Emerging Roles of lncRNA MSC-AS1 in Cancer: Molecular Mechanisms, Clinical Implications, and Therapeutic Potential
Download
Abstract
Overview: Long non-coding RNAs (lncRNAs) are key regulators of tumor biology, affecting proliferation, apoptosis, metastasis, and treatment resistance. Among them, Musculin Antisense RNA 1 (MSC-AS1) has attracted increasing attention for its oncogenic roles across multiple cancers. This review summarizes current knowledge on MSC-AS1, emphasizing molecular mechanisms, clinical relevance, and therapeutic potential.
Methods: A comprehensive literature search of PubMed, Scopus, and Web of Science identified studies published 2015–2024 that investigated MSC-AS1 in cancer. Eligible articles examined its functional roles, implicated signaling pathways, regulatory interactions (e.g., lncRNA–miRNA–mRNA axes), and diagnostic, prognostic, or therapeutic implications.
Results: Evidence consistently shows that MSC-AS1 is frequently upregulated in gastric, hepatic, pancreatic, and colorectal cancers. Mechanistically, MSC-AS1 contributes to tumor progression mainly through lncRNA–miRNA–mRNA regulatory networks, promoting malignant phenotypes and therapeutic resistance. Clinically, elevated MSC-AS1 expression correlates with poor prognosis, supporting its utility as a biomarker for diagnosis and prognosis and as a candidate target for precision oncology.
Conclusion: MSC-AS1 functions as an oncogenic lncRNA across multiple gastrointestinal and hepatobiliary malignancies, driving disease through competitive regulatory networks and associating with adverse outcomes and resistance. Current evidence positions MSC-AS1 as a promising biomarker and a potential therapeutic target; future work should validate its clinical performance and evaluate targeted interventions against MSC-AS1-mediated pathways.
Introduction
Cancer is one of the most prevalent and deadly diseases, ranking first or second among noncommunicable diseases in terms of morbidity and mortality [1, 2]. Despite significant advances in cancer therapy over the past several decades, the survival rate, especially for those with advanced cancer and metastasis, remains poor [2].
Therefore, developing more effective clinical strategies and gaining a deeper understanding of the molecular mechanisms driving tumor growth are imperative [3, 4]. Among them, recent studies have highlighted that noncoding RNAs could be pivotal players in cancer pathogenesis [5]. Advances in epigenetic engineering,
such as zinc finger–based control of immune genes, show how targeted gene regulation can be used therapeutically. Similar mechanisms of transcriptional and epigenetic modulation are also central to the oncogenic functions of lncRNAs like MSC-AS1 [6].
lncRNAs, which are RNA molecules longer than 200 nucleotides, have been found to play a crucial role in regulating various biological processes, including growth, chromatin dynamics, differentiation, progression, and gene expression [7, 8]. Unlike mRNAs, lncRNAs lack protein-coding capacity [9]. However, recent research using innovative molecular approaches has revealed the presence of small open reading frames (sORFs) in some lncRNAs, enabling them to interact with ribosomes and encode short peptides [10, 11]. Numerous investigations have demonstrated that lncRNAs function as principal regulators of the treatment response, onset, and tumor progression in cancer [12, 13]. For instance, lncRNA MALAT1 suppresses metastasis and shows an inverse correlation with breast cancer progression, underscoring its potential as a metastasis-inhibiting lncRNA [14]. Moreover, research revealed that the HOXB-AS3-encoded peptide inhibits colorectal cancer (CRC) development, and loss of this peptide is correlated with a worse prognosis for CRC [15]. MSC-AS1, a novel lncRNA molecule, has recently emerged as an important factor in tumor development [16-19]. A study found that MSC- AS1 overexpression promotes the development of liver cancer by increasing the expression of Phosphoglycerate Kinase 1 (PGK1) [20]. Another study revealed that MSC-AS1 regulates the miR-524-5p/NR4A2 axis, thereby promoting nasopharyngeal carcinoma [21]. Additionally, MSC-AS1 may enhance the differentiation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts [22]. It has also been shown to aggravate nasopharyngeal carcinoma by sponging miR-524-5p, leading to increased NR4A2 expression [21]. Furthermore, Hu et al. demonstrated that MSC-AS1 regulates the migration and proliferation of kidney renal clear cell carcinoma (KIRC) cells through the miR-3924/WNT5A/ β-catenin axis [23].
This review aims to provide an in-depth overview of recent findings on the abnormal expression, molecular mechanisms, and clinical relevance of MSC-AS1 in various cancers.
Materials and Methods
This review aims to provide a comprehensive overview of the role of MSC-AS1 in various human cancers. To ensure broad coverage and minimize selection bias, no restrictions were applied regarding cancer type, experimental model, or publication date. A thorough search of the scientific literature was conducted using well-established databases, including PubMed, Scopus, Web of Science, and Google Scholar. The search strategy employed a combination of keywords such as “MSC-AS1,” “long non-coding RNA,” “cancer,” “tumor progression,” “prognostic biomarker,” “chemoresistance,” and “lncRNA–miRNA–mRNA axis.” Studies published up to May 20, 2024, were considered. Only peer-reviewed articles written in English and focusing on the biological functions, molecular mechanisms, diagnostic and prognostic relevance, and therapeutic potential of MSC-AS1 were included in this review.
LncRNA biogenesis and function
RNA polymerase II (Pol II) is responsible for the transcription of lncRNAs, which are characterized by the addition of a 5′ methyl-cytosine cap and 3′ poly (A) tail [24]. These molecules can be classified based on their genetic origin into long intergenic, intronic, and exonic lncRNA, and based on transcriptional orientation as sense or anti-sense lncRNA. Additionally, they can be classified into macro and retained intron lncRNAs based on splicing-based classification [25]. LncRNAs are crucial in numerous physiological and pathological processes by either activating or suppressing gene expression [26]. Primarily, they function as transcriptional regulators through interactions with transcriptional complexes, chromatin remodeling, or by serving as scaffolds that recruit various molecules [27]. Moreover, lncRNAs regulate the expression of genes by interacting with DNA, RNA, and protein near enhancers or promoters of target genes [28]. These interactions occur via a variety of methods, such as guide, scaffold, decoy, and signal [26]. Enzymatically or regulatory active protein complexes are bound by guide lncRNAs, which then direct the complexes to specific target gene promoters or chromosomal areas to regulate downstream signaling pathways and expression of genes [8]. Moreover, lncRNAs may be related to pancreatic cancer (PC) via regulating various post-transcriptional protein molecules, promoting or suppressing gene transcription, or interacting with miRNA to regulate or increase its expression. In this manner, lncRNAs can act as regulators for cancer suppressors or proto-oncogene genes [29] (Figure 1 and 2).
LncRNAs in cancer
There is no doubt that cancer is a life-threatening disease that poses a major risk to human health. Since the coding genome accounts for less than 2 percent of all gene sequences, most cancer phenotypes are driven by mutations in non-coding regions [30]. lncRNAs have been demonstrated to have a significant effect on various cellular processes, including angiogenesis, differentiation, migration, and proliferation [31, 32]. Aberrant lncRNA expression is common in cancer and can act as factors that either promote or inhibit tumor growth. In oral cancers, for example, the lncRNA ELDR is overexpressed and activates the ILF3-cyclin E1 axis signaling. ELDR suppression markedly reduces the growth of oral cancer cells [33]. In colorectal cancer, it has been discovered that the lncRNA ZFAS1 stimulates tumor growth and metastasis while inhibiting apoptosis. Additionally, studies have indicated that ZFAS1 overexpression is associated with poor prognosis in patients with colorectal cancer [34]. LINC00460 has been shown to promote the growth of cervical cancer cells and suppress apoptosis by downregulating miRNA-503-5p to advance cervical cancer progression. LINC00511 has been shown to upregulate MMP-13 and decrease miRNA-150 expression, driving invasion and proliferation [35, 36]. Conversely, lncRNA TUSC7 has been shown to sponge miRNA-23b to suppress epithelial-mesenchymal transition ( EMT) and metastasis in CRC. TSLC8 lncRNA has also been shown to increase stability, leading to reduced colony formation and survival of CRC. Collectively, these findings indicate that lncRNAs have a bright future as cancer therapeutic targets [37-39].
MSC-AS1
MSC-AS1 is classified as an lncRNA that does not code for proteins but performs other cellular functions. It has been associated with several types of cancer, including laryngeal cancer, colorectal cancer, and gastric cancer. MSC-AS1 may also influence the ability of mesenchymal stem cells to differentiate into various tissues, such as bone, cartilage, and fat. Evidence suggests that MSC-AS1 affects cancer progression and stem cell function by modulating the expression of genes involved in the PI3K/Akt pathway, which is essential for cell survival, proliferation, and division (Figure 1). MSC-AS1 is classified as a long non-coding RNA (lncRNA) that does not code for proteins but performs other cellular functions. It has been associated with several types of cancer, including laryngeal cancer, colorectal cancer, and gastric cancer. MSC-AS1 may also influence the ability of mesenchymal stem cells to differentiate into various tissues, such as bone, cartilage, and fat. Evidence suggests that MSC-AS1 affects cancer progression and stem cell function by modulating the expression of genes involved in the PI3K/Akt pathway, which is essential for cell survival, proliferation, and division (Figure 1).
Figure 1. Role of MSC-AS1 in Key Cancer-Related Cellular Processes. This diagram summarizes the key cellular processes regulated by MSC-AS1 in cancer. MSC-AS1 modulates the cell cycle, suppresses apoptosis, and promotes cell proliferation. It also enhances cell migration and induces EMT. Together, these functions promote tumor growth, metastasis, and treatment resistance, highlighting MSC-AS1’s oncogenic potential in various cancers.
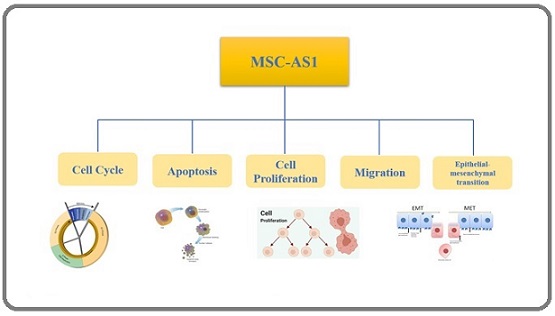
Numerous signals, including growth factors, cytokines, and hormones, can activate the PI3K/Akt pathway, which regulates various cellular functions such as angiogenesis, migration, metabolism, and cell death [16-18]. MSC-AS1 may influence the PI3K/Akt pathway by binding to and inhibiting microRNAs (miRNAs), which are small RNAs that suppress target gene expression by binding to messenger RNAs (mRNAs). For example, MSC-AS1 may bind to and inhibit miR-330-3p, which targets and reduces YAP1, a key factor in the PI3K/Akt pathway. By doing so, MSC-AS1 can increase YAP1 expression, enhancing the PI3K/Akt pathway. This promotes cancer cell survival and proliferation, as well as stem cell differentiation and function. Another mechanism by which MSC-AS1 affects cancer and stem cells is through a competing endogenous RNA (ceRNA) network. This network of interactions among lncRNAs, miRNAs, and mRNAs regulates gene expression at the post-transcriptional level. In a ceRNA network, lncRNAs act as sponges for miRNAs, thereby relieving repression of target mRNAs. For instance, MSC-AS1 may act as a sponge for miR-143-3p, which targets and reduces KRAS, another important factor in the PI3K/Akt pathway [40, 41].
Roles of MSC-AS1 in cancers
MSC-AS1 has been reported as an oncogene or tumor suppressor depending on the context, due to its diverse effects on tumor cell phenotypes (Figure 2).
Figure 2. Molecular Targets and Regulatory Pathways of MSC-AS1 in Various Human Cancers. MSC-AS1 contributes to the progression of multiple cancers by regulating specific microRNA–mRNA axes. In hepatocellular carcinoma, MSC-AS1 upregulates PGK1; in pancreatic cancer, it modulates miR-29b-3p/PFTK1. Similar regulatory axes are observed in melanoma (miR-302a-3p/LEF1), glioma (miR-373-3p/CPEB4), colorectal cancer (miR-325), nasopharyngeal carcinoma (miR-524-5p/NR4A2), osteosarcoma (miR-142/PI3K/AKT), ovarian cancer (miR-425-5p), and gastric cancer (miR-142-5p/DDX5, PFKFB3). The figure summarizes the cancer-specific downstream targets and regulatory functions of MSC-AS1 in tumor biology.
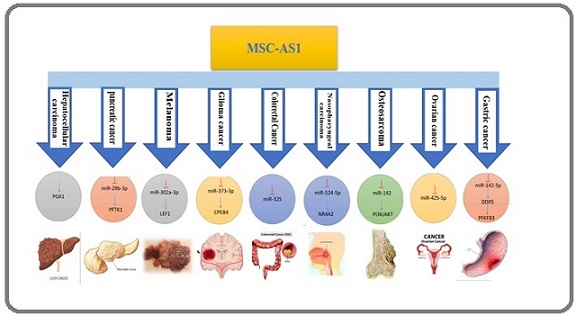
Furthermore, an accumulating body of research suggests that MSC-AS1 plays a role in the processes of cell division, apoptosis, invasion and metastasis, and resistance to therapy in a variety of malignancies. The changes in the expression level of lncRNA in various cancers are presented in Table 1.
| Tumor | Origin | Changes in Expression Level | Healthy Group | Illness Group | Ref |
| GC | Tissue | Upregulated | 80 normal adjacent tissues | 80 GC | [42] |
| Cell | Upregulated | GES-1 | MKN-45, AGS, SGC-7901, and MGC-803 | [42] | |
| GC | Tissue | Upregulated | 27 normal adjacent tissues | 27 GC | [41] |
| Cell | Upregulated | - | - | [41] | |
| GC | Tissue | Upregulated | 40 normal adjacent tissues | 40 GC | [43] |
| Cell | Upregulated | GES-1 | MGC-803, MKN-45 HGC-27, SGC-7901, and GES | [43] | |
| HCC | Tissue | Upregulated | 60 normal adjacent tissues | 60 HCC | [20] |
| Cell | Upregulated | HL-7702cells | HepG2, BEL-7404, SNU449 HUH- 7, and QGY-7701 | [20] | |
| HCC | Tissue | Upregulated | - | - | [44] |
| Cell | Upregulated | L02 | BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H | [44] | |
| PDAC | Tissue | Upregulated | 45 normal adjacent tissues | 45 PDAC | [16] |
| Cell | Upregulated | - | PANC-1 and BxPC-3 | [16] | |
| OC | Tissue | Downregulated | 25 normal adjacent tissues | 25 OC | [45] |
| cell | Downregulated | IOSE80 | OVCAR3, A2780, SKOV3 | [45] | |
| LC | Tissue | Upregulated | 37 normal adjacent tissues | 37 LC | [40] |
| Cell | Upregulated | - | - | [40] | |
| CRC | Tissue | Upregulated | 46 normal adjacent tissues | 46 CRC | [46] |
| Cell | Upregulated | NCM460 | HT29, SW620, HCT116, and SW480 | [46] | |
| NPC | Tissue | Upregulated | 34 normal adjacent tissues | 34 NPC | [21] |
| Cell | Upregulated | NP69 and NP460 | SUNE1, CNE-2, 5-8F, CNE-1 | [21] | |
| Glioma | Tissue | Upregulated | 25 normal adjacent tissues | 50 Glioma | [47] |
| cell | Upregulated | - | SHG-44, LN229, and 293T | [47] | |
| Melanoma | Tissue | Upregulated | - | - | [48] |
| Cell | Upregulated | HEMa-LP | A375, A2058, SK-MEL-5 and SKMEL-2 | [48] | |
| OS | Tissue | Downregulated | 45 normal adjacent tissues | 45 OS | [19] |
| Cell | Downregulated | hFOB1.19 | MG63, SOSP-9607, HOS, U2OS, and SaOS2 | [19] |
There has been evidence that the MSC-AS1 plays a crucial role in the development of several forms of cancer [41]. Several cancers have been associated with this compound, including OC, PC, Nasopharyngeal Cancer (NPC), and GC. The lncRNA-miRNA-mRNA network of MSC-AS1 has been demonstrated to modulate gemcitabine-induced apoptosis and cell proliferation in pancreatic cancer [16]. In PC, the lncRNA–miRNA– mRNA network of MSC-AS1 has been shown to modulate gemcitabine-induced apoptosis and cell proliferation [49]. Additionally, in ovarian cancer, MSC-AS1 has been shown to inhibit cancer progression by targeting miR-425-5p [45]. Furthermore, in gastric cancer, high expression of MSC-AS1 has been associated with a poor prognosis and might be used as a diagnostic biomarker and a predictor of unfavorable outcomes [41]. In NPC, MSC-AS1 promotes disease progression by sponging miR-524-5p and thereby upregulating NR4A2 expression [21]. Recent findings have revealed the involvement of MSC-AS1 in various tumor progressions. Research has indicated that MSC-AS1 may enhance the process of osteogenic differentiation in BMSCs [17]. In recent years, Hu et al have shown that MSC-AS1 regulates KIRC migration and cell proliferation through the miR3924- WNT5A-catenin axis [18].
Collectively, these findings underscore the critical role of MSC-AS1 in tumorigenesis and stem cell biology. They further suggest that MSC-AS1 may serve as a promising therapeutic target and prognostic biomarker. However, additional studies are required to elucidate the precise molecular mechanisms and validate its therapeutic potential.
The role of MSC-AS1 in cancer development and progression
Long non-coding RNAs (lncRNAs) have been extensively studied for their role in regulating gene expression, with important implications for diverse biological processes and diseases, including cancer. Among them, MSC-AS1 has emerged as a key regulator implicated in the pathogenesis of several cancer types. Multiple mechanisms have been identified to explain its involvement in cancer progression, primarily through interactions with microRNAs (miRNAs) and modulation of gene expression (Table 2).
| Disease | miR | Target | In vitro effect | Cell | In vivo effect | Animal | Ref |
| GC | - | PFKFB3 | Knockdown of MSC-AS1 inhibited glucose consumption, proliferation, lactate production, and pyruvate production. | AGS, GES-1/MKN-45, MGC-803and SGC-7901 | - | - | [42] |
| GC | miR-142-5p | DDX5 | induce cell cycle progression, cell growth, and inflammation | MKN-45, GES-1/HGC-27SGC-7901, GES and MGC-803 | - | - | [43] |
| HCC | - | PGK1 | Knockdown of MSC-AS1 inhibits colony formation capacity, cell proliferation, and triggers cell apoptosis | HL-7702cells /HUH-7, BEL-7404, SNU449, HepG2, and QGY-7701 | Decrease tumor volume and weight | nude mice | [20] |
| HCC | - | - | Knockdown of MSC-AS1 inhibits migration, cell proliferation, invasion and promotes apoptosis. | BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H | - | - | [44] |
| PDAC | miR-29b-3p | PFTK1 | Knockdown of MSC-AS1 inhibits cell proliferation, migration, and promotes apoptosis and invasion. | PANC-1 and BxPC-3 | - | - | [16] |
| OC | miR-425-5p | - | Promote apoptosis and inhibit cell proliferation, | OVCAR3, A2780, SKOV3 | - | - | [45] |
| CRC | miR-325 | TRIM14 | Knockdown of MSC-AS1 inhibits invasion, migration, and cell proliferation | NCM460/HT29, SW620, HCT116, and SW480 | - | - | [46] |
| NPC | miR-524-5p | NR4A2 | Knockdown of MSC-AS1 promotes apoptosis and inhibits cell proliferation | NP69 and NP460/SUNE1, CNE-2, 5-8F, CNE-1 | - | - | [21] |
| Glioma | miR‑373‑3p | CPEB4 | Knockdown of MSC-AS1 promotes apoptosis, TMZ sensitivity, and inhibits cell proliferation | LN229/ TR and SHG-44/TR | reducing tumor volume and weight | nude mice | [47] |
| Melanoma | miR-302a-3p | LEF1 | Knockdown of MSC-AS1 inhibits migration, cell proliferation, and EMT | HEMa-LP /A375, A2058, SK-MEL-5 and SKMEL-2 | Decrease tumor growth | nude mice | [48] |
| OS | miR-142 | PI3K/AKT | migration, cell proliferation, promote apoptosis, and invasion | hFOB1.19/MG63, SOSP-9607, HOS, U2OS, and SaOS2 | reduce tumor volume and weight | nude mice | [19] |
Results
Pancreatic Cancer
Approximately 64,050 new instances of PC are projected to arise globally in the United States in 2023 [50]. PC remains one of the deadliest cancers worldwide, largely because patients typically do not exhibit symptoms until advanced stages. Its incidence is higher in industrialized regions such as Europe, North America, and Australia, partly due to aging populations [23, 51, 52]. Non-coding RNAs (ncRNAs) play a crucial role in the progression and development of PC. Significantly, lncRNAs and microRNAs have particular relevance among them.
Sun et al. reported a significant correlation between the long non-coding RNA (lncRNA) MSC-AS1 and CDK14 in pancreatic ductal adenocarcinoma (PDAC) tissues, where MSC-AS1 was upregulated. Patients with higher MSC-AS1 expression had a poor prognosis. Suppressing MSC-AS1 reduced cell proliferation in BxPC-3 and Panc-1 cells. Additionally, the tumor suppressor miR-29b-3p was negatively correlated with MSC-AS1 levels; its reduced expression in PDAC patients was linked to unfavorable outcomes. Functional assays revealed that miR-29b- 3p negatively regulates both MSC-AS1 and CDK14. Knockdown of MSC-AS1 led to decreased CDK14 protein levels, inhibited PDAC proliferation, and increased gemcitabine-induced apoptosis [16].
Gastric cancer
GC ranks among the five most common malignancies and represents a major cause of cancer-related mortality worldwide, irrespective of a country’s level of development [52]. GC has a high fatality rate and poor prognosis [53]. ncRNAs play a crucial role in the progression and development of GC. Significantly, lncRNAs and microRNAs have particular relevance among them.
Jin et al. reported that MSC-AS1 was significantly overexpressed in GC tissues compared to adjacent non-tumor tissues and normal gastric mucosa. Elevated MSC-AS1 expression was associated with advanced tumor stage and poor prognosis. Functional assays showed that silencing MSC-AS1 suppressed GC cell proliferation and glycolytic activity, including lactate production, pyruvate production, and glucose consumption. Conversely, MSC-AS1 overexpression enhanced these processes. Mechanistically, MSC-AS1 promoted glycolysis by specifically upregulating PFKFB3, a key glycolytic enzyme, without affecting PKM2 or HK2. Restoration of PFKFB3 expression rescued glycolysis and proliferation in MSC-AS1–silenced cells [42].
Collectively, these findings indicate that MSC-AS1 promotes glycolysis and proliferation in GC cells by sustaining PFKFB3 expression, highlighting its potential as a therapeutic target in gastric cancer.
Yang et al. demonstrated that MSC-AS1 was significantly overexpressed in GC tissues compared to normal controls. Elevated MSC-AS1 expression promoted cell proliferation, cell cycle progression, and the release of inflammatory mediators such as TNF-α, IL-1β, and IL-6. Mechanistically, MSC-AS1 suppressed miR-142- 5p expression in HGC-27 cells, a microRNA that targets DDX5. Overexpression of miR-142-5p reduced DDX5 activity, whereas this effect was abolished upon DDX5 mutation, confirming the specificity of this regulatory axis. Furthermore, miR-142-5p levels were significantly reduced in GC tissues and showed an inverse correlation with MSC-AS1 expression. Collectively, these findings suggest that MSC-AS1 functions as an oncogene in gastric cancer through modulation of the MSC-AS1/miR-142-5p/ DDX5 pathway, highlighting its potential as a therapeutic target in GC [43].
Ovarian cancer
Ovarian cancer (OC) is one of the most lethal gynecologic malignancies worldwide and represents the leading cause of death among women with gynecologic cancers [45]. Patients are often diagnosed at an advanced stage, and diagnostic accuracy is low during the early stages [54]. Despite the progress made in treatment approaches for ovarian cancer, such as radiation, chemotherapy, and surgery, the rate of five-year survival for patients with this disease has remained unsatisfactory for an extended period [55-57]. The development and progression of OC are significantly influenced by ncRNAs. Among them, lncRNAs and microRNAs are of particular significance.
Zhao et al. observed that MSC-AS1 was downregulated in ovarian cancer cells and tissues, while miR-425-5p exhibited elevated expression. Overexpression of MSC-AS1 was found to significantly inhibit proliferation and induce apoptosis. Additionally, the study identified MSC-AS1 as a target and negative regulator of miR-425-5p, which partially reversed the tumor-suppressive effects of MSC-AS1. Collectively, these results suggest that MSC- AS1 acts as a tumor suppressor in OC by modulating the MSC-AS1/miR-425-5p axis, highlighting its potential as a therapeutic target [45].
Laryngeal cancer
There is a high morbidity and mortality rate associated with LC is a common type of head and neck cancer. In 2018, the global prevalence of LC accounted for 1% of all newly diagnosed cancer cases, while the fatality rate accounted for 1% of all cancer-related fatalities. The development and progression of LC are significantly influenced by ncRNAs [58].
Liu et al. found that MSC-AS1 lncRNA could be used as a biomarker for lung cancer (LC), and a ceRNA network involving MSC-AS1, miR-429, TGAV, and COL4A1 was constructed. Additionally, the MEblue module of a WGCNA network was related to the clinical stages of LC, with particular emphasis placed on the exploration of PI3K/Akt pathways. It was demonstrated by RT-qPCR that these genes were highly expressed in LC tissues, highlighting their importance for research [40].
Hepatocellular carcinoma
According to the World Health Organization, HCC is a major contributor to global cancer-related deaths. Annually, more than 500,000 new cases of HCC are documented [59, 60]. ncRNAs have significant involvement in the development and advancement of HCC. Notably, lncRNAs and microRNAs have special importance among them [61]. Cao et al. reported a notable increase in MSC-AS1 expression in both HCC tissues and cells. Inhibition of MSC-AS1 in the BEL-7404 and HepG2 cell lines resulted in a significant reduction in colony formation and cell proliferation, while simultaneously inducing apoptosis. Additionally, the cell cycle of BEL-7404 and HepG2 cells was effectively arrested in the G1 phase, leading to a marked decrease in cell migration and invasion. In vivo experiments further demonstrated that the suppression of MSC-AS1 impeded HCC growth by activating phosphoglycerate kinase 1 (PGK1). The data indicated that silencing MSC-AS1 in HCC cells was associated with a decrease in PGK1 expression. Collectively, these findings suggest that MSC-AS1 plays a critical role in promoting HCC development by enhancing PGK1 expression [20].
Kou et al. discovered that the expression of MSC-AS1 was significantly higher in HCC cells than in comparison with L02 cells. As compared to the blank and NC-siRNA groups, the colonization and proliferation of HCC cells were inhibited; however, the rate of apoptosis was significantly higher in the MSC-AS1 siRNA group. A significant reduction in wound healing rates and invasion rates was observed in the MSC-AS1 siRNA group when compared to the blank and NC-siRNA groups. These findings suggest that MSC-AS1 exhibited increased expression in HCC cells, and suppressing its expression may inhibit migration, cell proliferation, invasion, and enhance apoptosis in HCC cells [44].
Colorectal Cancer
According to the World Health Organization (WHO), CRC was projected to cause over 1.93 million deaths worldwide in 2020, making it the third leading cause of cancer-related mortality. The incidence and mortality rates of CRC are influenced by several risk factors, including poor dietary habits, obesity, and physical inactivity [62-65]. ncRNAs play a crucial role in the progression and development of HCC. Significantly, lncRNAs and microRNAs have particular relevance among them.
He et al. reported that the expression levels of TRIM14 and MSC-AS1 were elevated in tissues containing CRCs, whereas miR-325 exhibited reduced expression in these tissues. Functional experiments indicated that the suppression of MSC-AS1 led to decreased migration, invasion, and proliferation of cancer cells. Furthermore, miR-325 was identified as the specific microRNA targeted by MSC-AS1, with TRIM14 being the gene regulated by miR-325. It was also demonstrated that MSC-AS1 counteracted the inhibitory effects of miR-325 on cell growth and TRIM14 expression. Consequently, MSC-AS1 was shown to activate miR-325, thereby enhancing TRIM14 expression and promoting CRC progression. These findings suggest that MSC-AS1 could be a promising therapeutic target for the treatment of CRC [46].
Nasopharyngeal carcinoma
NPC is an aggressive malignancy of the head and neck that arises from the epithelial lining of the Yao et al. reported that MSC-AS1 expression was significantly elevated in both NPC cells and patient-derived tissues. Functional assays demonstrated that MSC-AS1 inhibited apoptosis, promoted cell proliferation, induced epithelial– mesenchymal transition (EMT), and enhanced invasive capacity. Mechanistically, MSC-AS1 acted as a sponge for miR-524-5p, thereby upregulating NR4A2 expression. Notably, overexpression of NR4A2 reversed the suppressive effects of MSC-AS1 knockdown on NPC progression, confirming the oncogenic role of NR4A2 in NPC cells. Collectively, these findings indicate that MSC- AS1 promotes NPC development through the miR-524- 5p/NR4A2 axis and may represent a promising therapeutic target for lncRNA-based interventions in NPC [21].
Glioma cancer
Glioma is a prevalent kind of brain cancer, making up around 40-50% of tumors that occur inside the skull [66]. Despite advancements in medical treatment techniques, the median survival duration for patients with glioma remains below one year [67]. ncRNAs play a crucial role in the progression and development of Glioma. Significantly, lncRNAs and microRNAs have particular relevance among them [68].
Li et al. found that MSC-AS1 expression was elevated in glioma tissues and cells that exhibited resistance to temozolomide (TMZ). Suppression of MSC-AS1 led to decreased resistance to TMZ and increased cell death. The influence of miR-373-3p was found to be reversible by silencing this microRNA, which directly targeted CPEB4 and affected the PI3K/Akt signaling pathway. Furthermore, knocking down miR-373-3p reversed the effects of CPEB4 or MSC-AS1 suppression on TMZ sensitivity. The study indicated that the PI3K/Akt pathway regulates the miR-373-3p/CPEB4 axis through MSC-AS1 knockdown, ultimately inhibiting the growth of TMZ- resistant glioma cells. Overall, MSC-AS1 inhibition suppressed glioma growth and enhanced TMZ sensitivity both in vitro and in vivo, highlighting its potential as a therapeutic target [47].
Melanoma
Melanoma is one of the most aggressive and lethal forms of skin cancer, and its incidence and mortality rates continue to rise despite considerable preventive efforts [69]. Early detection and treatment of melanoma might somewhat decrease the occurrence rate [70]. ncRNAs have significant involvement in the development and advancement of melanoma. Notably, lncRNAs and microRNAs have special importance among them.
Ma et al. demonstrated that the expression levels of MSC-AS1 and lymphoid enhancer-binding factor 1 (LEF1) were elevated in melanoma cell lines, while the expression of microRNA 302a-3p (miR-302a-3p) was reduced. Their study revealed that silencing MSC-AS1 inhibited migration, cell proliferation, and EMT in vitro, as well as tumor growth in vivo. Additionally, MSC-AS1 was found to modulate LEF1 expression by functioning as a sponge that recruits insulin-like growth factor 2 mRNA- binding protein 2 (IGF2BP2) and miR-302a-3p. The tumor progression rescue facilitated by the overexpression of LEF1 was hindered by the knockdown of MSC-AS1. Collectively, these findings indicate that the MSC-AS1/ miR-302a-3p/IGF2BP2/LEF1 axis plays a crucial role in melanoma progression and that MSC-AS1 may represent a promising biomarker and therapeutic target in melanoma [48].
Osteosarcoma
Osteosarcoma (OS) is the most common primary malignant bone tumor, characterized by multiple genetic abnormalities and the uncontrolled proliferation of mesenchymal cells that produce immature bone or malignant osteoid [71, 72]. ncRNAs play a crucial role in the progression and development of OS. Significantly, lncRNAs and microRNAs have particular relevance among them.
Zhang et al. demonstrated that silencing MSC-AS1 led to decreased proliferation, invasion, and migration of OS cells, while increasing apoptosis. Additionally, the suppression of MSC-AS1 heightened the susceptibility of OS cells to cisplatin (DDP). Their findings indicated that MSC-AS1 knockdown resulted in reduced levels of CDK6 and miR-142 expression, which subsequently decreased the protein expression of pPI3K/t-PI3K and pAKT/t-AKT. Furthermore, the suppression of MSC-AS1 impeded the progression of osteosarcoma in vivo by enhancing miR-142 levels, lowering CDK6 expression, and inactivating the PI3K/AKT signaling pathway. these findings highlight MSC-AS1 as a potential therapeutic target and suggest novel treatment strategies for osteosarcoma [19].
MSC-AS1 as a promising diagnostic biomarker in cancer prognosis
Early cancer detection is critical for effective treatment, yet current diagnostic methods often lack the specificity and sensitivity required for reliable early-stage identification [73]. This highlights the need for novel biomarkers that can improve early diagnosis and prognosis. Recent investigations have identified MSC-AS1 as a promising diagnostic and prognostic biomarker across several cancer types [41]. ncRNAs play a crucial role in the progression and development of hepatocellular carcinoma (HCC). Significantly, lncRNAs and microRNAs have particular relevance among them. MSC-AS1 is elevated in PDAC tissues, associated with a worse prognosis. Silencing of MSC-AS1 suppresses cell proliferation, but the elevated expression of miR-29b-3p is associated with improved prognosis. In gastric cancer, increased MSC-AS1 levels are substantially correlated with T stage, histological type, and grade, TP53 status, and PIK3CA status. Elevated MSC-AS1 expression forecasts reduced overall survival and progression-free interval, with its independent association with overall survival validated by multivariate analysis. Zhang et al. discovered that MSC-AS1 lncRNA is markedly elevated in OS tissues relative to surrounding normal bone tissues and correlates with worse prognosis. Increased MSC-AS1 levels were detected in osteosarcoma cell lines relative to normal osteoblasts. Likewise, MSC-AS1 levels are elevated in NPC tissues, with heightened levels associated with advanced tumor grade and worse prognosis.
SUN et al. found that MSC-AS1 expression was increased in pancreatic ductal adenocarcinoma (PDAC) tissues. Elevated MSC-AS1 levels were linked to worse prognosis in PDAC patients. Silencing MSC-AS1 in Panc-1 and BxPC-3 cells led to a significant reduction in cell proliferation. Furthermore, miR-29b-3p, which is known for its tumor-suppressing role, was predicted to bind to both MSC-AS1 and CDK14. Unlike MSC-AS1, elevated levels of miR-29b-3p were correlated with improved prognosis in patients with PDAC [16]. A different study revealed that MSC-AS1 expression was significantly elevated in GC tissues compared to non-tumor tissues. Additionally, higher MSC-AS1 levels were observed in GC cell lines relative to normal gastric mucosal GES-1 cells. Data from The Cancer Genome Atlas (TCGA) also indicated that elevated MSC-AS1 expression is strongly associated with advanced poor prognosis and tumor stages in GC patients [42].
Another study showed that MSC-AS1 levels were higher in gastric cancer. They showed that a significant association between elevated MSC-AS1 expression and factors such as histological classification, histological grade, T stage, and the status of TP53 and PIK3CA. Moreover, they revealed that patients with high MSC- AS1 expression had worse overall survival and shorter progression-free intervals. Additionally, multivariate survival analysis confirmed that MSC-AS1 expression independently predicted overall survival [41].
Zhang et al. revealed that MSC-AS1 lncRNA levels were significantly higher in OS tissues than in the surrounding normal tissues. The analysis suggested that increased MSC-AS1 expression correlated with worse prognosis in OS patients [19]. Likewise, in nasopharyngeal carcinoma (NPC), elevated MSC-AS1 expression is associated with advanced tumor grade and poor prognosis. Collectively, these findings support the role of MSC-AS1 as a clinically relevant biomarker for cancer diagnosis and prognosis across multiple tumor types.
MSC-AS1 in cancer chemotherapy
Chemotherapy remains one of the most widely used and effective treatment strategies for various cancers; however, its efficacy is often limited by the emergence of drug resistance. Although the mechanisms underlying this resistance are not fully understood, alterations within the tumor microenvironment (TME) are considered major contributors [74]. MSC-AS1 has been found to enhance chemotherapy resistance in various types of cancer, including glioma. In particular, MSC-AS1 levels are elevated in temozolomide (TMZ)-resistant cells and tissues. Patients with high MSC-AS1 expression tend to have shorter overall survival. Furthermore, reducing MSC-AS1 via knockdown impairs cell viability, proliferation, and sensitivity to TMZ in resistant glioma cells [47].
In gastric cancer, MSC-AS1 promotes cell proliferation and inflammatory mediator secretion while enhancing cisplatin resistance through the miR-142-5p/DDX5 axis [43]. MSC-AS1 interacts with miRNAs like miR-142-5p, miR-373-3p, and miR-425-5p to regulate chemotherapy resistance genes [45, 47]. MSC-AS1 promotes fatty acid oxidation (FAO) and stemness in gastric cancer cells, contributing to 5-FU and oxaliplatin resistance. MSC-AS1 influences cell cycle progression, apoptosis, and expression of proliferation markers like PCNA and Ki-67 to modulate chemosensitivity [45]. Collectively, these findings identify MSC-AS1 as a critical regulator of chemotherapy resistance in diverse cancers. Targeting MSC-AS1 and its downstream signaling pathways may provide a promising strategy to overcome resistance and improve the effectiveness of chemotherapeutic agents.
In conclusion, cancer research continues to evolve with emphasis on targeted therapies, epigenetic mechanisms, and environmental influences on tumor development across various types like ovarian, oral, and hepatocellular cancers. Biosensing advancements and regulatory networks further aid in diagnosis and treatment, highlighting nanotechnology’s broad potential in combating cancer [75-95]. lncRNAs are increasingly recognized as key regulators of tumor development, and MSC-AS1 has consistently demonstrated a strong association with tumor aggressiveness across multiple cancer types. Elevated MSC-AS1 expression correlates with adverse clinical features, including higher histological grade, larger tumor size, and reduced overall survival. Functional studies further support its oncogenic potential, showing that silencing MSC-AS1 significantly impairs cancer cell proliferation, invasion, and metastasis both in vitro and in vivo. These findings highlight MSC-AS1 as a promising candidate for prognostic biomarker development and therapeutic intervention. Although initial studies suggest its potential for early cancer detection, current evidence is insufficient for clinical application. Further validation through large-scale clinical trials and mechanistic studies is required to fully elucidate its diagnostic value, therapeutic feasibility, and underlying molecular pathways. Future investigations of MSC-AS1 may not only clarify lncRNA- driven mechanisms in tumorigenesis but also facilitate the development of innovative RNA-based biomarkers and precision therapies tailored to specific cancer subtypes.
Conflicts of Interest
The authors state that they have no competing interests or financial relationships relevant to this work.
Declaration
This manuscript was prepared without the use of any artificial intelligence (AI) tools or technologies.
Funding
Not applicable
References
- The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth Visser KE , Joyce JA . Cancer Cell.2023;41(3). CrossRef
- Immunotherapy for Ovarian Cancer: Disappointing or Promising? Deng M, Tang F, Chang X, Liu P, Ji X, Hao M, Wang Y, et al . Molecular Pharmaceutics.2024;21(2). CrossRef
- The covert symphony: cellular and molecular accomplices in breast cancer metastasis Si H, Esquivel M, Mendoza Mendoza E, Roarty K. Frontiers in Cell and Developmental Biology.2023;11. CrossRef
- Acquired Resistance to Clinical Cancer Therapy: A Twist in Physiological Signaling Wicki A, Mandalà M, Massi D, Taverna D, Tang H, Hemmings BA , Xue G. Physiological Reviews.2016;96(3). CrossRef
- Non-coding RNA in cancer Yan H, Bu P. Essays in Biochemistry.2021;65(4). CrossRef
- Enhanced epigenetic modulation via mRNA-encapsulated lipid nanoparticles enables targeted anti-inflammatory control Mokhtari T, Taheri MN , Akhlaghi S, Aryannejad A, Xiang Y, Mahajan V, Keshavarz K, et al . bioRxiv: The Preprint Server for Biology.2025. CrossRef
- Molecular mechanisms of long noncoding RNAs Wang KC , Chang HY . Molecular Cell.2011;43(6). CrossRef
- Long Noncoding RNA and Cancer: A New Paradigm Bhan A, Soleimani M, Mandal SS . Cancer Research.2017;77(15). CrossRef
- Long non-coding RNAs: definitions, functions, challenges and recommendations Mattick JS , Amaral PP , Carninci P, Carpenter S, Chang HY , Chen L, Chen R, et al . Nature Reviews. Molecular Cell Biology.2023;24(6). CrossRef
- Pervasive functional translation of noncanonical human open reading frames Chen J, Brunner A, Cogan JZ , Nuñez JK , Fields AP , Adamson B, Itzhak DN , et al . Science (New York, N.Y.).2020;367(6482). CrossRef
- A Hidden Human Proteome Signature Characterizes the Epithelial Mesenchymal Transition Program Vergara D, Verri T, Damato M, Trerotola M, Simeone P, Franck J, Fournier I, Salzet M, Maffia M. Current Pharmaceutical Design.2020;26(3). CrossRef
- Regulatory function of glycolysis-related lncRNAs in tumor progression: Mechanism, facts, and perspectives Peng X, Li S, Zeng A, Song L. Biochemical Pharmacology.2024;229. CrossRef
- lncRNAs as prognostic markers and therapeutic targets in cuproptosis-mediated cancer Bhat AA , Afzal M, Moglad E, Thapa R, Ali H, Almalki WH , Kazmi I, et al . Clinical and Experimental Medicine.2024;24(1). CrossRef
- Long noncoding RNA MALAT1 suppresses breast cancer metastasis Kim J, Piao H, Kim B, Yao F, Han Z, Wang Y, Xiao Z, et al . Nature Genetics.2018;50(12). CrossRef
- A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth Huang J, Chen M, Chen D, Gao X, Zhu S, Huang H, Hu M, Zhu H, Yan G. Molecular Cell.2017;68(1). CrossRef
- The role of lncRNA MSC-AS1/miR-29b-3p axis-mediated CDK14 modulation in pancreatic cancer proliferation and Gemcitabine-induced apoptosis Sun Y, Wang P, Yang W, Shan Y, Zhang Q, Wu H. Cancer Biology & Therapy.2019;20(6). CrossRef
- LncRNA MSC-AS1 promotes osteogenic differentiation and alleviates osteoporosis through sponging microRNA-140-5p to upregulate BMP2 Zhang N, Hu X, He S, Ding W, Wang F, Zhao Y, Huang Z. Biochemical and Biophysical Research Communications.2019;519(4). CrossRef
- lncRNA MSC-AS1 activates Wnt/β-catenin signaling pathway to modulate cell proliferation and migration in kidney renal clear cell carcinoma via miR-3924/WNT5A Hu Z, Li L, Cheng P, Liu Q, Zheng X, Peng F, Zhang Q. Journal of Cellular Biochemistry.2020;121(10). CrossRef
- Downregulated Long Non-Coding RNA MSC-AS1 Inhibits Osteosarcoma Progression and Increases Sensitivity to Cisplatin by Binding to MicroRNA-142 Zhang L, Zhao G, Ji S, Yuan Q, Zhou H. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research.2020;26. CrossRef
- Long noncoding RNA MSC-AS1 promotes hepatocellular carcinoma oncogenesis via inducing the expression of phosphoglycerate kinase 1 Cao C, Zhong Q, Lu L, Huang B, Li J, Meng L, Wei H. Cancer Medicine.2020;9(14). CrossRef
- LncRNA MSC-AS1 aggravates nasopharyngeal carcinoma progression by targeting miR-524-5p/nuclear receptor subfamily 4 group A member 2 (NR4A2) Yao H, Yang L, Tian L, Guo Y, Li Y. Cancer Cell International.2020;20(1). CrossRef
- Hacking the Cancer Genome: Profiling Therapeutically Actionable Long Non-coding RNAs Using CRISPR-Cas9 Screening Esposito R, Bosch N, Lanzós A, Polidori T, Pulido-Quetglas C, Johnson R. Cancer Cell.2019;35(4). CrossRef
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- Long Non-Coding RNAs in Pancreatic Cancer: Biologic Functions, Mechanisms, and Clinical Significance Li J, Hou S, Ye Z, Wang W, Hu X, Hang Q. Cancers.2022;14(9). CrossRef
- A Single Cell but Many Different Transcripts: A Journey into the World of Long Non-Coding RNAs Alessio E, Bonadio RS , Buson L, Chemello F, Cagnin S. International Journal of Molecular Sciences.2020;21(1). CrossRef
- Long non-coding RNAs: Biogenesis, functions, and clinical significance in gastric cancer Liu Y, Ding W, Yu W, Zhang Y, Ao X, Wang J. Molecular Therapy Oncolytics.2021;23. CrossRef
- Emerging roles of lncRNAs in the post-transcriptional regulation in cancer He R, Luo D, Mo Y. Genes & Diseases.2019;6(1). CrossRef
- Long Non-Coding RNAs: Biogenesis, Mechanism of Action and Role in Different Biological and Pathological Processes Shah IM , Dar MA , Bhat KA , Dar TA , Ahmad F, Ahmad SM . 2022.
- Role of non-coding RNA in pancreatic cancer Lv Y, Huang S. Oncology letters.2019;18(4):3963-73.
- Long Noncoding RNAs in Cancer Pathways Schmitt AM , Chang HY . Cancer Cell.2016;29(4). CrossRef
- Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Ranjbar A, Seyed Saleh SH , et al . Cancer Letters.2021;509. CrossRef
- Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition Cheng J, Wang L, Wang H, Tang F, Cai W, Sethi G, Xin H, Ma Z. Cells.2019;8(10). CrossRef
- Long non-coding RNA ELDR enhances oral cancer growth by promoting ILF3-cyclin E1 signaling Sur S, Nakanishi H, Steele R, Zhang D, Varvares MA , Ray RB . EMBO reports.2020;21(12). CrossRef
- Long non-coding RNA ZFAS1 promotes colorectal cancer tumorigenesis and development through DDX21-POLR1B regulatory axis Wang X, Wu Z, Qin W, Sun T, Lu S, Li Y, Wang Y, et al . Aging.2020;12(22). CrossRef
- Long non-coding RNA LINC00460 promotes proliferation and inhibits apoptosis of cervical cancer cells by targeting microRNA-503-5p Lin L, Xin B, Jiang T, Wang X, Yang H, Shi T. Molecular and Cellular Biochemistry.2020;475(1-2). CrossRef
- Long non-coding RNA LINC00511/miR-150/MMP13 axis promotes breast cancer proliferation, migration and invasion Shi G, Cheng Y, Zhang Y, Guo R, Li S, Hong X. Biochimica Et Biophysica Acta. Molecular Basis of Disease.2021;1867(3). CrossRef
- Long non-coding RNA MIAT promotes gastric cancer proliferation and metastasis via modulating the miR-331-3p/RAB5B pathway Li X, Jiao Y, Luan B, Wu H, Wang R, Zhong J. Oncology Letters.2020;20(6). CrossRef
- Long non-coding RNA DDX11-AS1 facilitates gastric cancer progression by regulating miR-873-5p/SPC18 axis Ren Z, Liu X, Si Y, Yang D. Artificial Cells, Nanomedicine, and Biotechnology.2020;48(1). CrossRef
- Long Non-coding RNA LINC01503 Promotes Gastric Cancer Cell Proliferation and Invasion by Regulating Wnt Signaling Ding J, Shi F, Xie G, Zhu Y. Digestive Diseases and Sciences.2021;66(2). CrossRef
- Identification of MSC-AS1, a novel lncRNA for the diagnosis of laryngeal cancer Liu Y, Meng W, Cao H, Wang B. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery.2021;278(4). CrossRef
- LncRNA MSC-AS1 Is a Diagnostic Biomarker and Predicts Poor Prognosis in Patients With Gastric Cancer by Integrated Bioinformatics Analysis Yang W, Ge F, Lu S, Shan Z, Peng L, Chai J, Liu H, et al . Frontiers in Medicine.2021;8. CrossRef
- Long non-coding RNA MSC-AS1 facilitates the proliferation and glycolysis of gastric cancer cells by regulating PFKFB3 expression Jin X, Qiao L, Fan H, Liao C, Zheng J, Wang W, Ma X, et al . International Journal of Medical Sciences.2021;18(2). CrossRef
- MSC-AS1 induced cell growth and inflammatory mediators secretion through sponging miR-142-5p/DDX5 in gastric carcinoma Liu Y, Li L, Wu X, Qi H, Gao Y, Li Y, Chen D. Aging.2021;13(7). CrossRef
- Expression of lncRNA MSC-AS1 in hepatocellular carcinoma cell lines and its effect on proliferation, apoptosis, and migration Kou X, Zhu J, Xie X, Hao M, Zhao Y. The Turkish Journal of Gastroenterology: The Official Journal of Turkish Society of Gastroenterology.2020;31(12). CrossRef
- LncRNA-MSC-AS1 inhibits the ovarian cancer progression by targeting miR-425-5p Zhao Y, Yuan D, Zhu D, Xu T, Huang A, Jiang L, et al . Journal of Ovarian Research.2021;14(1). CrossRef
- LncRNA MSC-AS1 Promotes Colorectal Cancer Progression by Regulating miR-325/TRIM14 Axis He C, Wang X, Du M, Dong Y. Journal of Oncology.2021;2021. CrossRef
- MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway Li C, Feng S, Chen L. Molecular and Cellular Biochemistry.2021;476(2). CrossRef
- LncRNA MSC-AS1 motivates the development of melanoma by binding to miR-302a-3p and recruiting IGF2BP2 to elevate LEF1 expression Ma Y, Jin Y, Li C, Liu Y, Wang D. Experimental Dermatology.2021;30(12). CrossRef
- Long Chain Non-Coding RNA MSC-AS1 Promote Invasion and Migration of Prostate Cancer Through Regulating microRNA-190a-3p Zhu Q, Yu R, He R, Song D, Liu W. Journal of Biomedical Nanotechnology.2025;19(10):1730-7. CrossRef
- ACS Report Shows Prostate Cancer on the Rise, Cervical Cancer on the Decline Clancy E. Renal & Urology News. 2023:NA-NA..
- Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages Yu J, Blackford AL , Dal Molin M, Wolfgang CL , Goggins M. Gut.2015;64(11). CrossRef
- Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS): A population-based comparison Pearce A, Sharp L, Hanly P, Barchuk A, Bray F, Camargo Cancela M, Gupta P, et al . Cancer Epidemiology.2018;53. CrossRef
- Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, et al . Lancet (London, England).2018;391(10125). CrossRef
- Neoadjuvant chemotherapy in elderly women with ovarian cancer: Rates of use and effectiveness Meyer LA , He W, Sun CC , Zhao H, Wright AA , Suidan RS , Dottino J, et al . Gynecologic Oncology.2018;150(3). CrossRef
- Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement Grossman DC , Curry SJ , Owens DK , Barry MJ , Davidson KW , Doubeni CA , Epling JW , et al . JAMA.2018;319(6). CrossRef
- Factors that influence survival in high-grade serous ovarian cancer: A complex relationship between molecular subtype, disease dissemination, and operability Torres D, Wang C, Kumar A, Bakkum-Gamez J, Weaver A, McGree M, et al . Gynecologic Oncology.2019;154(1):e18.
- Epigenetic Pathways Offer Targets for Ovarian Cancer Treatment Gyparaki M, Papavassiliou AG . Clinical Breast Cancer.2018;18(3). CrossRef
- Laryngeal Cancer: Epidemiology, Etiology, and Prevention: A Narrative Review Igissin N, Zatonskikh V, Telmanova Z, Tulebaev R, Moore M. Iranian Journal of Public Health.2023;52(11). CrossRef
- From Bedside to Boardroom: advancing Liver health for a hepatitis free future Lesi O. A.. 2022.
- Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals Kishta S, Tabll A, Omanovic Kolaric T, Smolic R, Smolic M. Biomedicines.2020;8(6). CrossRef
- Non-Coding RNAs: Regulating Disease Progression and Therapy Resistance in Hepatocellular Carcinoma Manna D, Sarkar D. Cancers.2020;12(5). CrossRef
- Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer Zheng X, Hur J, Nguyen LH , Liu J, Song M, Wu K, Smith-Warner SA , et al . Journal of the National Cancer Institute.2021;113(5). CrossRef
- Obesity and colorectal cancer Bardou M, Barkun AN , Martel M. Gut.2013;62(6). CrossRef
- Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States Knudsen MD , Wang L, Wang K, Wu K, Ogino S, Chan AT , Giovannucci E, Song M. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2022;20(6). CrossRef
- Beyond colonoscopy: Physical activity as a viable adjunct to prevent colorectal cancer Chang W, Chiu H. Digestive Endoscopy: Official Journal of the Japan Gastroenterological Endoscopy Society.2023;35(1). CrossRef
- Insights into molecular therapy of glioma: current challenges and next generation blueprint Rajesh Y., Pal I, Banik P, Chakraborty S, Borkar SA , Dey G, Mukherjee A, Mandal M. Acta Pharmacologica Sinica.2017;38(5). CrossRef
- Krüppel-like factor 9 inhibits glioma cell proliferation and tumorigenicity via downregulation of miR-21 Huang S, Wang C, Yi Y, Sun X, Luo M, Zhou Z, Li J, et al . Cancer Letters.2015;356(2 Pt B). CrossRef
- Insights into the Role of LncRNAs and miRNAs in Glioma Progression and Their Potential as Novel Therapeutic Targets Kciuk M, Yahya EB , Mohamed MMI , Abdulsamad MA , Allaq AA , Gielecińska A, Kontek R. Cancers.2023;15(13). CrossRef
- Melanoma biology and treatment: a review of novel regulated cell death-based approaches Hsieh M, Hsu S, Liu T, Wu C, Chiu C. Cancer Cell International.2024;24(1). CrossRef
- Primary malignant melanoma Mısır AF , Durmuşlar MC , Zerener T, Gün BD . Saudi Medical Journal.2016;37(4). CrossRef
- Clinical and Pathological Profile of Children and Adolescents with Osteosarcoma Ivan A, Cojocaru E, Sirbu PD , Al Namat DR , Tîrnovanu SD , Butnariu LI , Bernic J, et al . Diagnostics.2025;15(3). CrossRef
- Osteosarcoma: a comprehensive review Misaghi A, Goldin A, Awad M, Kulidjian AA . SICOT-J.2018;4. CrossRef
- Cancer Screening and Early Detection in the 21st Century Loud JT , Murphy J. Seminars in Oncology Nursing.2017;33(2). CrossRef
- Chemotherapy: a double-edged sword in cancer treatment Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, Falak R. Cancer immunology, immunotherapy: CII.2022;71(3). CrossRef
- Paclitaxel-Loaded PBCA Nanoparticles for Targeted Drug Delivery in Ovarian Cancer Ghahremani H, Ourang Z, Izadidehkordi S, Zandi S, Ebadi M, Ebrahimzade M. Asian Pacific Journal of Cancer Biology.2025;10(3). CrossRef
- Enhanced Antibacterial, Anti-Biofilm, and Anticancer Activities of Liposome-Encapsulated Selenium Nanoparticles: A Novel Therapeutic Approach Motavaf F, Abbasi M, Asadalizadeh H, Zandi S, Charmduzi F, Asadi M, Jafarlou M, Ghanbarikondori P, Ebrahimifar M. Asian Pacific journal of cancer prevention: APJCP.2025;26(8). CrossRef
- NUTM2A-AS1 as a potential key regulator in cancer: unraveling its ceRNA networks and impact on tumor biology Pourianazar NT , Radmehr S, Ourang Z, Jaseb K, Asadi A. European Journal of Medical Research.2025;30(1). CrossRef
- Recent advances in bioactive materials: Future perspectives and opportunities in oral cancer biosensing Ghahramani Y, Tabibi SS , Khan MMR , Asadi A, Mohammadi E, Khaksar E, Khaksar E, et al . Talanta.2025;286. CrossRef
- Aluminum Nanoparticles, a New Approach in Sustainable Chemistry and Usage in Medicine Asadi A, Khaksar E, Abbasi, R., & Ghahramani, Y S, Abbasi R, Ghahramani Y. Advances in Applied Nano-Bio Technologies.2025. CrossRef
- Gold nanoparticles: A powerful biosensor in oral medicine and dentistry Asadi A, Ghahramani Y. Journal of Oral and Dental Health Nexus.2025. CrossRef
- Visible-Light Responsive La₀.₇Sr₀.₃MnO₃@TiO₂/g-C₃N₄ Nanocomposite for Photocatalytic Antibiotic Degradation and Bioactivity Applications Nejat R, Zandi S. Journal of Alloys and Compounds.2025. CrossRef
- A Review of Poly Butyl Cyanoacrylate Nanoparticles as a Cancer Drug Delivery and Targeting Semyari S, Azizi S, Kundu D, Boroumandmoghaddam A, Moniri M, Ebrahimifar M, Toofani Milani A. Journal of Nanostructures.2021;11(4). CrossRef
- Bleomycin-loaded folic acid-conjugated nanoliposomes: a novel formulation for targeted treatment of oral cancer Saberian E, Jenčová J, Jenča A, Jenča A, Salehipoor F, Zare-Zardini H, Petrášová A, et al . Frontiers in Bioengineering and Biotechnology.2025;13. CrossRef
- Environmental Determinants of Oral Cancer Development: An Overview Maddahi M, Ghanbarikondori P, Amiri F, Abdi N, Jahromi AN , Pour NS , Allahyartorkaman M, Moazzam F. Asian Pacific Journal of Environment and Cancer.2024;7(1). CrossRef
- The Application of Polybutyl Cyanoacrylate (PBCA) Nanoparticles in Delivering Cancer Drugs Salehi V, Izadkhah M, Salehi H, Pour NS , Ghanbarikondori P. Asian Pacific Journal of Cancer Biology.2024;9(2). CrossRef
- Synergistic Effects of Platinum-Based Drugs and Curcumin on Liposomal Delivery in HSC-3 Oral Cancer Cells Amiri F, Ghanbarikondori P, Amoozegar H, Kazemi K, Sadrian S, Afshari-BehbahaniZadeh S, Akbarzadehkhayavi A. Indian Journal of Clinical Biochemistry.2025. CrossRef
- Improved Antitumor Efficacy of Liposome-Encapsulated Selenium Nanoparticles Asadalizadeh M, Ghahremani H, Ghanbarikondori P, Asadalizadeh H, Rahmani P, Motlagh FR . Asian Pacific Journal of Cancer Biology.2025;10(2). CrossRef
- Integrative Cancer Care: Leveraging Nutrition and Positive Psychology for Optimal Outcomes Arabmoorchegani M, Abbasi M, Asadalizadeh M, Motavaf F. Asian Pacific Journal of Cancer Nursing.2025. CrossRef
- Epigenetic regulation of hepatocellular carcinoma progression: MicroRNAs as therapeutic, diagnostic and prognostic factors Hashemi M, Daneii P, Asadalizadeh M, Tabari K, Matinahmadi A, Bidoki SS , Motlagh YSM , et al . The International Journal of Biochemistry & Cell Biology.2024;170. CrossRef
- Compression of radio and photo sensitivity of 5-aminolevulinic acid (5-ALA) conjugated hollow gold nanoparticles (HGNs) on KYSE cell line of oesophageal cancer Mohammadi Z, Imanparast A, Talebian H, Sobhani N, Shabanzadeh M, Sazgarnia A. Nanomedicine Research Journal.2025;9(3). CrossRef
- Reduction of Breast Surface Dose, Cancer and Mortality Risks Using Lead Apron during the Head Scanning a Computed Tomography Technique Asghari F, Gorji KE , Mehraeen R, Kiapour M, Talebian H, Monfared AS . Iranian Journal of Medical Physics/Majallah-I Fīzīk-I Pizishkī-i Īrān.1401;19(5). CrossRef
- Efficacy of 153Sm-EDTMP on Bone Pain Palliation in Metastatic Patients: Breast and Prostate Cancers Rokni M, Amiri M, Gorji KE , Talebian H, Bijani A, Vakili M, Shafiei H, et al . Frontiers in Biomedical Technologies.2023;10(3). CrossRef
- Cervical Cancer Tissue Analysis Using Photothermal Midinfrared Spectroscopic Imaging Reihanisaransari R, Gajjela CC , Wu X, Ishrak R, Zhong Y, Mayerich D, Berisha S, Reddy R. Chemical & Biomedical Imaging.2024;2(9). CrossRef
- Cancer-associated glomerulopathy; an updated review on current knowledge Dahmardnezhad M, Foodeh T, Afshinpoor S, Fooladivanda N. Journal of Nephropathology.2024;13(2). CrossRef
- Hypericin and resveratrol anti-tumor impact through E-cadherin, N-cadherin, galectin-3, and BAX/BCL-ratio; possible cancer immunotherapy succor on Y79 retinoblastoma cells Shahriari F, Barati M, Shahbazi S, Arani HZ , Dahmardnezhad M, Javidi MA , Alizade A. Precision Medical Sciences.2025;14(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details