossRef
- Predicting pathologic complete response in triple negative breast cancer Karhan O, Bilen E, İleri S, Tunç S, Balçık OY , Arak H. Eastern Journal Of Medicine.2024;29(3). CrossRef
- Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis Spring LM , Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL , Smith BL , et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2020;26(12). CrossRef
- Factors Affecting Pathological Complete Response in Locally Advanced Breast Cancer Cases Receiving Neoadjuvant Therapy: A Comprehensive Literature Review Alamoodi M. European Journal of Breast Health.2024;20(1). CrossRef
- The Relationship Between Peripheral Neuropathy Induced by Docetaxel and Systemic Inflammation-based Parameters in Patients with Breast Cancer Yamanouchi K, Kuba S, Sakimura C, Morita M, Kanetaka K, Kobayashi K, Takatsuki M, Hayashida N, Eguchi S. Anticancer Research.2017;37(12). CrossRef
- Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Journal of Cellular and Molecular Medicine.2020;24(5). CrossRef
- The Systemic Inflammation Response Index as an Independent Predictor of Survival in Breast Cancer Patients: A Retrospective Study Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, Wang J. Frontiers in Molecular Biosciences.2022;9. CrossRef
- Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy Ray-Coquard I., Borg C., Bachelot Th, Sebban C., Philip I., Clapisson G., Le Cesne A., et al . British Journal of Cancer.2003;88(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details