An Experimental Study on Apoptosis in Lymphoid Tissues of SPF Chicken after Avian Influenza Serotypes H9N2 Infection
Download
Abstract
Background and purpose: Influenza virus induces cell death in animals and human through necrosis and apoptosis. The present study is an attempt to experimentally examine the type of cell death in lymphoid tissue of chicken infected by avian influenza serotype H9N2 (A/chicken/Iran/772/2000).
Methodology: Twenty SPF three weeks old chickens were grouped in two identical groups. The treatment group was infected by influenza virus (0.2ml, dilution 1:10, titration EID 50 107.5) and the control group received saline serum of the same volume through intranasal inoculation. Spleen, thymus, bursa of fabricius tissue specimens were collected after 72hrs. Afterward, microscopic intersections (5-6µm) stabilized in buffer formalin (10%) and marked by hematoxylin-eosin were prepared from the specimens.
Findings: Histopathological examination of lymphoid tissues of the treatment and control chickens showed necrosis, apoptosis, and lymphoid depletion in lymphoid tissues of the treatment group. The difference between treatment and control groups in terms of apoptosis in spleen tissue was significant (p<0.001); however, the difference in thymus and bursa of fabricius tissues was not significant. As to necrosis and lymphoid depletion, there was a significant difference between the two groups with regard to spleen, thymus, and bursa fabricius tissues. (p<0.001)
Conclusion: The survey showed that avian influenza serotype H9N2 can cause damages to lymphoid tissues through inducing apoptosis and necrosis
Introduction
Influenza as a viral disease was discovered in 1901 and a specific type of pathogenic influenza virus was discovered in 1955, which was then named as avian plague due to great losses it caused. There is no doubt about significance of influenza viruses as a global pathogen in human, domestic animals, and birds. Avian influenza virus is from orthomyxoviridae family, genus A. H9N2 strain of influenza virus A caused large scale loss of birds in Korea and China in 1994. Between 2001 and 2002, H9N2 was widely found in meat and bone marrow of the chickens imported from China to Japan. In March 1999, two separated influenza virus were obtained from girls at age range 1-4 in Hong Kong diagnosed with an influenza like disease. In addition, five H9N2 influenza viruses were extracted from human in 1998 [2].
There are proven evidences of intensification of pathogenic effect of H9N2 strain of influenza virus A separated from chickens in China by co-infections caused by golden staphylococcus and haemophilus paragallinarum [10]. Developing our knowledge about the ways that influenza viruses interact with the host cells at cell surface and the mechanisms and the pathways to induce cell death in the host helps us to find more efficient ways to deal with the disease. It is important to take into account that cell death in most of pathological cases (e.g. damaging viral infections) happens through necrosis; however, recent studies have suggested that many viruses such as influenza induce cell death through apoptosis or programmed cell death [3, 5, 11]. Influenza virus induces pathological changes in different tissues and lymphoid tissues are not an exception. Given the growing prevalence of influenza in human and livestock population, it is imperative to develop our knowledge about the pathogen and its viral strains such as H9N2 in terms of cell damages. The present study is an attempt to examine cell damages caused by avian influenza virus (AIV) serotype H9N2 infection; therefore, it can be helpful for expanding knowledge of some of pathogenic aspects of avian influenza.
Materials and Methods
Twenty SPF chicken three-weeks old (Valo Lohman Germany) were grouped randomly in groups of treatment and control (n = 10). The treatment group was infected through intranasal inoculation by influenza virus type H9N2 (0.2ml, dilution 1:10; titration EID50 107.5; A/chicken/Iran/772/2000), which was cloned two times in fertilized eggs. Simultaneously, the control group received saline solution of the same volume and through the same method. Three days after inoculation, the chickens were dissected simultaneously and lymphoid tissue samples were collected. The specimens were stabilized in buffer formalin (10%). Following dehydration, impregnation in paraffin, staining, and sectioning, 5-6µm thick layers of the specimens were prepared and marked by hematoxylin eosin and tunel. Based on Cohen’s table (effect coefficient: 0.7 and test power: 90), total sample size was obtained equal to 10. The collected data was analyzed in SPSS (v 13) using Mann-Whitney U test [1, 2, 4].
Results
The major tissue change in Bursa of fabricius was lymphoid depletion, which was due to necrosis and apoptosis of lymphoid cells (Fig 1-2).
Figure 1 :Microscopic view of bursa fabricius tissue of the treatment samples infected by influenza virus serotype H9N2 (hematoxylin eosin; magnification:x100). Note lymphoid depletions (arrows) induced by necrosis and apoptosis of lymphocyte cells.
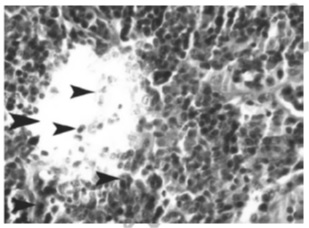
Figure 2 :Microscopic view of thymus tissue in the sample infected by influenza virus serotype H9N2 (hematoxylin-eosin; magnification: 20x)
Lymphoid depletion due simultaneous incidence of two models of cell death (necrosis and apoptosis) was observed in thymus of the samples infected by influenza virus (Fig 2-3).
Figure 3 :Microscopic view of thymus tissue of the sample infected by influenza virus serotype H9N2 (hematoxylin-eosin; magnification: x100). Note the intense lymphoid tissue depletion (arrows), necrosis, and apoptosis of the lymphocyte cells
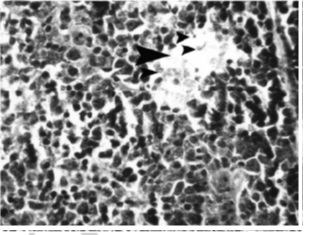
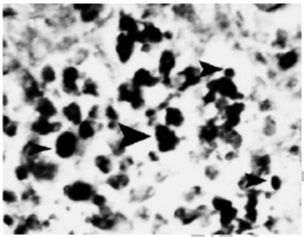
Apoptosis in lymphoid cells of spleen was considerable; the cells are demonstrated as condense partitioned nucleus in the samples marked by hematoxylin-eosin and brown in the samples marked by tunel (Fig 4-5).
Figure 4 :Microscopic view of spleen tissue in the samples infected by influenza virus serotype H9N2 (hematoxylin-eosin; magnification: 100X). Note sinusoidal histiocytosis and apoptosis of lymphocytes along with bleeding (arrows)
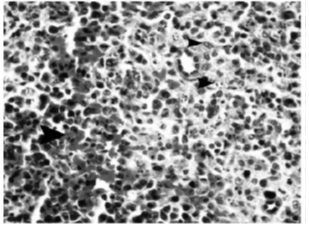
Figure 5 :Microscopic view of spleen tissue in the samples infected by influenza virus serotype H9N2 (marked by tunel; magnification: 1250X). Note lymphocyte positive tunel cells and histiocytosis (arrows)
Statistical analysis using Mann-Whitney U test showed that apoptotic changes of spleen tissue between the control and treatment groups was significant (p<0.001); while the changes were not significant in thymus and bursa of fabricius. In terms of lymphoid depletion and necrosis of cells in spleen, bursa of fabricius, and thymus, the difference between the treatment and control groups was significant. (p<0.001)
Discussion
Mangkol et al. (2007) noted that influenza virus serotype H5N1 induced apoptosis in lymphocyte cells of spleen red pulp and white pulp in some cases. They also reported that apoptosis was indirectly effective on over activation of immunity responses and discharge of excessive volume of inflammatory cytokines following influenza infection [12]. Roberto et al. (2004) examined the effects of influenza virus on necrotic damages in spleen tissue and other lymphoid organs along with severe bleeding and hyperemia [14]. Perkins et al. (2003) traced influenza virus serotype H5N1 in spleen tissue and reported that in 2 or 5 days after inoculation, viral antigens were cloned in histiocytosis of spleen and induced histiocytosis, endothelial cells, and myocytes of spleen arterial straight muscle death [13]. Mehranpour et al. (2007) studied the effects of H5N1 virus in turkeys and showed that the main damages happened in brain, pancreas and tonsil cecum [10]. The results of the present study were consistent with Mangkol, Roberto, Perkins, and Mehranpour and the role of H9N2 virus in damaging lymphoid tissue was explained. Apoptosis is the main finding in the present study, which might be coincident with several mechanisms. Kunio et al. highlighted hemagglutinin inducing effect of influenza virus and argued that adding the viral factor to sialoglycoprotein at cellular level and expression of inflammatory cytokines (e.g. IL-1β, IL-6, TNF.α, INF.γ, INF β, and GM.CSF) were of the factors effective on apoptosis [7]. However, the most prominent mechanism in this regard is the role of neuraminidase factor mediated by TGF. Β. Free radicals generated by oxidative stressors, which are probably effective on inducing cell death. Kunio mentioned that expression of GST-Д and MN-SOD depended mRNA decreased in presence of influenza infection; therefore, increase of cell oxidative stresses and oxygen free radicals lead to activation of caspases 8 & 9 and caspases 3, 6, 7 consequently and activation of apoptosis pathway [7]. Zhirnov et al. (2002) mentioned that M1 protein or matrix protein of influenza virus attach to caspases 3, 6, and 7 and apoptosis pathway is triggered when this set is attached to caspase 8. They also showed that non-structural protein of the virus (i.e. NS1) may be one of the main initiators of cell death in the case of influenza virus infection [19]. In this regard and along with the effect of NS1 protein, Takizaw et al. (2003) mentioned the role of protein Bcl2, ligand FAS, and activation of kinase protein enzyme and other viral proteins such as neuraminidase and PB1-F2 [18]. Consistently, Zhou et al. (2006) noted that by influencing macrophage cells, influenza virus generates proteins that are depended on TNF.α and TRIAL exclusive receptors and increases sensitivity of DRLs receptor and apoptosis subsequently [20]. Through a comparative study on other serotypes of influenza virus (H7N7, H4H8, H5N2, H5N0) Mo et al. (1997) found that there were moderate damages by serotypes H5N2 and H7N7 in lymphoid tissues in the form of histiocytosis and lymphocytes necrosis [11]. Our results indicated that along with cell necrosis, there was apoptosis in lymphoid tissues cells following experimental infection by avian influenza virus H9N2. Pazani et al. (2008) showed that influenza virus serotype A/Chicken/Tehran/ZMF173/99 (H9N2) induced mild lymphoid depletion, and atrophy of lymph follicle in most of lymphatic tissues. Histopathology studies by the same research team on spleen tissue also showed lymphoid depletion and mild hyperemia in sinusoids tissue. They also reported extreme hyperemia along with necrosis of lymphoid tissue in Thymus tissue and the major damages were reported in thymus and bursa tissues. Results of the present study, however, showed that influenza virus serotype H9N2 (A/chicken/Iran/772/2000) caused the major tissue damages in spleen white pulp mostly in the form of necrosis and apoptosis of lymphocyte and macrophage cells. Pazani et al.’s reported consistent results as to thymus and bursa tissue; while they noted that damage in spleen tissue was more of reactive hyperplasia rather that tissue necrosis. On the other hand, majority of researchers in this field such as Shalaby et al. (1994), Swayne et al. (1995), and To et al. (2001) reported necrosis and lymphocyte depletion of spleen tissue. Clearly, diversity of serotypes of influenza virus may influence diversity of tissue damages [15-17]. Given the above, one can say that cell apoptosis in lymphoid tissues following infection by influenza virus serotype (H9N2) is more probable through the mentioned pathways while detail explanation of the molecular mechanisms in the case of H9N2 serotype needs supplementary studies. One conclusion is that there are several serotypes of influenza virus effective on cell death and H9N2 is not an exemption. In short, the both types of cell death (necrosis and apoptosis) happened following influenza infection in lymphoid tissues of chicken and there is a need for large sale molecular studies on serotype H9N2 to achieve a clearer image of cell death mechanism.
Acknowledgements
This article is part of thesis work of student of Msc Yusef Doustar. The authors express their gratitude to all their colleagues in research department of the Islamic Azad University.
References
[1]. Doustar Y. Experimental study of apoptosis induced by infectious bursal disease virus using Tunel assay and electron microscope [dissertation]. Tehran: Tehran University; 2004.
[2]. Hablolvarid MH, Sohraby Haghdost I, Pourbakhsk SA, Gholami MR. A study on Histopathologic changes in chicken following intravenous inoculation with avian influenza virus A/chicken/Iran/1988(H9N2). Archives of Razi Institute. 2003;55:41-51.
[3]. Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8(2):113-26.
[4]. Frankfurt OS, Krishan A. Identification of apoptotic cells by formamide-induced dna denaturation in condensed chromatin. J Histochem Cytochem. 2001;49(3):369-78.
[5]. Ito T, Kobayashi Y, Morita T, Horimoto G, Kawaoka Y. Virulent influenza A viruses induces apoptosis in chickens. Virus Res. 2002;84(1-2):27-35.
[6]. Kwon YK, Joh SJ, Kim MC, Sung HW, Lee YJ, Choi JG, et al. Highly pathogenic avian influenza (H5N1) in the commercial domestic ducks of South Korea. Avian Pathol. 2005;34(4):367-70.
[7]. Kunio O, Manabu N, Bo Y, Toshio B, Toshio Y. Apoptosis induced by influenza virus-hemagglutin stimulation may be related to fluctuation of cellular oxidative condition. Biol Pharmacol Bull. 2003;26:141-47.
[8]. Liu J, Okazaki K, Ozaki H, Sakoda Y, Wu Q, Chen F, et al. H9N2 influenza viruses prevalent in poultry in china is phylogenetically distinct from A/quail/ Hong Kong/G1/97 presumed to be the donor of the internal protein genes of the H5N1 Hong Kong/ 97virus. Avian Pathol. 2003;32:552-60.
[9]. Morris J, Nightingale S, Harry S, Clive S. Influenza A virus induced apoptosis is a multifactorial process: Exploiting reverse genetics to elucidate the role of influenza A virus proteins in virus . induced apoptosis. Virology .2005;335(2):198-211.
[10]. Mehranpour MJ, Dadras H, Khodakaram-Tafti A, Rahimian A, Toffan A. Pathological finding of highly pathogenic avian influenza virus A/Duck/Vietnam/12/2005(H5N1) in turkey. Int J Poultr Sci .2007;6(9):679-83.
[11]. Mo OI, Brugh M, Fletcher J, Rowland GN, Swayne G. Comparative pathology of chickens experimentally inoculated with avian influenza virus of low and high pathogenicity. Avian Dis. 1997;41(1):125-36.
[12].Uiprasertkul M, Kitphati R, Puthavathana P, Kriwong R, Kongchanagul A, Ungchusak K, et al. Apoptosis and pathgenesis of avian influenza A(H5N1) virus in humans. Emerg Infect Dis.2007;13(5):708-12.
[13]. Perkins LEL, Swayne D. Varied pathogenecity of a Hong Kong-origin H5N1 avian influenza virus in four passé ring species and budgerigars. Vet Pathol.2003;40:14-24.
[14]. Roberto P, Enriqe A, Moises F, Luis P. Avian influenza: Histopathology and viral detection in formalin-fixed paraffin-embedded tissue by RT-PCR. Vet Med. 2004;35:1-9.
[15]. Shalaby AA, Slemons RD, Swayne DE. Pathological studies of A/chicken/Alabama/7395/75 (H4N8) influenza virus in specific-pathogen-free laying hens. Avian Dis. 1994;38(1):22-32.
[16]. Swayne DE, Slemons RD. Comparative pathology of intravenously inoculated wild duck- and turkey-origin types A influenza viruses in chickens. Avian Dis. 1995;39:74-84.
[17]. To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63(3):242-6.
[18]. Takizawa T. [Influenza virus infection and apoptosis]. Nihon Rinsho. 2003;61(11):2001-5.
[19]. Zhirnov OP, Ksenofontov AL, Kuzmina SG, Klenk HD. Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry (Mosc). 2002;67(5):534-9.
[20]. Zhou J, Law HK, Cheung CY, Ng IH, Peiris JS, Lau YL. Functional tumor necrosis factor-related apoptosis-inducing ligand production by avian influenza virus-infected macrophages. J Infect Dis. 2006;193(7):945-53.
License
Copyright
© ,
Author Details