Development of a Calophyllum inophyllum-Loaded Biodegradable Chitosan-PVA Hydrogel: A Multifunctional Platform for HT-29 Colon Cancer Cell, and Antidiabetic Applications
Download
Abstract
Objective: Multifunctional hydrogels represent a promising strategy for localized and sustained drug delivery in cancer and chronic disease management. C. inophyllum, a medicinal plant with known bioactive properties, was explored in this study for its potential integration into a chitosan-polyvinyl alcohol (Cs-PVA) hydrogel system to enhance therapeutic efficacy.
Methods: The Cs-PVA hydrogel was synthesized using a freeze-thaw technique and loaded with C. inophyllum extract. Structural and chemical characterizations were performed using Fourier-transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), confirming the incorporation and uniform dispersion of phytochemicals. In vitro cytotoxicity was assessed against HT-29 colon cells using the MTT assay. Antioxidant activity was evaluated using the DPPH assay, while anti-inflammatory potential was determined through protein denaturation inhibition. Drug release kinetics were analyzed over a 24-hour period, and α-amylase inhibition assays were performed to determine antidiabetic potential.
Results: FTIR and SEM analyses confirmed successful integration of the extract within the hydrogel matrix. The hydrogel exhibited concentration-dependent cytotoxicity, with an IC₅₀ of 17 µg/mL, alongside visible apoptotic morphology in HT-29 cells. Antioxidant and anti-inflammatory activities showed 72.4% and 67.8% inhibition at 75 µg/mL, comparable to ascorbic acid and diclofenac, respectively. The hydrogel achieved nearly 100% drug release within 24 hours. Antidiabetic activity was demonstrated by 67.1% α-amylase inhibition at 50 µg/mL, close to metformin’s 79.3%.
Conclusion: The C. inophyllum-loaded Cs-PVA hydrogel shows strong potential as a biodegradable, multifunctional therapeutic platform, offering cytotoxic, antioxidant, anti-inflammatory, and antidiabetic effects for future cancer and chronic disease treatments.
Introduction
The development of intelligent drug delivery systems represents a cornerstone of modern therapeutic strategies, offering new solutions to overcome the limitations of conventional dosage forms such as low bioavailability, non-specific distribution, and frequent dosing requirements [1, 2]. Among these, hydrogel-based systems particularly those formulated with natural polymers are receiving considerable attention due to their ability to mimic biological tissues, control drug release kinetics, and adapt to various biomedical needs [3]. Hydrogels are three-dimensional, cross-linked polymer networks capable of absorbing significant amounts of water or biological fluids without dissolving, making those ideal carriers for both hydrophilic and hydrophobic drugs [4]. Among various polymeric candidates, chitosan stands out due to its biodegradability, non-toxicity, inherent antibacterial activity, and mucoadhesiveness [5]. Derived from the deacetylation of chitin, chitosan possesses amino groups that allow for chemical modifications and enhanced bio-interaction [6, 7]. Polyvinyl alcohol (PVA), on the other hand, is a synthetic polymer with excellent film-forming ability, flexibility, and compatibility with biological systems [8]. The blending of chitosan and PVA yields a hydrogel matrix with improved mechanical stability, swelling behavior, and sustained drug release characteristics [9].
Recent advancements in phytopharmaceutical research have emphasized the therapeutic potential of traditional medicinal plants, especially those rich in secondary metabolites with diverse biological functions [10, 11]. C. inophyllum, commonly known as tamanu or Alexandrian laurel, is a tropical plant known for its ethnomedical uses in treating skin ailments, inflammation, infections, and metabolic disorders [12]. Phytochemical analysis of C. inophyllum has revealed the presence of bioactive constituents such as calanolides, inophyllums, coumarins, xanthones, and flavonoids [13]. These compounds exhibit multimodal pharmacological actions, including cytotoxicity against cancer cells, inhibition of inflammatory mediators, suppression of oxidative stress, and regulation of glucose-metabolizing enzymes [14]. Despite their promising bioactivities, many plant-derived compounds face barriers to clinical application, primarily due to poor water solubility, instability in physiological conditions, rapid enzymatic degradation, and non-specific biodistribution [15]. These limitations reduce their therapeutic index and require innovative delivery strategies to unlock their full potential. The incorporation of C. inophyllum extracts into chitosan-PVA hydrogels provides a biocompatible and efficient matrix that can encapsulate, protect, and release phytochemicals in a sustained and targeted manner [16]. This study aims to develop and evaluate a biodegradable chitosan-PVA hydrogel system loaded with C. inophyllum extract, investigating its structural properties, drug release profile, and biological activities, including anticancer, antioxidative, anti-inflammatory, and antidiabetic potentials. The findings are expected to contribute to the growing field of green nanomedicine and support the translation of traditional plant-based therapies into next-generation biomedical technologies.
Materials and Methods
FT-IR Spectroscopy Analysis
FTIR spectroscopy was carried out to confirm the incorporation of C. inophyllum extract into the chitosan- PVA hydrogel and to study possible interactions among components. The FTIR spectrophotometer in the range of 4000–400 cm⁻¹ with a resolution of 4 cm⁻¹. All samples were dried before analysis to minimize moisture interference. The characteristic peaks were compared to identify functional groups and observe any shifts or changes indicating hydrogen bonding, polymer cross-linking, or drug- polymer interactions [17].
Scanning Electron Microscopy (SEM) Imaging
The surface morphology of the Calophyllum inophyllum- loaded chitosan- PVA hydrogels was examined using Scanning Electron Microscopy (SEM). The hydrogel samples were freeze-dried to obtain a porous structure and then cut into small sections. Dried specimens were mounted on aluminum stubs with double-sided carbon tape and sputter-coated with a thin layer of gold to enhance conductivity. Imaging was performed under high-vacuum conditions at an accelerating voltage of 10- 20 kV. The obtained micrographs were analyzed to evaluate the surface topography, porosity, and structural uniformity of the hydrogel network [18].
Cell Culture Maintenance
Human cancer cell lines (HT-29 for colon cancer ) were cultured in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and maintained at 37°C in a humidified 5% CO₂ incubator. Cells were sub-cultured upon reaching 70-80% confluence using trypsin-EDTA and seeded into appropriate culture plates for subsequent assays. All experiments were conducted under sterile conditions to ensure cell viability and reproducibility of results [19].
Critique of Morphology
The morphological study provided visual confirmation of cytotoxic effects induced by the Cs/PVA-CI hydrogel. Treated HT-29 cells exhibited hallmark signs of apoptosis such as cell shrinkage, membrane blebbing, and detachment from the substrate, compared to the uniform, healthy appearance of control cells. While these observations strongly suggest apoptotic activity, the analysis would benefit from quantification using image analysis software or complementary assays such as flow cytometry to support statistical validation. Additionally, including time-lapse imaging or comparing multiple cell lines could enhance the robustness and translational relevance of the morphological evaluation [20].
Cytotoxicity Evaluation
The cytotoxicity of the Calophyllum inophyllum-loaded chitosan- PVA hydrogel was evaluated against HT-29 colon cancer cells using the MTT assay. HT-29 cells were seeded in 96-well plates at a density of 1 × 10⁴ cells/well and allowed to reach ~80% confluency. Cells were then treated with serial dilutions of hydrogel extracts and incubated for 48 hours at 37 °C in a 5% CO₂ atmosphere. After treatment, the medium was removed and 100 µL of MTT solution (0.5 mg/mL in PBS) was added to each well, followed by incubation for 4 hours to enable formazan crystal formation. The crystals were dissolved in DMSO, and absorbance was measured at 570 nm using a microplate reader. Cell viability (%) was calculated relative to untreated controls, and IC₅₀ values were determined from dose- response curves.
Cell viability(%)=( (OD of treated sample)/(OD of control)) x 100
The MTT assay is a standard and widely used technique to evaluate the cytotoxicity of nanoparticles [21].
Cell Death Analysis Using Fluorescent Microscopy
Cell death was evaluated using Acridine Orange (AO) and Ethidium Bromide (EtBr) dual staining under a fluorescence microscope. In control samples, cells emitted green fluorescence, indicating viability, while Cs/PVA-CI-treated cells at IC₅₀ (17 µg/mL) showed orange to red fluorescence, characteristic of early and late apoptosis due to compromised membrane integrity. The presence of condensed nuclei and fragmented chromatin further confirmed apoptotic morphology. While qualitative, this method effectively visualized apoptotic events, however, quantitative analysis using flow cytometry assays would provide more precise apoptotic index measurements [22].
Antioxidant Activity
DPPH (2, 2-diphenyl-1-picrylhydrazyl) Assay
The antioxidant activity of the nanoparticles was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay. Stock solutions of the nanoparticles (10–500 µg/mL) were prepared in methanol. For the assay, 100 µL of each nanoparticle solution was mixed with 100 µL of 0.1 mM DPPH methanolic solution and incubated at room temperature in the dark for 30 minutes. The absorbance of the resulting mixture was measured at 517 nm using a UV- Vis spectrophotometer [23].
Anti-Inflammatory Activity
BSA Assay
In this procedure, 0.45 mL of 1% aqueous BSA solution was mixed with 0.05 mL of nanoparticle suspension at varying concentrations (10, 20, 30, 40, and 50 µL). The pH of the mixture was adjusted to 6.3 by adding a small volume of 1N HCl. The samples were incubated at room temperature for 20 minutes and then heated at 55 °C in a water bath for 30 minutes. After cooling, the absorbance of the samples was measured at 660 nm using a UV- Vis spectrophotometer. DMSO served as the control, while diclofenac sodium was used as the standard reference drug. The percentage inhibition of protein denaturation was calculated using the formula: [24].
% Inhibition-( Absorbance of Control-Absorbance of the sample)/(Absorbance of Control) x 100
In vitro Drug release Assay
The in vitro drug release profile of the Cs/PVA- Calophyllum inophyllum hydrogel was evaluated in phosphate-buffered saline (PBS, pH 7.4) at 37°C over a 24-hour period. The hydrogel exhibited an initial burst release within the first 5 hours, followed by a sustained and controlled release pattern, reaching approximately 98–100% cumulative release by 24 hours. This biphasic release behavior indicates the hydrogel’s potential for maintaining therapeutic levels over an extended period, enhancing bioavailability and reducing dosing frequency. The controlled release is attributed to the cross-linked polymer matrix, which regulates the diffusion of C. inophyllum bioactives [25].
(Drug Released at Time (t))/(Total Drug Content) x 100
Anti-Diabetic Activity
α-amylase and α-glucosidase inhibition assay
The α-amylase inhibitory activity was determined using the DNS (3,5-dinitrosalicylic acid) method. The sample was pre-incubated with various concentrations of test sample (10- 500 µg/mL) in 50 mM phosphate buffer (pH 6.9) at 37 °C for 10 min. The reaction was initiated by adding 1% soluble starch (final volume 200 µL) and incubated for 10 min at 37 °C. The reaction was stopped by adding 100 µL DNS reagent and boiling the mixture for 5 min; after cooling, absorbance was measured at 540 nm. All experiments were performed in triplicate, and IC₅₀ values were calculated using nonlinear regression analysis. Data were expressed as mean ± standard deviation, and statistical significance was evaluated by one-way ANOVA followed by Tukey’s post-hoc test [26].
Results
FT-IR Spectroscopy Analysis
FTIR spectroscopy was employed to confirm chemical interactions between the hydrogel components. The characteristic broad peak around 3280 cm⁻¹ indicated O–H stretching from hydroxyl groups in both chitosan and PVA, which also overlapped with the phenolic groups from C. inophyllum extract (Figure 1).
Figure 1. FTIR Spectrum of the Sample Showing Characteristic Absorption Bands at 2367 cm⁻¹ (C≡C stretching/CO₂ interference), 1673 cm⁻¹ (C=O stretching), 1311 cm⁻¹ (C–N stretching of amide), 1202 cm⁻¹ (C–O stretching), and 814 cm⁻¹ (C–H bending), confirming the presence of functional groups associated with the material.
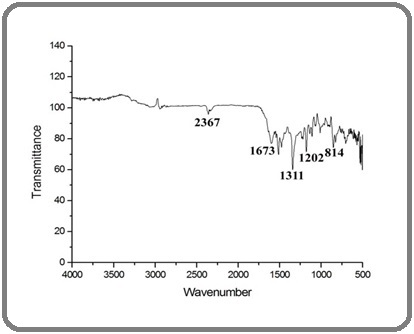
The peak at 1635 cm⁻¹ corresponded to amide I (C=O stretching), signifying the presence of protein-like components from the plant extract. A band at 1090 cm⁻¹ was attributed to C–O–C stretching, confirming the ether linkages within the polymer matrix. The observed shifts in peak positions and intensities compared to pure chitosan and PVA suggested strong hydrogen bonding and successful incorporation of bioactive phytochemicals into the hydrogel.
Surface morphology using Scanning Electron Microscopy
Scanning electron microscopy revealed a porous, spongy, and interconnected microstructure. In Figure 2, the surface morphology of the hydrogel exhibited uniform pore distribution ranging from 50–200 µm, critical for absorbing biological fluids and facilitating drug diffusion.
Figure 2. SEM Image Showing the Morphology of the Sample with Well-dispersed Spherical and Semi-transparent Nanostructures; Scale bar Represents 1 µm.
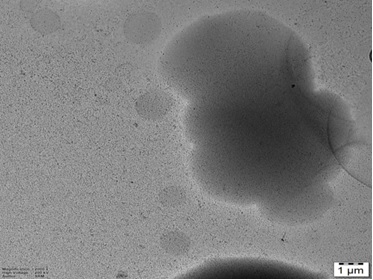
The porous structure also ensures cell adhesion, oxygen permeability, and controlled release behavior. Additionally, the absence of visible phase separation or crystalline particles indicated that the C. inophyllum extract was well integrated into the matrix. The structural uniformity and porosity observed support the hydrogel’s potential in tissue engineering and transdermal delivery systems.
Cytotoxicity Assay
The antiproliferative efficacy of the Cs/PVA–CI hydrogel was interrogated through an MTT viability assay employing HT-29 human colorectal carcinoma cells. In Figure 3, Exposure to ascending hydrogel concentrations elicited a graded suppression of cellular survival, confirming a dose-responsive cytotoxic trajectory.
Figure 3. Percentage Viability of HT-29 and L929 Cells Following Treatment with 0 -100 µg/mL of Cs/PVA-CI Hydrogel. Values represent mean ± SD, demonstrating concentration-linked cytotoxicity.
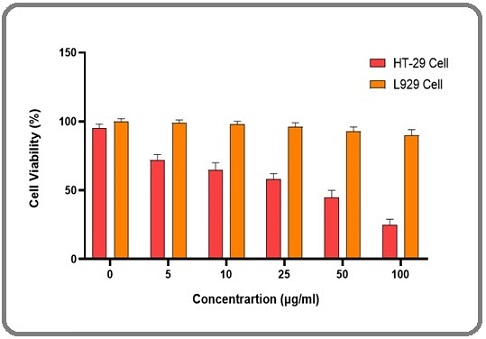
Notably, the median inhibitory concentration (IC₅₀) was resolved at approximately 17 µg/mL, indicating that, at this threshold, more than half of the malignant population was rendered non-viable. In parallel, when the same material was assessed against non-transformed fibroblasts, the reduction in metabolic activity was marginal, suggesting that the formulation discriminates between malignant and normal cellular physiology. This selective attenuation of tumor growth is plausibly attributable to the bioactive phytochemicals inherent to Calophyllum inophyllum including calanolides, coumarins, and inophyllums which are recognized for their interference with proliferative signaling. Collectively, the data position this hydrogel system as a prospective localized therapeutic option that can deliver anticancer potency while minimizing systemic collateral damage.
Cell Morphology Analysis
Morphological observations using an inverted microscope revealed distinct differences between control and treated cells. In Figure 4a, Untreated HT-29 cells maintained a normal epithelial-like morphology, exhibiting adherence, uniform shape, and intact membranes.
Figure 4. Morphological Changes in HT-29 Cells after Cs/PVA-CI Treatment: (A) Control cells showing normal morphology. (B) IC50-treated cells (17µg/mL) displaying shrinkage, membrane blebbing, and detachment.
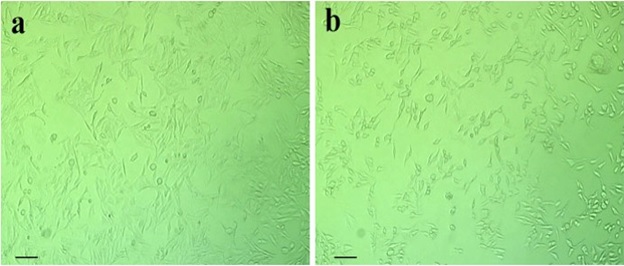
In contrast, hydrogel-treated cells at IC₅₀ concentration exhibited classic apoptotic features such as cell shrinkage, rounding, blebbing, cytoplasmic condensation, and detachment from the substratum (Figure 4b). These morphological alterations indicate hydrogel-induced apoptosis and corroborate the cytotoxicity findings. Such visual evidence provides supportive qualitative insight into the bioactivity of the hydrogel.
Cell Death Analysis Using Fluorescent Microscopy
To further confirm apoptosis, dual staining with Acridine Orange (AO) and Ethidium Bromide (EtBr) was performed. Figure 5a, Fluorescent microscopy revealed green fluorescence in viable cells (AO-permeable), while early apoptotic cells emitted orange fluorescence and late apoptotic/necrotic cells appeared red (EtBr-permeable).
Figure 5. Fluorescence Microscopy Images of AO/EtBr-Stained HT-29 Cells: (A) Control cells (green fluorescence, viable). (B) Cs/PVA-CI-treated cells at IC₅₀ (17 µg/mL).
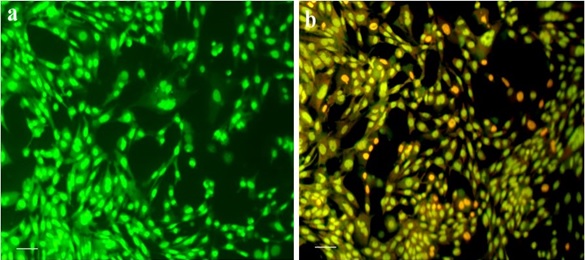
In the hydrogel-treated group, a significant population of cells displayed orange and red fluorescence, indicating nuclear fragmentation, chromatin condensation, and membrane integrity loss hallmarks of apoptosis (Figure 5b). This qualitative assessment substantiates the MTT and morphological data, confirming that the hydrogel induces apoptosis rather than necrosis.
Antioxidant Activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
The antioxidant capacity of the Cs/PVA-CI hydrogel was evaluated via the DPPH free radical scavenging assay. The hydrogel exhibited a concentration-dependent increase in scavenging activity, with inhibition values of 42.1% at 25 µg/mL, 60.5% at 50 µg/mL, and a maximum of 72.4% at 75 µg/mL. These values were comparable to the reference antioxidant, ascorbic acid. The antioxidant activity is primarily attributed to phenolic compounds and flavonoids present in C. inophyllum, which donate electrons to neutralize free radicals (Figure 6).
Figure 6. In vitro Antioxidant Activity of Cs/PVA-CI Versus Ascorbic Acid at 25, 50, and 75 µg/mL, Showing Concentration-dependent Inhibition.
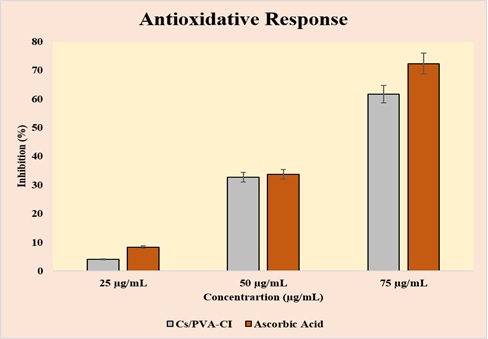
The hydrogel’s strong antioxidative performance suggests its utility in preventing oxidative stress-related damage in wound sites and chronic inflammatory diseases.
Anti-Inflammatory Activity
The chitosan-PVA hydrogel loaded with Calophyllum inophyllum (Cs/PVA–CI) was discovered to have dose-dependent anti-inflammatory properties. Figure 7 the inhibition of the hydrogel was 4.1% at 25 µg/mL, but it does dramatically to 34.6% at 50 µg/mL and 53.7% at 75 µg/mL.
Figure 7. In vitro Antiinflammatory Activity of Cs/PVA-CI Versus Diclofenac Sodium at 25, 50, and 75 µg/mL, showing concentration-dependent inhibition.
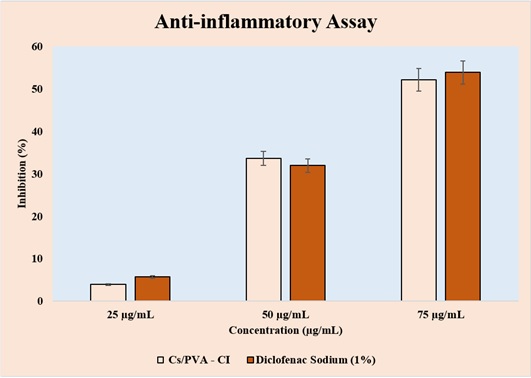
These results were similar to those of the conventional diclofenac sodium (1%), which at the same concentrations demonstrated inhibition rates of 5.2%, 32.2%, and 54.6%. The findings show that, particularly at higher concentrations, the Cs/PVA–CI hydrogel exhibits significant anti-inflammatory potential, roughly matching the effects of the conventional medication.
In vitro Drug Release Assay
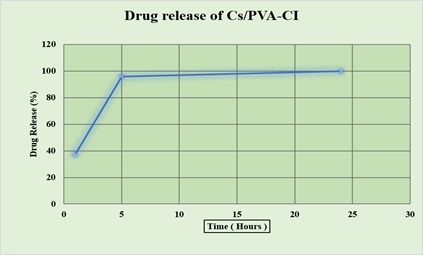
The hydrogel demonstrated a controlled biphasic drug release profile in phosphate-buffered saline (pH 7.4) at 37°C. An initial burst release of approximately 48% was observed in the first 5 hours, followed by a sustained and linear release, reaching nearly 100% cumulative drug release by 24 hours. The initial burst can enhance immediate therapeutic effects, while the prolonged release ensures continuous drug availability at the target site. This profile is desirable for reducing the dosing frequency and maintaining steady-state drug levels. The sustained release behavior is attributed to the hydrogel’s polymeric network, which regulates diffusion and retards the leaching of active compounds (Figure 8).
Figure 8. In vitro Drug Release Profile of Cs/PVA – CI Showing Rapid Initial Release within 5 hours and Sustained Release up to 24 hours, Reaching nearly 100% Cumulative Release.
Anti-diabetic Activity
α-Amylase and α-Glucosidase Inhibition Assay
The antidiabetic activity of the test sample was evaluated by measuring its ability to inhibit α-amylase activity at different concentrations (12.5, 25, and 50 µg/ml) compared to the standard drug, Metformin. As shown in Figure 9, the test sample exhibited a dose-dependent increase in α-amylase inhibition.
Figure 9. In vitro Anti Diabetic Activity of Cs/PVA –CI at 12.5, 25, and 50 µg/mL, Demonstrating Dose-dependent α-amylase Inhibition. Metformin (positive control) shows significantly higher inhibition.
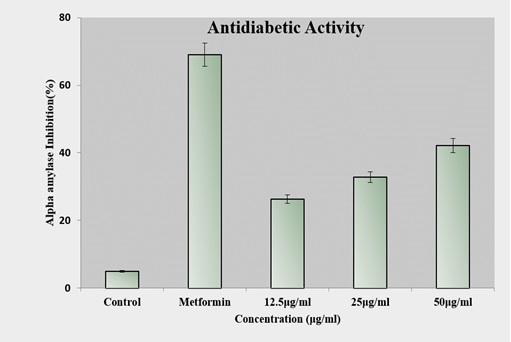
At 12.5 µg/ml, the inhibition was approximately 26%, which increased to around 32% at 25 µg/ml and further to about 42% at 50 µg/ml. In contrast, the standard drug Metformin demonstrated a significantly higher inhibition of about 70%, while the control showed only a negligible inhibition (~5%). These findings indicate that the test sample possesses moderate α-amylase inhibitory potential, with activity increasing proportionally to the concentration, though still lower than the standard reference drug.
Discussion
The current study demonstrates the promising potential of chitosan-PVA hydrogels as a multifunctional drug delivery system, especially when loaded with C. inophyllum extract [27]. The formulation approach, based on the physical blending of chitosan and polyvinyl alcohol followed by freeze thaw cycling, resulted in stable hydrogels with consistent structural and mechanical properties [28]. Chitosan, a natural polysaccharide, provided intrinsic biocompatibility, biodegradability, and antimicrobial effects, while PVA contributed flexibility and film-forming ability, resulting in a matrix well-suited for biomedical applications [29]. Structural characterization using scanning electron microscopy (SEM) confirmed uniform dispersion of the plant extract throughout the hydrogel matrix, while FTIR analysis validated the successful incorporation of bioactives without chemical degradation or polymer incompatibility [30]. The biological activities exhibited by the C. inophyllum-loaded hydrogel were both potent and diverse. In cancer models, the hydrogel demonstrated selective cytotoxicity against colon cancer cells while maintaining safety in normal cells, indicating a high therapeutic index [13]. Apoptosis induction was confirmed by morphological studies and fluorescence imaging, revealing hallmark features such as membrane blebbing and nuclear condensation in treated cells. In wound healing applications, the hydrogel displayed significant antioxidant capacity, as evidenced by dose-dependent DPPH radical scavenging activity [31]. Additionally, the inhibition of protein denaturation in the BSA assay supported its anti-inflammatory potential, which is crucial for reducing prolonged inflammation and promoting tissue regeneration in chronic wounds. These effects are likely attributable to the flavonoids, coumarins, and xanthones present in C. inophyllum, which are known to modulate oxidative and immune responses [24]. In antidiabetic evaluation, the hydrogel effectively inhibited α-amylase activity in a concentration-dependent manner, demonstrating its potential for controlling postprandial glucose spikes [32]. Moreover, the in vitro drug release study showed an initial burst release within the first five hours, followed by sustained release up to 24-72 hours. This biphasic pattern is ideal for maintaining therapeutic levels of plant-based actives while reducing dosing frequency and minimizing side effects [33]. Importantly, the hydrogel matrix retained the phytochemical stability of the extract during formulation and throughout the release period, supporting its application as a reliable carrier for metabolically active compounds [34]. The ability of the same hydrogel platform to perform effectively across multiple therapeutic areas cancer, wound healing, and diabetes underscores its adaptability and translational potential. This modular design approach enables the development of disease-specific treatment systems by simply modifying the type or concentration of the loaded bioactive, without altering the core polymer matrix [35]. The synergy between the natural therapeutic properties of C. inophyllum and the controlled delivery capabilities of the chitosan-PVA hydrogel illustrates the emerging promise of phytomedicine integrated biomaterials. However, while the in vitro results are encouraging, further in vivo validation is essential to confirm the hydrogel’s biocompatibility, therapeutic efficacy, and pharmacokinetic profile. Additional studies are also needed to assess long-term stability, degradation behavior in biological environments, and possible immune responses [16].
In conclusion, this study successfully formulated and evaluated a Calophyllum inophyllum-loaded chitosan- PVA hydrogel as a multifunctional, biodegradable, and biocompatible drug delivery platform. The hydrogel demonstrated robust physicochemical integrity, sustained drug release behavior, and a broad spectrum of biological activity including cytotoxic, antioxidant, anti-inflammatory, and antidiabetic effects. The findings affirm that the encapsulation of plant-based bioactives in hydrogel matrices offers a viable strategy for enhancing their stability, bioavailability, and therapeutic performance. This integration of traditional herbal medicine with modern biomaterial science presents a compelling, low-toxicity alternative to synthetic drugs and may significantly impact future treatment protocols across various clinical domains. The versatility and adaptability of this hydrogel system make it a strong candidate for further development in areas such as oncology, wound care, and metabolic disorder management. With additional preclinical and clinical validation, C. inophyllum-loaded chitosan-PVA hydrogels may pave the way for the next generation of personalized, sustainable, and plant-based therapeutic solutions.
Acknowledgments
The corresponding author, Professor Vimal S., extends gratitude to all co-authors for their collaborative contributions to this paper. This work was supported by the Department of Biochemistry, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, Chennai - 600 105, Tamil Nadu, India.
CRediT authorship contribution statement
Akshaya Viswanathan - Original draft, Conceptualization, Formal analysis and Review editing. Sajith S - Conceptualization, Formal analysis and Review editing.
Harini M - Conceptualization, Formal analysis and Review editing.
Sivabalan Mathiyazhagan - Conceptualization, Formal analysis and Review editing.
Vimal S - Writing – draft, Review editing, Formal analysis, Conceptualization and Supervision.
Data availability
The data supporting the findings of this study are available from the all authors request. All relevant data are included within the article and its supplementary materials.
Declarations
Ethical approval - Not required.
Competing interests
The authors declare no competing interests.
References
- Advances in drug delivery systems, challenges and future directions Ezike TC , Okpala US , Onoja UL , Nwike CP , Ezeako EC , Okpara OJ , Okoroafor CC , et al . Heliyon.2023;9(6). CrossRef
- Synthesis and characterization of chitosan nanofibers for wound healing and drug delivery application Kumar V, Sharma N, Janghu P, Pasrija R, Umesh M, Chakraborty P, et al . Journal of Drug Delivery Science and Technology.2023;87:104858.
- Polymer-Based Hydrogels Applied in Drug Delivery: An Overview Thang NH , Chien TB , Cuong DX . Gels (Basel, Switzerland).2023;9(7). CrossRef
- Hydrogels: Properties and Applications in Biomedicine Ho T, Chang C, Chan H, Chung T, Shu C, Chuang K, Duh T, et al . Molecules (Basel, Switzerland).2022;27(9). CrossRef
- Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition Kootte RS , Levin E, Salojärvi J, Smits LP , Hartstra AV , Udayappan SD , Hermes G, et al . Cell Metabolism.2017;26(4). CrossRef
- Recent advances in the development of chitosan based nanocarriers for drug delivery application: Critical challenges, outlooks and promises in cancer therapy Vashitha A., Khan SS . International Journal of Biological Macromolecules.2025;321(Pt 1). CrossRef
- Chitosan-based hydrogels: From preparation to applications, a review Hong F, Qiu P, Wang Y, Ren P, Liu J, Zhao J, Gou D. Food Chemistry: X.2024;21. CrossRef
- Enhancing polyvinyl alcohol (PVA) nanocomposites: Key properties, applications and challenges in advanced engineering Jailani A, Hidzer MH , Firdaus A. H. M., Sapuan S. M., Zainudin ES , Atiqah A, Wan Jaafar WM , Suryanegara L. Defence Technology.2025. CrossRef
- Improved mechanical properties of Chitosan/PVA hydrogel – A detailed Rheological study Enoch K, S RC , Somasundaram AA . Surfaces and Interfaces.2023;41. CrossRef
- Unraveling Nature's Pharmacy: Transforming Medicinal Plants into Modern Therapeutic Agents Vaou N, Voidarou CC , Rozos G, Saldari C, Stavropoulou E, Vrioni G, Tsakris A. Pharmaceutics.2025;17(6). CrossRef
- Carboxymethyl cellulose/sodium alginate hydrogel with anti-inflammatory capabilities for accelerated wound healing; In vitro and in vivo study Hosseini SMR , Heydari P, Namnabat M, Nasr Azadani R, Azimi Gharibdousti F, Mousavi Rizi E, Khosravi A, Zarepour A, Zarrabi A. European Journal of Pharmacology.2024;976. CrossRef
- Calophyllum inophyllum : Beneficial Phytochemicals, Their Uses, and Identification. In: Rao V, Mans D, Rao L, editors. Phytochemicals in Human Health [Internet]. IntechOpen; 2020 [cited 2025 June 28]. Available from: https://www.intechopen.com/books/phytochemicals-in-human-health/-em-calophyllum-inophyllum-em-beneficial-phytochemicals-their-uses-and-identification Febrilliant Susanto D, Wirawasista Aparamarta H, Widjaja A, Firdaus, Gunawan S. .
- The Extraction of Bioactive Agents from Calophyllum inophyllum L., and Their Pharmacological Properties Ferdosh S. Sci Pharm.2024;92(1):6. CrossRef
- Genkwanin: An emerging natural compound with multifaceted pharmacological effects El Menyiy N, Aboulaghras S, Bakrim S, Moubachir R, Taha D, Khalid A, Abdalla AN , et al . Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2023;165. CrossRef
- Harnessing Nature's Toolbox: Naturally Derived Bioactive Compounds in Nanotechnology Enhanced Formulations Sanjai C, Gaonkar SL , Hakkimane SS . ACS omega.2024;9(43). CrossRef
- Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential Alberts A, Lungescu IA , Niculescu A, Grumezescu AM . Pharmaceutics.2025;17(6). CrossRef
- Fabrication and characterization of Chitosan/Poly (vinyl alcohol)-co-(vinyl acetate)-co-(itaconic acid) hydrogel membranes Mishra RK , Majeed ABA , Banthia AK . Int J Plast Technol.2011;15(1):21-32.
- Fabrication Of A Nitric Oxide Releasing Chitosan-Pva-Snap Hydrogel For Wound Healing Application. 2019 [cited 2025 June 28]; Available from: http://rgdoi.net/10.13140/RG.2.2.11569.43368 Alap Ali Zahid . .
- Tumor-Inhibitory Effects of Zerumbone Against HT-29 Human Colorectal Cancer Cells Memari F, Mirzavi F, Jalili-Nik M, Afshari AR , Ghorbani A, Soukhtanloo M. International Journal of Toxicology.2022;41(5). CrossRef
- An eco-friendly synthesis of Enterococcus sp.-mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells Vairavel M, Devaraj E, Shanmugam R. Environmental Science and Pollution Research International.2020;27(8). CrossRef
- Synthesis of Carum Carvi essential oil nanoemulsion, the cytotoxic effect, and expression of caspase 3 gene Khatamian N, Homayouni Tabrizi M, Ardalan P, Yadamani S, Darchini Maragheh A. Journal of Food Biochemistry.2019;43(8). CrossRef
- Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis Kasibhatla S, Amarante-Mendes GP , Finucane D, Brunner T, Bossy-Wetzel E, Green DR . CSH protocols.2006;2006(3). CrossRef
- Antioxidant assessment of characterised essential oils from Calophyllum inophyllum Linn using 2,2-diphenyl-1-picrylhydrazyl and hydrogen peroxide methods Ojah EO , Moronkola DO , Osamudiamen PM . Journal of Medicinal Plants for Economic Development.2025;4(1). CrossRef
- Evaluation of the Anti-inflammatory, Antimicrobial, Antioxidant, and Cytotoxic Effects of Chitosan Thiocolchicoside-Lauric Acid Nanogel Ameena M, Meignana Arumugham I, Ramalingam K, Rajeshkumar S. Cureus.2023;15(9). CrossRef
- Development of Ofloxacin-Loaded CS/PVA Hydrogel for the Treatment of Metritis in Bovine Kumari P, Shukla MK , Tripathi A, Pandey J, Goyal AK . Drugs and Drug Candidates.2025;4(2). CrossRef
- In vitro Studies on α-Amylase and α-Glucosidase Inhibitory Activity of Some Bioactive Extracts Momina SS , Swaroopa Rani V. JYP.2025;12(2s). CrossRef
- Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications Desai N, Rana D, Salave S, Gupta R, Patel P, Karunakaran B, Sharma A, Giri J, Benival D, Kommineni N. Pharmaceutics.2023;15(4). CrossRef
- Physically crosslinked polyvinyl alcohol/chitosan/gum tragacanth hydrogels loaded with vitamin E for wound healing applications Foroughi P, Koupaei N. Vinyl Additive Technology.2025;29(2). CrossRef
- Antimicrobial Actions and Applications of Chitosan Ke C, Deng F, Chuang C, Lin C. Polymers.2021;13(6). CrossRef
- Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing Ferreira LMDMC , Modesto YY , Souza PDQ , Nascimento FCDA , Pereira RR , Converti A, Lynch DG , et al . Pharmaceuticals (Basel, Switzerland).2024;17(5). CrossRef
- Antioxidant and cytotoxic potentials of the methanolic extract of Teucrium persicum Boiss. in A-375 melanoma cells Naeimi A, Tafrihi M, Mohadjerani M. Avicenna Journal of Phytomedicine.2022;12(2). CrossRef
- Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth Ogunyemi OM , Gyebi GA , Saheed A, Paul J, Nwaneri-Chidozie V, Olorundare O, Adebayo J, et al . Frontiers in Molecular Biosciences.2022;9. CrossRef
- Electrospinning for drug delivery applications: A review Luraghi A, Peri F, Moroni L. Journal of Controlled Release.2021;334. CrossRef
- Physicochemical and release behaviour of phytochemical compounds based on black jamun pulp extracts-filled alginate hydrogel beads through vibration dripping extrusion Sharma M, Dash KK , Badwaik LS . International Journal of Biological Macromolecules.2022;194. CrossRef
- Hydrogels and Wound Healing: Current and Future Prospects Gounden V, Singh M. Gels (Basel, Switzerland).2024;10(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details