The Impact of Chemotherapy on the Chronic Inflammation Caused by Escherichia coli Infection and Insulin Resistance in Type 2 Diabetes-Associated Prostate Cancer Patients
Download
Abstract
Background: The most common malignancy in men is prostate cancer. Chronic inflammation and bacterial infections exacerbate insulin resistance in type 2 diabetes (T2D) patients, potentially worsened by chemotherapy in those with prostate cancer. This study investigates the effects of Docetaxel chemotherapy on systemic inflammation, Insulin Resistance, and bacterial resistance in T2D patients with prostate cancer.
Methods: Eighty participants (aged 50–75 years) were enrolled in a cross-sectional study and divided into four groups: Group 1 (T2D without cancer, n=40), Group 2 (T2D with prostate cancer, pre-chemotherapy, n=40), Group 3 (Group 2 subset post-two Docetaxel cycles), and Group 4 (Group 2 subset post-five Docetaxel cycles). Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), procalcitonin (PCT), fasting glucose, fasting insulin, and prostate-specific antigen (PSA) were measured via immunoassays. Insulin resistance was assessed using the Homeostatic Model Assessment (HOMA-IR). Urine samples were analyzed for Escherichia coli isolation and antibiotic resistance via the Kirby-Bauer method. Statistical significance was determined using t-tests (P≤0.05).
Results: Group 4 exhibited notably elevated inflammatory markers (PCT, IL-6, TNF-α), fasting glucose, fasting insulin, PSA, and the value of HOMA-IR compared to Groups 1–3 (P≤0.01). E. coli isolation rates increased from 67.5% (Group 2) to 80% (Group 4, P=0.20), with antibiotic resistance rising, notably for Amikacin (17.5% to 35%, P=0.08) and Nitrofurantoin (25% to 30%, P=0.61), though these differences were not statistically significant. Multidrug-resistant (MDR) and extensively drug-resistant (XDR) isolates also increased slightly post-chemotherapy.
Conclusion: Docetaxel chemotherapy in T2D prostate cancer patients is associated with heightened systemic inflammation, insulin resistance, and bacterial resistance, underscoring the need for integrated therapeutic strategies to mitigate these effects.
Introduction
Prostate cancer, a predominant cause of illness and death among men, originates in the prostate a gland the size of a walnut that generates seminal fluid to sustain and convey sperm. Early detection through screening improves treatment success [1], but advanced metastatic castration- resistant prostate cancer (mCRPC) often necessitates systemic therapies like chemotherapy [2]. Docetaxel, a cornerstone treatment for prostate cancer, exerts its anticancer effects by binding to microtubules, stabilizing them, and preventing their disassembly, which halts cancer cell division during mitosis [3]. However, this mechanism also disrupts rapidly dividing normal cells, such as bone marrow precursors, leading to myelosuppression characterized by neutropenia and increased susceptibility to infections which can exacerbate systemic inflammation [4]. This dynamic is especially important in type 2 diabetes (T2D), a common comorbidity with hyperglycemia, insulin resistance, and chronic low-grade inflammation [5]. T2D may affect prostate cancer prognosis due to overlapping inflammatory pathways and altered androgen signaling [6]. Increased pro-inflammatory cytokines, such as TNF-α and IL-6, impair insulin signaling in T2D by phosphorylating insulin receptor substrate-1 (IRS-1) at serine residues, causing oxidative stress and ROS [7]. This metabolic abnormality may worsen cancer inflammation and therapy responses. Prostate-specific antigen (PSA), a serine protease and key biomarker, is elevated in both malignancy and non-cancerous conditions like bacterial prostatitis [8]. Chronic bacterial infections, often caused by Escherichia coli a frequent uropathogen isolated from prostate tissue are implicated in sustained prostatic inflammation [9]. These infections activate innate immune responses, releasing cytokines that may promote carcinogenesis by fostering a pro-tumorigenic microenvironment or enhancing angiogenesis [10, 11]. In prostate cancer patients undergoing chemotherapy, Docetaxel-induced neutropenia heightens infection risk, potentially increasing bacterial persistence and antibiotic resistance. Moreover, chemotherapy suppresses adaptive immunity by depleting T-lymphocytes and impairing antigen presentation, weakening the body’s ability to resolve infections and control inflammation [12]. This effect may be more pronounced in T2D patients whose baseline immune function is already compromised by hyperglycemia and chronic inflammation [13]. Despite these interconnections, few studies have examined how Docetaxel chemotherapy influences inflammation, glucose metabolism, PSA dynamics, and bacterial resistance in prostate cancer patients with T2D. Our study addresses this gap by evaluating Docetaxel’s impact on inflammatory markers (TNF-α, IL-6, procalcitonin [PCT]), metabolic parameters (fasting glucose, insulin, HOMA-IR), PSA levels, and E. coli antibiotic resistance patterns in this population.
Materials and Methods
Subjects and study design
Participants ranged in age from 50 to 75 and were sourced from specialized hospitals in Baghdad. The study was designed to be cross-sectional. Table 1 shows the four groups of participants classified by their health status and chemotherapy exposure.
| Group | Description | Number of Participants | Age Range | Chemotherapy Details | Dose of Chemotherapy |
| Group 1 | Patients with type 2 diabetes (T2D) but without cancer | 40 | 50-75 years | None | None |
| Group 2 | Patients with type 2 diabetes (T2D) and prostate cancer (pre-chemotherapy) | 40 | 50-75 years | None | None |
| Group 3 | Patients from Group 2 with T2D and prostate cancer who underwent two cycles of chemotherapy | 40 (subset of Group 2) | 50-75 years | 2 cycles of Docetaxel (3 weeks apart) | 75 mg/m² per cycle |
| Group 4 | Patients from Group 2 with T2D and prostate cancer who underwent five cycles of chemotherapy | 40 (subset of Group 2) | 50-75 years | 5 cycles of Docetaxel (3 weeks apart) | 75 mg/m² per cycle |
Sample Collection
Ten milliliters of blood were put into tubes free of anticoagulants following venipuncture. After 30 minutes of coagulation at room temperature, the samples were spun at 3000 rpm for 10 minutes in a centrifuge. Preserved at-80°C until analysis, portions of the serum supernatant were transferred to labeled tubes. The midstream clean-catch approach was used to collect urine samples following genital cleaning with antiseptic wipes. In order to prevent the growth of bacteria, samples were placed in sterile, airtight containers that could not leak. A patient’s ID, date, and time were written on the labels. If the samples were not processed within two hours, they were refrigerated at 4°C.
Escherichia coli Isolation and Identification
Urine specimens from Groups 2 and 4 (n=40 each) were examined for E. coli. A 10 µL sample was inoculated onto MacConkey agar and blood agar, then incubated at 37°C for 18 to 24 hours. E. coli was detected through lactose-fermenting pink colonies on MacConkey agar and grey, smooth colonies on blood agar, verified by Gram staining (Gram-negative rods) and the VITEK 2 Compact system (bioMérieux, France).
Antibiotic Susceptibility Testing
In accordance with the Clinical and Laboratory Standards Institute, as outlined in CLSI M100TM (2024) [14], the antibiotic susceptibility of E. coli isolates was assessed using the Kirby-Bauer disc diffusion method. [14]. The isolates were adjusted to a 0.5 McFarland standard, introduced onto Mueller-Hinton agar, and tested with discs that contained the following compounds: 25 µg of Trimethoprim-Sulfamethoxazole, 5 µg of Ciprofloxacin, 30 µg of Ceftriaxone, 30 µg of Cefotaxime, 300 µg of Nitrofurantoin, 20/10 µg of Amoxicillin-Clavulanate, and 30 µg of Amikacin. As a result of measuring inhibitory zone widths after 16–18 hours of incubation at 37°C, plates were categorized as susceptible, intermediate, or resistant based on CLSI breakpoints.
Biomarker Analysis
Serum levels of procalcitonin (PCT), interleukin-6 (IL-6), prostate-specific antigen (PSA), and fasting insulin were quantified using the Cobas e411 analyzer (Roche, Germany) with electrochemiluminescence immunoassays (ECLIA). Tumor necrosis factor-α (TNF-α) was measured using a Human TNF-α ELISA Kit, Invitrogen- Thermo Fisher Scientific - US, with colorimetric detection.
Fasting glucose was determined on the Cobas c311 analyzer (Roche, Germany) using a photometric method at 550 nm,. All assays were performed in duplicate, with intra-assay coefficients of variation <5%.
HOMA-IR Calculation
The Homeostasis Model Assessment (HOMA-IR) used the following calculation to measure insulin resistance:
Interpreting HOMA-IR Values:
• < 1.0 → Highly insulin sensitive (ideal)
• 1.0 – 1.9 → Normal insulin sensitivity
• 2.0 – 2.9 → Early insulin resistance
• ≥ 3.0 → Significant insulin resistance (risk for metabolic disorders like type 2 diabetes)
Statistical Analysis
The statistical analysis was done using Prism 10.4 from GraphPad Software (San Diego, CA, USA). For continuous variables, the data are presented as the mean with the standard deviation shown as plus or minus. We employed one-way ANOVA and Tukey’s post hoc test for multiple pairwise comparisons among the four groups. In all two-tailed statistical tests, a p-value less than 0.05 was deemed significant. The groups exhibit substantial differences when the significance threshold is elevated (p < 0.0001).
Results
This study evaluated differences in inflammatory markers (procalcitonin [PCT], interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α]), metabolic parameters (fasting glucose, fasting insulin, HOMA-IR), prostate-specific antigen (PSA) and E.coli isolation with antibiotic resistance profiles across four distinct groups: Group 1 (n=40, type 2 diabetes without cancer), Group 2 (n=40, type 2 diabetes with untreated cancer), Group 3 (subset of Group 2 after two cycles of Docetaxel chemotherapy), and Group 4 (subset of Group 2 after five cycles of Docetaxel chemotherapy). Results are summarized in Table 2, Table 3 and illustrated in Figures 1–3.
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | P value |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| PCT (less than 0.05 ng/ml) | 0.03 ± 0.01 | 0.47± 0.3 | 1.15 ± 0.51 | 49.15 ± 5.8 | <0.0001 |
| IL-6 (less than 7 pg/ml) | 3.62 ± 1.4 | 9.63 ± 1.63 | 27.88 ± 9.5 | 131.5 ± 37.3 | <0.0001 |
| TN-F (less than 8.1 pg/ml) | 5.38 ± 1.7 | 2.3 ± 0.36 | 19.2 ± 3 | 37.3 ± 5.9 | <0.0001 |
| Fasting glucose (70-99 md/dl) | 63.07 ± 9.9 | 55.9 ± 8.8 | 88.1 ± 13.9 | 123.2 ± 19.5 | <0.0001 |
| Fasting insulin (2–20 μIU/ml) | 28.18 ± 5.9 | 33.25 ± 6.2 | 38.5 ± 5.1 | 48.75 ± 9.4 | <0.0001 |
| HOMA-IR ≥ 3.0 → Significant insulin resistance | 11.83 ± 5.4 | 19.10 ± 5.7 | 28.7 ± 10.4 | 40.26 ± 15.7 | <0.0001 |
| Prostate-Specific Antigen (PSA) | 3.8 ± 0.85 | 12.96 ± 3.5 | 10.67 ± 2.4 | 9.48 ± 1.8 | <0.0001 |
| Parameter | Group 2 (Pre-Chemotherapy) | Group 4 (Post-Chemotherapy) | Change (%) | P-Value |
| E. coli isolation rate | 67.5% (27/40) | 80% (32/40) | 12.5 | 0.2 |
| TMP-SMX Resistance | 80% (32/40) | 85% (34/40) | 5 | 0.54 |
| Ciprofloxacin Resistance | 87.5% (35/40) | 90% (36/40) | 2.5 | 0.72 |
| Ceftriaxone Resistance | 57.5% (23/40) | 62.5% (25/40) | 5 | 0.66 |
| Cefotaxime Resistance | 52.5% (21/40) | 55% (22/40) | 2.5 | 0.82 |
| Nitrofurantoin Resistance | 25% (10/40) | 30% (12/40) | 5 | 0.61 |
| Amoxicillin-Clavulanate Resistance | 72.5% (29/40) | 72.5% (29/40) | 0 | 1 |
| Amikacin Resistance | 17.5% (7/40) | 35% (14/40) | 17.5 | 0.08 |
| MDR Rate | 55% (22/40) | 57.5% (23/40) | 2.5 | 0.82 |
| XDR Rate | 20% (8/40) | 25% (10/40) | 5 | 0.6 |
Figure 1. Levels of Inflammatory Markers Across Study Groups.
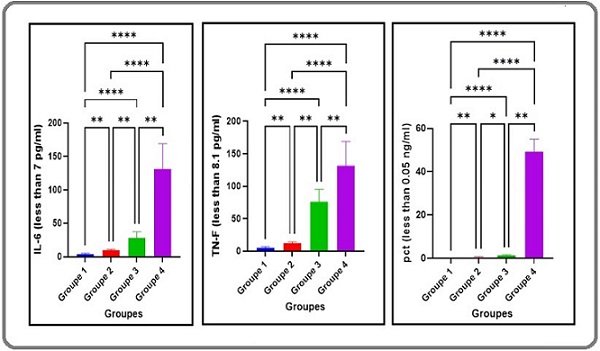
Figure 2. Metabolic Parameters Across Study Groups.
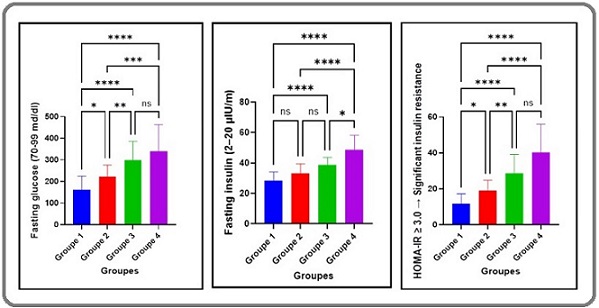
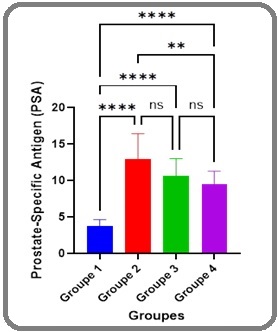
Figure 3. Prostate-Specific Antigen (PSA) Levels Across Study Groups .
Inflammatory Markers
The levels of PCT, IL-6, and TNF-α exhibited substantial variation among the groups (P≤0.01 for all comparisons, Table 2). Group 4 exhibited the highest concentrations of these markers, with substantially elevated levels compared to Groups 1, 2, and 3 (P≤0.01; Figure 1). Group 3 showed a substantial increase in PCT, IL-6, and TNF-α relative to Groups 1 and 2 (P≤0.01) but a modest reduction compared to Group 4 (P≤0.01). In contrast, Group 2 displayed a marked elevation in inflammatory markers compared to Group 1 (P≤0.01), though levels were significantly lower than in Groups 3 and 4 (P≤0.01). Group 1 consistently demonstrated the lowest levels of PCT, IL-6, and TNF-α among all groups (P≤0.01 vs. Groups 2–4). These findings suggest a progressive increase in systemic inflammation with cancer presence and chemotherapy duration (Figure 1).
Metabolic Parameters
Fasting glucose, fasting insulin, and HOMA-IR values also varied significantly across groups (P≤0.01, Table 2). Group 4 exhibited the highest levels of these parameters, with substantial increases compared to Groups 1, 2, and 3 (P≤0.01). Group 3 showed a significant rise in fasting glucose, fasting insulin, and HOMA-IR relative to Groups 1 and 2 (P≤0.01), but a slight decline compared to Group 4 (P≤0.01). Notably, No notable variations were detected between Groups 1 and 2 for any metabolic parameter (P>0.05), while Group 2 values were significantly lower than those in Groups 3 and 4 (P≤0.01). These results indicate that chemotherapy, particularly with extended cycles, exacerbates insulin resistance and glycemic dysregulation in diabetic patients with cancer (Figure 2).
Prostate-Specific Antigen (PSA)
PSA levels were significantly elevated in Groups 2, 3, and 4 compared to Group 1 (P≤0.01, Table 2; Figure 3), reflecting the presence of cancer in these cohorts. However, no notable differences in PSA were observed among Groups 2, 3, and 4 (P>0.05), suggesting that Docetaxel chemotherapy, regardless of cycle number, does not significantly alter PSA concentrations in this population. Group 1 maintained the lowest PSA levels (P≤0.01 vs. Groups 2–4), consistent with the absence of cancer.
Bacterial Isolation and Antibiotic Resistance in E. coli
E. coli was isolated from urine samples in 27 out of 40 patients (67.5%) in Group 2 (pre-chemotherapy) and 32 out of 40 patients (80%) in Group 4 (post-five cycles of Docetaxel chemotherapy), reflecting a 12.5% increase post-treatment (P=0.20, two-sample proportion test). Antibiotic resistance patterns were assessed among all isolates (n=40 per group) using the Kirby-Bauer method (Table 3). In Group 2, resistance rates were 80% for Trimethoprim-Sulfamethoxazole (TMP-SMX), 87.5% for Ciprofloxacin, 57.5% for Ceftriaxone, 52.5% for Cefotaxime, 25% for Nitrofurantoin, 72.5% for Amoxicillin-Clavulanate, and 17.5% for Amikacin, with 55% of isolates classified as multidrug-resistant (MDR) and 20% as extensively drug-resistant (XDR). In Group 4, resistance rates increased to 85% for TMP-SMX, 90% for Ciprofloxacin, 62.5% for Ceftriaxone, 55% for Cefotaxime, 30% for Nitrofurantoin, 72.5% for Amoxicillin-Clavulanate, and 35% for Amikacin, with 57.5% MDR and 25% XDR isolates. Notable increases post-chemotherapy included Amikacin (17.5% to 35%, P=0.08) and Nitrofurantoin (25% to 30%, P=0.61), though differences were not statistically significant (P>0.05). Resistance to Amoxicillin-Clavulanate remained stable (72.5%), while MDR and XDR rates rose slightly (55% to 57.5% and 20% to 25%, respectively). These findings suggest chemotherapy enhances both E. coli isolation and antibiotic resistance in T2D prostate cancer patients.
Discussion
This study elucidates the multifaceted effects of Docetaxel chemotherapy in type 2 diabetes (T2D) patients with prostate cancer. It demonstrates a dose-dependent escalation of systemic inflammation, metabolic dysregulation, and Escherichia coli infection risk alongside persistent PSA elevation. This novel study clarifies chemotherapy’s dual role in targeting malignancy while inadvertently exacerbating comorbidities, offering critical insights into optimizing treatment strategies.
The notable increase in inflammatory markers (PCT, IL- 6, TNF-α) across Groups 1–4 (P≤0.01), peaking in Group 4 post-five Docetaxel cycles, underscores chemotherapy’s role in amplifying systemic inflammation. This aligns with prior evidence that taxanes induce cytokine release via cytotoxicity and immune activation [15]. The concurrent rise in PCT, a biomarker of bacterial infection, with IL-6 and TNF-α in Group 4 (Table 2, Figure 1) suggests a synergistic interaction between chemotherapy-induced immunosuppression and infection-driven inflammation [16]. Neutropenia, a common sequela of Docetaxel due to bone marrow suppression [17], likely contributes by impairing neutrophil-mediated bacterial clearance, as evidenced by the increased E. coli isolation rate (80% in Group 4 vs. 67.5% in Group 2, P=0.20; Table 2). This inflammatory cascade, exacerbated by prolonged treatment, may perpetuate a chronic state that complicates cancer and diabetes management [18].
The dose-dependent rise in fasting glucose, insulin, and HOMA-IR value from Group 2 to Group 4 (P≤0.01), with no substantial variation between Groups 1 and 2 (P>0.05), indicates that chemotherapy, rather than prostate cancer alone, drives metabolic deterioration in T2D patients (Table 2, Figure 2). This mirrors findings linking chemotherapy to insulin resistance via oxidative stress and pro-inflammatory cytokines [19]. TNF-α, elevated across cancer groups, disrupts insulin signaling by promoting lipolysis and impairing receptor function, while IL-6 inhibits glucose uptake in skeletal muscle [20]. The peak HOMA-IR in Group 4 reflects a vicious cycle where inflammation and metabolic stress reinforce each other, potentially fueling cancer progression by providing glucose to tumor cells [21]. These metabolic shifts underscore the need for glycemic control during chemotherapy to mitigate adverse outcomes [22].
The elevated PSA levels in Groups 2–4 compared to Group 1 (P≤0.01), absent notable disparities among the cancer groups (P>0.05; Figure 3), deviate from the expected PSA decline with effective chemotherapy [23]. This stability may reflect Docetaxel resistance, a known challenge in advanced prostate cancer [24], or interference from infection-induced inflammation. The 12.5% increase in E. coli isolation and rising resistance (Amikacin: 17.5% to 35%, P=0.08; Table 3) in Group 4 suggests that chronic bacterial prostatitis, exacerbated by immunosuppression, sustains PSA elevation independently of tumor burden [25]. IL-6, a key modulator of acute-phase responses, may further amplify prostate inflammation [19, 26], masking chemotherapeutic efficacy. This dual contribution of malignancy and infection to PSA levels complicates its utility as a treatment response marker in this cohort, necessitating complementary diagnostics like imaging or biopsy.
The trend toward increased E. coli isolation and antibiotic resistance post-chemotherapy (XDR isolates: 20% to 25%, P=0.60; Table 3) highlights an underappreciated risk in T2D cancer patients. Chemotherapy’s depletion of lymphocytes and neutrophils [27], impairs adaptive immunity, fostering bacterial persistence and resistance development. The notable rise in Amikacin resistance (P=0.08), though not statistically significant, is clinically alarming given its role as a reserve antibiotic. This may stem from chemotherapy-induced gut dysbiosis, which alters microbial composition and promotes resistant strains [28]. The correlation between PCT elevation and
E. coli prevalence in Group 4 (Table 2, Table 3) supports a model where immunosuppression drives infection, which in turn sustains inflammation and metabolic decline, forming a feedback loop with broad systemic effects. Docetaxel may drive inflammation and bacterial resistance through gut microbiota dysbiosis and immune pathway disruption. Chemotherapy can reduce beneficial gut microbes, increasing pathogenic E. coli and resistance gene dissemination, potentially explaining elevated isolation rates (Table 3).
Inflammation, metabolic dysfunction, PSA stability, and bacterial resistance reflect a complicated pathophysiological network. ROS and cytokines (TNF-α, IL-6) from chemotherapy might generate oxidative inflammatory conditions that impair insulin signaling and immune function [29]. Hypoxic tumor regions prevent Docetaxel penetration, and CAFs or P-glycoproteins may promote tumor resistance [10]. Immunosuppression increases the risk of prostatitis and PSA levels from E. coli infections, possibly offsetting the tumor-suppressing effects.
This suggests using anti-inflammatory medications (IL-6 inhibitors), tight glycemic management, and preventive bacterial prophylaxis to decrease chemotherapy adverse effects in clinical practice. The recurring PSA and resistance patterns emphasize the need for personalized treatment regimens, which may involve resistance testing or alternative treatments if Docetaxel fails.
The study’s limitations include small sample size (n=40/group), reducing statistical power, a cross-sectional design limiting temporal insights, single-center recruitment affecting generalizability, and the absence of microbiome data restricting mechanistic understanding of bacterial resistance. Future studies with larger sample sizes, longitudinal methodologies, and metagenomic investigations can provide a better understanding of the metabolic effects of microbial changes. Analyzing tumor microenvironment alterations with clinical results may help type 2 diabetic patients understand chemotherapy’s efficacy and side effects.
In conclusion, in type 2 diabetes (T2D) prostate cancer patients, Docetaxel, systemic inflammation, metabolic dysregulation, and bacterial resistance interact in complex ways. The data show that chemotherapy promotes insulin resistance, chronic inflammation, and Escherichia coli prevalence and antibiotic resistance. Prolonged Docetaxel exposure led to significant side effects for Group 4, including increased inflammatory markers (TNF-α, IL-6, PCT), insulin, fasting glucose, HOMA-IR, and bacterial resistance rates after five treatment rounds. This study recommends targeting inflammation, glucose metabolism, and bacterial resistance in tailored cancer therapy. Future research should focus on helping this at-risk group.
Acknowledgments
Mustansiriyah University and Ibn Sina University of Medical and Pharmaceutical Sciences in Baghdad, Iraq, were much appreciated by the authors for their assistance with this endeavor.
Conflicts of interest
The authors report no conflicts of interest.
Authors’ contributions
Study conception & design: (Anwer Jaber Faisa, Baraa Ahmed Saeed). Literature search: (Anwer Jaber Faisal, Baraa Ahmed Saeed, Rabiah Muayad Sabri, Noor Kareem Kadhim, and Bassam Shaker Mahmood). Data acquisition: (Baraa Ahmed Saeed). Data analysis & interpretation: (Anwer Jaber Faisa, Baraa Ahmed Saeed, and Abbas A Mohammed). Manuscript preparation: (Anwer Jaber Faisal, Baraa Ahmed Saeed, Abbas A Mohammed, and Bassam Shaker Mahmood). Manuscript editing & review: (Baraa Ahmed Saeed and Anwer Jaber Faisal).
Data Availability
All data were included in this investigation.
References
- Epidemiology of Prostate Cancer Rawla P. World Journal of Oncology.2019;10(2). CrossRef
- Metronomic Chemotherapy in Prostate Cancer Wysocki PJ , Lubas MT , Wysocka ML . Journal of Clinical Medicine.2022;11(10). CrossRef
- Targeted Therapy for Cancers: From Ongoing Clinical Trials to FDA-Approved Drugs Choi HY , Chang J. International Journal of Molecular Sciences.2023;24(17). CrossRef
- Treatment of infections in cancer patients: an update from the neutropenia, infection and myelosuppression study group of the Multinational Association for Supportive Care in Cancer (MASCC) Rapoport BL , Cooksley T, Johnson DB , Anderson R, Shannon VR . Expert Review of Clinical Pharmacology.2021;14(3). CrossRef
- Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes Zatterale F, Longo M, Naderi J, Raciti GA , Desiderio A, Miele C, Beguinot F. Frontiers in Physiology.2019;10. CrossRef
- Clinical and biological risk factors associated with inflammation in patients with type 2 diabetes mellitus Ellulu MS , Samouda H. BMC endocrine disorders.2022;22(1). CrossRef
- Evaluation of the relationship of cytokines concentrations tumor necrosis factor-alpha, interleukin-6, and C-reactive protein in obese diabetics and obese non-diabetics: A comparative study Mohammad IJ , Kashanian S, Rafipour R, Aljwaid H, Hashemi S. Biotechnology and Applied Biochemistry.2024;71(2). CrossRef
- Biomarkers for prostate cancer: prostate-specific antigen and beyond Duffy MJ . Clinical Chemistry and Laboratory Medicine.2020;58(3). CrossRef
- A uropathogenic E. coli UTI89 model of prostatic inflammation and collagen accumulation for use in studying aberrant collagen production in the prostate Ruetten H, Sandhu J, Mueller B, Wang P, Zhang HL , Wegner KA , Cadena M, et al . American Journal of Physiology. Renal Physiology.2021;320(1). CrossRef
- Advances in Nanocarriers for Effective Delivery of Docetaxel in the Treatment of Lung Cancer: An Overview A Razak SA , Mohd Gazzali A, Fisol FA , M Abdulbaqi I, Parumasivam T, Mohtar N, A Wahab H. Cancers.2021;13(3). CrossRef
- Anticancer Activity of Secondary Metabolites Extracted from Endophytic Fungus in Pongamia pinnata Barks Mahmood BS , Faisal AJ , Thanoon AH , Saeed BA , Sulaiman GM , Malla S. Asian Pacific journal of cancer prevention: APJCP.2025;26(7). CrossRef
- Effects of Chemotherapy on the Immune System: Implications for Cancer Treatment and Patient Outcomes Sharma A, Jasrotia S, Kumar A. Naunyn-Schmiedeberg's Archives of Pharmacology.2024;397(5). CrossRef
- Wound Chronicity, Impaired Immunity and Infection in Diabetic Patients Rodríguez-Rodríguez N, Martínez-Jiménez I, García-Ojalvo A, Mendoza-Mari Y, Guillén-Nieto G, Armstrong DG , Berlanga-Acosta J. MEDICC review.2021;24(1). CrossRef
- Outer membrane protein genes and MDR resistance of Enterobacter cloacae in UTI of bladder cancer patients Faisal AJ Anwer Jaber, Jarallah ET Eman Thamer, Mahmood BS Bassam Shaker. Microbes and Infectious Diseases.2024. CrossRef
- Chemotherapy: a double-edged sword in cancer treatment Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, Falak R. Cancer immunology, immunotherapy: CII.2022;71(3). CrossRef
- Infections in Cancer Patients with Solid Tumors: A Review Rolston KVI . Infectious Diseases and Therapy.2017;6(1). CrossRef
- Efficacy of Plinabulin vs Pegfilgrastim for Prevention of Docetaxel-Induced Neutropenia in Patients With Solid Tumors: A Randomized Clinical Trial Blayney DW , Mohanlal R, Adamchuk H, Kirtbaya DV , Chen M, Du L, Ogenstad S, et al . JAMA network open.2022;5(1). CrossRef
- Microbes and the fate of neutrophils Kobayashi SD , DeLeo FR , Quinn MT . Immunological Reviews.2023;314(1). CrossRef
- Chronic Inflammation induced by Escherichia coli Blood Infections as a Risk Factor for Pancreatic Cancer Progression and the Diagnostic Role of CA19-9, Amylase, and Inflammatory Markers Saeed BA , Faisal AJ , Mahmood BS , Thanoon AH . Iraqi Journal of Cancer & Medical Genetics.2024;17.
- Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha Bruce CR , Dyck DJ . American Journal of Physiology. Endocrinology and Metabolism.2004;287(4). CrossRef
- Metabolic regulation of cell growth and proliferation Zhu J, Thompson CB . Nature Reviews. Molecular Cell Biology.2019;20(7). CrossRef
- Chemotherapy and Metabolic Syndrome: A Comprehensive Review of Molecular Pathways and Clinical Outcomes Trehan S, Singh G, Singh A, Bector G, Jain A, Antil P, Kalpana F, Farooq A, Singh H. Cureus.2024;16(8). CrossRef
- Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study Matsubara N, Chi KN , Özgüroğlu M, Rodriguez-Antolin A, Feyerabend S, Fein L, Alekseev BY , et al . European Urology.2020;77(4). CrossRef
- Overcoming Immune Resistance in Prostate Cancer: Challenges and Advances Movassaghi M, Chung R, Anderson CB , Stein M, Saenger Y, Faiena I. Cancers.2021;13(19). CrossRef
- The Molecular Basis and Clinical Consequences of Chronic Inflammation in Prostatic Diseases: Prostatitis, Benign Prostatic Hyperplasia, and Prostate Cancer Oseni SO , Naar C, Pavlović M, Asghar W, Hartmann JX , Fields GB , Esiobu N, Kumi-Diaka J. Cancers.2023;15(12). CrossRef
- Changes in Interleukins and Follicle Stimulating Hormone in Toxoplasmosis Male Patients Altemeemi AS , Kadhim NK , Nsaif GS . Indian Journal of Forensic Medicine & Toxicology.2021;15(1):803-907.
- Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC Kargl J, Zhu X, Zhang H, Yang GHY , Friesen TJ , Shipley M, Maeda DY , et al . JCI insight.2019;4(24). CrossRef
- Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways Wei L, Wen X, Xian CJ . International Journal of Molecular Sciences.2021;22(17). CrossRef
- ROS-associated immune response and metabolism: a mechanistic approach with implication of various diseases Banerjee S, Ghosh S, Mandal A, Ghosh N, Sil PC . Archives of Toxicology.2020;94(7). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details